Changes in and Predictors of HIV among People Who Inject Drugs in Mizoram, Northeast India, from 2007 to 2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Study Region
2.4. Ethical Considerations
2.5. Outcome and Exploratory Variables
3. Statistical Analysis
4. Results
4.1. Sociodemographic Characteristics and Injecting and Sexual Behaviours of Study Participants (Years 2007–2021)
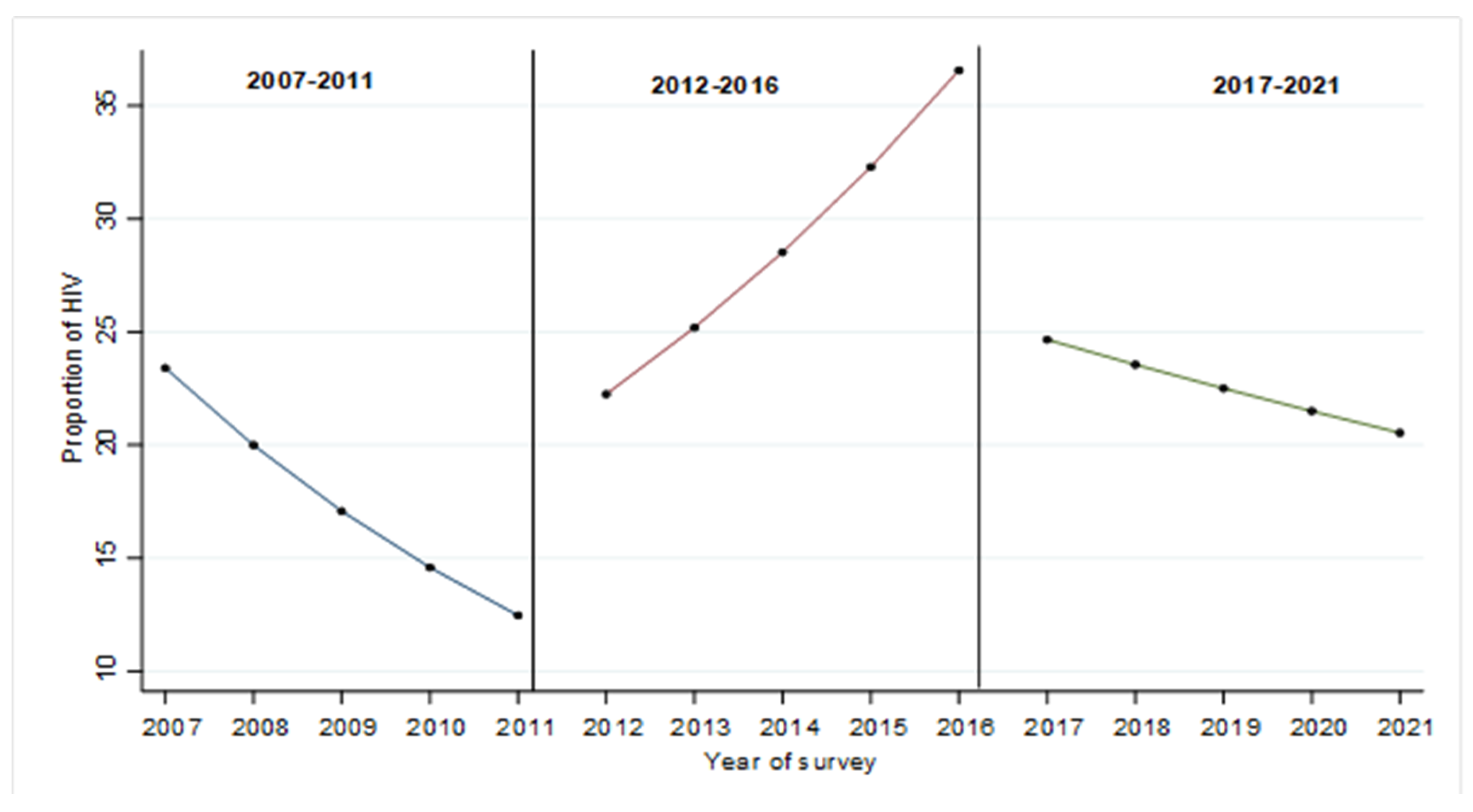
4.2. Changes in the Proportion of HIV (2007–2021)
4.3. Changes in HIV Prevalence among PWID by Sociodemographic Characteristics and Injecting and Sexual Behaviour (2007–2021)
4.4. Multivariable Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simoes, E.A.; Babu, P.G.; John, T.J.; Nirmala, S.; Solomon, S.; Lakshminarayana, C.; Quinn, T.C. Evidence for HTLV-III infection in prostitutes in Tamil Nadu (India). Ind. J. Med. Res. 2012, 136, 335–338. [Google Scholar]
- Avert. HIV and AIDS in India. 2017. Available online: https://www.avert.org/professionals/hiv-around-world/asia-pacific/india (accessed on 6 December 2020).
- National AIDS Control Organisation. National Integrated Biological and Behavioural Surveillance (IBBS) 2014–2015. 2015. Available online: http://naco.gov.in/sites/default/files/IBBS%20Report%202014-15.pdf (accessed on 6 December 2020).
- National AIDS Control Organisation. India HIV Estimates 2020. Techincal Brief. 2020. Available online: http://naco.gov.in/sites/default/files/India%20HIV%20Estimates%202020__Web_Version_0.pdf (accessed on 6 December 2020).
- National AIDS Control Organization; Ministry of Health and Family Welfare; Government of India. India HIV Estimates 2019 Report. 2019. Available online: http://naco.gov.in/sites/default/files/Estimation%20Report%202019.pdf (accessed on 4 December 2020).
- Paranjape, R.S.; Challacombe, S.J. HIV/AIDS in India: An overview of the Indian epidemic. Oral Dis. 2016, 22, 10–14. [Google Scholar] [CrossRef]
- Department of Health and Family Welfare MoHaFW, Government of India. Annual Report 2019–2020. 2020. Available online: https://main.mohfw.gov.in/sites/default/files/Annual%20Report%202019-2020%20English.pdf (accessed on 10 November 2020).
- National AIDS Control Organisation. National Strategic Plan for HIV/AIDS and STI 2017–2024. “Paving Way for an AIDS Free India”. 2017. Available online: http://naco.gov.in/national-strategic-plan-hivaids-and-sti-2017-24 (accessed on 10 November 2020).
- Biswas, S.; Ghosh, P.; Chakraborty, D.; Kumar, A.; Aggarwal, S.; Saha, M.K. Variation in injecting drug use behavior across different North-eastern States in India. Indian J. Public Health 2020, 64, 71. [Google Scholar]
- Kermode, M.; Longleng, V.; Singh, B.C.; Bowen, K.; Rintoul, A. Killing time with enjoyment: A qualitative study of initiation into injecting drug use in north-east India. Subst. Use Misuse 2009, 44, 1070–1089. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, A.A.A.; Rao, R.; Mishra, A.K.; Khandelwal, S.K.; Chadda, R.K.; on behalf of the group of investigators for the National Survey on Extent and Pattern of Substance Use in India. Magnitude of Substance Use in India; Ministry of Social Justice and Empowerment, Ed.; Government of India: New Delhi, India, 2019.
- United Nations Office on Drugs and Crime. Association of Drug Use Pattern with Vulnerability and Service Uptake among Injecting Drug Users. 2012. Available online: https://www.unodc.org/documents/southasia/publications/research-studies/OR4_final_print_ready.pdf (accessed on 17 January 2021).
- MSACS. Mizoram State AIDS Control Society 2020. Available online: https://mizoramsacs.org/about/ (accessed on 4 January 2021).
- Mizoram State AIDS Control Society. Targetted Intervention. 2019. Available online: https://mizoramsacs.org/division/targetted-intervention/ (accessed on 10 December 2020).
- National AIDS Control Organisation; Ministry of Health and Family Wefare GoI. National Guidelines for HIV Testing. 2015. Available online: http://www.naco.gov.in/sites/default/files/National_Guidelines_for_HIV_Testing_21Apr2016.pdf (accessed on 26 April 2022).
- Government of Mizoram. Mizoram at a Glance. Aizawl. 2022. Available online: https://mizoram.gov.in/page/know-mizoram (accessed on 11 May 2023).
- Dumchev, K.; Sazonova, Y.; Salyuk, T.; Varetska, O. Trends in HIV prevalence among people injecting drugs, men having sex with men, and female sex workers in Ukraine. Int. J. STD AIDS 2018, 29, 1337–1344. [Google Scholar] [CrossRef]
- Mburu, G.; Chhoun, P.; Chann, N.; Tuot, S.; Mun, P.; Yi, S. Prevalence and risk factors of HIV infection among people who inject drugs in Cambodia: Findings from a national survey. Subst. Abus. Treat. Prev. Policy 2019, 14, 42. [Google Scholar] [CrossRef]
- Likindikoki, S.L.; Mmbaga, E.J.; Leyna, G.H.; Moen, K.; Makyao, N.; Mizinduko, M.; Mwijage, A.I.; Faini, D.; Leshabari, M.T.; Meyrowitsch, D.W. Prevalence and risk factors associated with HIV-1 infection among people who inject drugs in Dar es Salaam, Tanzania: A sign of successful intervention? Harm Reduct. J. 2020, 17, 18. [Google Scholar] [CrossRef]
- Corsi, D.J.; Neuman, M.; Finlay, J.E.; Subramanian, S. Demographic and health surveys: A profile. Int. J. Epidemiol. 2012, 41, 1602–1613. [Google Scholar] [CrossRef]
- Demographic and Health Surveys. DHS Survey Types. Available online: https://dhsprogram.com/Methodology/Survey-Types/DHS.cfm (accessed on 9 January 2021).
- Agho, K.E.; Ogeleka, P.; Ogbo, F.A.; Ezeh, O.K.; Eastwood, J.; Page, A. Trends and predictors of prelacteal feeding practices in Nigeria (2003–2013). Nutrients 2016, 8, 462. [Google Scholar] [CrossRef]
- Hasankhani, M.B.; Zayeri, F.; Rasouli, M.; Salehi, M. Trend Analysis of HIV/AIDS Burden in Iran: Results from the Global Burden of Disease 2017 Study. Med. J. Islam. Repub. Iran 2021, 35, 159. [Google Scholar]
- United Nations Office on Drugs and Crime. Mizoram, India: Making HIV Prevention and Care a Reality in Prisons. 2010. Available online: https://www.unodc.org/southasia/frontpage/2010/September/making-hiv-prevention-and-care-a-reality-in-prisons.html (accessed on 9 January 2021).
- Mukandavire, C.; Low, A.; Mburu, G.; Trickey, A.; May, M.T.; Davies, C.F.; French, C.E.; Looker, K.J.; Rhodes, T.; Platt, L.; et al. Impact of opioid substitution therapy on the HIV prevention benefit of antiretroviral therapy for people who inject drugs. Aids 2017, 31, 1181–1190. [Google Scholar] [CrossRef]
- Desrosiers, A.; Chooi, W.-T.; Zaharim, N.M.; Ahmad, I.; Mohd Yasin, M.A.; Syed Jaapar, S.Z.; Schottenfeld, R.S.; Vicknasingam, B.; Chawarski, M.C. Emerging drug use trends in Kelantan, Malaysia. J. Psychoact. Drugs 2016, 48, 218–226. [Google Scholar] [CrossRef]
- Hindu, T. Mizoram has 6 lakhs mobile phone users. The Hindu Businessline, 31 March 2011. [Google Scholar]
- Ghosh, S. How the ‘Jio Effect’ Brought Millions of Indians Online and Is Reshaping Silicon Valley and the Internet. 2020. Available online: https://www.businessinsider.com/reliance-jio-millions-of-indians-online-reshaped-internet-2019-8 (accessed on 7 August 2022).
- Sullivan, T.; Voce, A. Use of Mobile Phones to Buy and Sell Illicit Drugs; Australian Institute of Criminology: Canberra, Australia, 2020. [Google Scholar]
- Søgaard, T.F.; Kolind, T.; Haller, M.B.; Hunt, G. Ring and bring drug services: Delivery dealing and the social life of a drug phone. Int. J. Drug Policy 2019, 69, 8–15. [Google Scholar] [CrossRef]
- Standard, B. Mizoram Launches Scheme for Better Treament of HIV-Hit People. Aizawl. 2017. Available online: https://www.business-standard.com/article/pti-stories/mizoram-launches-scheme-for-better-treatment-of-hiv-hit-people-117062200741_1.html (accessed on 7 August 2022).
- Larney, S.; Mathers, B.M.; Poteat, T.; Kamarulzaman, A.; Degenhardt, L. Global epidemiology of HIV among women and girls who use or inject drugs: Current knowledge and limitations of existing data. J. Acquir. Immune Defic. Syndr. 2015, 69 (Suppl. 2), S100. [Google Scholar] [CrossRef]
- El-Bassel, N.; Gilbert, L.; Wu, E.; Go, H.; Hill, J. Relationship between drug abuse and intimate partner violence: A longitudinal study among women receiving methadone. Am. J. Public Health 2005, 95, 465–470. [Google Scholar] [CrossRef]
- Tlou, B. The influence of marital status on HIV infection in an HIV hyperendemic area of rural South Africa, 2000–2017. Afr. J. AIDS Res. 2019, 18, 65–71. [Google Scholar] [CrossRef]
- Ngurthangpuii, V.J.G. Divorce among Men and Women in Lawngtlai District, Mizoram: A Sociological Investigation into the Causes. Int. J. Eng. Dev. Res. 2017, 5, 1080–1086. [Google Scholar]
- Kposowa, A.J. Marital status and HIV/AIDS mortality: Evidence from the US National Longitudinal Mortality Study. Int. J. Infect. Dis. 2013, 17, e868–e874. [Google Scholar] [CrossRef]
- Liu, C.; Ma, Y.-L.; Liu, X.-H.; Duan, Y.-R.; Liu, P.-L.; Wang, X.; Yin, P. Sociodemographic Factors Associated with HIV/HCV High-Risk Behaviors among People Who Use Drugs on Methadone Maintenance Treatment: A 10-Year Observational Study. Front. Psychiatry 2021, 12, 707257. [Google Scholar] [CrossRef]
- Long, C.; DeBeck, K.; Feng, C.; Montaner, J.; Wood, E.; Kerr, T. Income level and drug related harm among people who use injection drugs in a Canadian setting. Int. J. Drug Policy 2014, 25, 458–464. [Google Scholar] [CrossRef]
- Fischer, B.; Medved, W.; Gliksman, L.; Rehm, J. Illicit opiates in Toronto: A profile of current users. Addict. Res. 1999, 7, 377–415. [Google Scholar] [CrossRef]
- Bretteville-Jensen, A.L.; Sutton, M. The income-generating behaviour of injecting drug-users in Oslo. Addiction 1996, 91, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.G.; Latkin, C.A. Drug users’ involvement in the drug economy: Implications for harm reduction and HIV prevention programs. J. Urban Health 2002, 79, 266–277. [Google Scholar] [CrossRef] [PubMed]
- DeBeck, K.; Shannon, K.; Wood, E.; Li, K.; Montaner, J.; Kerr, T. Income generating activities of people who inject drugs. Drug Alcohol Depend. 2007, 91, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Deering, K.N.; Shoveller, J.; Tyndall, M.W.; Montaner, J.S.; Shannon, K. The street cost of drugs and drug use patterns: Relationships with sex work income in an urban Canadian setting. Drug Alcohol Depend. 2011, 118, 430–436. [Google Scholar] [CrossRef]
- Debashis Mukherjee, L.S.; Laldikkimi; Vanlaldini; Lalnunthara; Chuauzikpui. Baseline Survey on Extent & Pattern of Drug Use in Mizoram; Social Welfare Department, Ed.; Government of Mizoram: Aizawl, India, 2017.
- Chakrapani, V.; Newman, P.A.; Shunmugam, M.; Dubrow, R. Social-structural contexts of needle and syringe sharing behaviours of HIV-positive injecting drug users in Manipur, India: A mixed methods investigation. Harm Reduct. J. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Kumar, P.; Sangal, B.; Ramanathan, S.; Ammassari, S.; Venkatesh, S.R. Unsafe injecting practices, sexual risk behaviours and determinants of HIV among men who inject drugs: Results from Integrated Biological and Behavioural Surveillance in India. Int. J. STD AIDS 2018, 29, 1066–1075. [Google Scholar] [CrossRef]
- Panda, S.; Kumar, M.S.; Lokabiraman, S.; Jayashree, K.; Satagopan, M.C.; Solomon, S.; Rao, U.A.; Rangaiyan, G.; Flessenkaemper, S.; Grosskurth, H.; et al. Risk factors for HIV infection in injection drug users and evidence for onward transmission of HIV to their sexual partners in Chennai, India. J. Acquir. Immune Defic. Syndr. 2005, 39, 9–15. [Google Scholar] [CrossRef]
- Kim, N.J.; Jin, H.; McFarland, W.; Raymond, H.F. Trends in sources and sharing of needles among people who inject drugs, San Francisco, 2005–2012. Int. J. Drug Policy 2015, 26, 1238–1243. [Google Scholar] [CrossRef]
- Kåberg, M.; Karlsson, N.; Discacciati, A.; Widgren, K.; Weiland, O.; Ekström, A.M.; Hammarberg, A. Significant decrease in injection risk behaviours among participants in a needle exchange programme. Infect. Dis. 2020, 52, 336–346. [Google Scholar] [CrossRef]
- Santoshini, S. India’s Hill Country Is the First Stop on Heroin’s Deadly Route. 2018. Available online: https://foreignpolicy.com/2018/10/02/indias-hill-country-is-the-first-stop-on-heroins-deadly-route/ (accessed on 7 August 2022).
- Pachuau, J.L. Being Mizo: Identity and Belonging in Northeast India; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Pachuau, L.N.; Tannous, C.; Agho, K.E. Factors Associated with Knowledge, Attitudes, and Prevention towards HIV/AIDS among Adults 15–49 Years in Mizoram, North East India: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 19, 440. [Google Scholar] [CrossRef]
- Scheibe, A.; Makapela, D.; Brown, B.; dos Santos, M.; Hariga, F.; Virk, H.; Bekker, L.G.; Lyan, O.; Fee, N.; Molnar, M.; et al. HIV prevalence and risk among people who inject drugs in five South African cities. Int. J. Drug Policy 2016, 30, 107–115. [Google Scholar] [CrossRef]
| Characteristics | Years 2007–2011 | Years 2012–2016 | Years 2017–2021 |
|---|---|---|---|
| Total n (%) | Total n (%) | Total n (%) | |
| Gender | |||
| Male | 4405 (95.16) | 3173 (89.41) | 6077 (92.01) |
| Female | 224 (4.84) | 376 (10.59) | 528 (7.99) |
| Age | |||
| 18–24 | 2522 (55.20) | 1691 (48.49) | 2522 (39.36) |
| 25–34 | 1912 (41.85) | 1546 (44.34) | 2974 (46.41) |
| >35 | 135 (2.95) | 250 (7.71) | 912 (14.23) |
| Marital status | |||
| Never married | 2736 (59.41) | 2250 (63.76) | 3986 (60.41) |
| Married | 1100 (23.91) | 667 (18.90) | 1498 (22.70) |
| Separated/divorced/widowed | 765 (16.63) | 612 (17.34) | 1114 (16.88) |
| Education status | |||
| Primary (0–6 years) | 924 (20.03) | 501 (14.12) | 572 (8.69) |
| Middle (7–9 years) | 1810 (39.24) | 1262 (35.46) | 1960 (29.78) |
| Higher (10–12 years) | 1824 (39.54) | 1678 (47.28) | 3662 (55.65) |
| Graduate and above | 55 (1.19) | 108 (3.04) | 387 (5.88) |
| Employment status | |||
| Unemployed | 2530 (54.85) | 1800 (50.75) | 3337 (50.53) |
| Employed | 1766 (38.28) | 1436 (40.46) | 2233 (33.81) |
| Self-employed | 317 (6.87) | 313 (8.82) | 1034 (15.65) |
| Average monthly income (INR) | |||
| None | 1978 (42.95) | 1311 (37.35) | 2028 (30.93) |
| <3000 | 1093 (23.74) | 1234 (35.16) | 2083 (31.77) |
| 3001–6000 | 1103 (23.95) | 612 (17.44) | 1435 (21.89) |
| 6001–10,000 | 357 (7.75) | 238 (6.78) | 608 (9.27) |
| >10,000 | 74 (1.61) | 115 (3.28) | 402 (6.13) |
| Sharing of needles/syringes | |||
| No | 3910 (85.02) | 3008 (85.12) | 3638 (55.51) |
| Yes | 689 (14.98) | 526 (14.88) | 2916 (44.49) |
| Condom use with regular partner | |||
| No | 1124 (25.28) | 1099 (35.54) | 2327 (38.85) |
| Yes | 3322 (74.72) | 1993 (64.46) | 3662 (61.15) |
| Characteristics | 2007–2011 HIV-Positive Prevalence % (95% CI) | 2012–2016 HIV-Positive Prevalence % (95% CI) | 2017–2020 HIV-Positive Prevalence % (95% CI) | 2007–2011 and 2012–2016 | 2012–2016 and 2017–2021 | 2007–2011 and 2017–2021 |
|---|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | % (95% CI) | ||||
| N = 4629 | N = 3549 | N = 6605 | ||||
| Gender | ||||||
| Male | 13.1 (12.1–14.1) | 26.9(25.4 -28.5) | 20.8 (19.8–21.8) | 13.8 (11.9 to 15.6) *** | −6.0 (−7.0 to −4.2) *** | 7.7 (6.2 to 9.1) *** |
| Female | 18.3 (13.7–23.9) | 53.9 (48.8–58.9) | 41.7 (37.6–46.0) | 35.6 (28.4 to 42.8) *** | −12.1 (−18.7 to −5.5) *** | 23.4 (16.8 to 30.1) *** |
| Age | ||||||
| 18–24 | 12.8 (11.5–14.1) | 29 (26.9–31.3) | 21.1 (19.6–22.8) | 16.2 (13.7 to 18.8) *** | −7.9 (−10.6 to −5.2) *** | 8.3 (6.2 to 10.4) *** |
| 25–34 | 14.6 (13.1–16.3) | 31.1 (28.8–33.5) | 23.0 (21.5–24.5) | 16.5 (13.6 to 19.3) *** | −8.1 (−10.8 to −5.3) *** | 8.4 (6.1 to 10.6) *** |
| >35 | 8.1 (4.6–14.1) | 25.5 (20.5–31.3) | 24.1 (21.5–27.0) | 17.3 (10.2 to 24.5) *** | −1.4 (−7.4 to 4.7) NS | 16 (10.6 to 21.4) *** |
| Marital status | ||||||
| Never married | 12.4 (11.2–13.7) | 27.0 (25.2–28.9) | 19.3 (18.1–20.5) | 14.7 (12.4 to 16.9) *** | −7.6 (−9.9 to −5.4) *** | 6.9 (5.1 to 8.6) *** |
| Married | 11.8 (10.0–13.9) | 26.0 (22.8–29.5) | 21.6 (19.6–23.8) | 14.2 (10.4 to 18.5) *** | −4.4 (−8.3 to −4.8) * | 9.7 (6.9 to 12.6) *** |
| Separated/divorced/widowed | 18.3 (15.7–21.2) | 43.9 (40.0–47.9) | 35.0 (32.2–37.8) | 25.6 (20.8 to 30.4) *** | −8.9 (−13.8 to −4.1) *** | 16.7 (12.7 to 20.6) *** |
| Education status | ||||||
| Primary (0–6 years) | 9.5 (7.8–11.6) | 25.0 (21.4–29.0) | 24.8 (21.5–28.5) | 15.5 (11.2 to 19.8) *** | −0.2(−5.37 to −5.1) NS | 15.3 (11.3 to 19.3) *** |
| Middle (7–9 years) | 13.9 (12.4–15.6) | 31.6 (29.1–34.3) | 23.4 (21.6–25.3) | 17.7 (14.7 to 20.7) *** | −8.2 (−11.3 to −5.0) *** | 9.4 (7.0 to 11.9) *** |
| Higher (10–12 years) | 14.7 (13.1–16.4) | 29.9 (27.8–32.2) | 21.8 (20.5–23.2) | 15.2 (12.5 to 17.9) *** | −8.1 (−10.8 to −5.3) *** | 7.1 (5.0 to 9.2) *** |
| Graduate and above | 12.7 (6.2–24.4) | 27.1 (19.5–36.3) | 21.2 (17.4–25.5) | 14.4 (2.2 to 26.6) * | −5.9(−15.2 to 3.4) NS | 8.4 (−1.2 to 18.2) NS |
| Employment status | ||||||
| Unemployed | 11.6 (10.4–12.9) | 28.9 (26.9–31.1) | 18.9-(17.6–20.3) | 17.3 (14.9 to 19.8) *** | −10.0 (−12.5 to 7.5) *** | 7.3 (5.4 to 9.1) *** |
| Employed | 16.5 (14.9–18.4) | 31.3 (28.9–33.7) | 27.7 (25.9–29.6) | 14.7 (11.7 to 17.7) *** | −3.5 (−6.6 to −0.7) * | 11.2 (8.6–13.7) *** |
| Self-employed | 9.2 (6.5–12.9) | 27.4 (22.7–32.7) | 22.6 (20.1–25.2) | 18.2 (12.3 to 24.1) *** | −4.8 (−10.4 to −0.7) NS | 13.4 (9.3–17.4) *** |
| Average monthly income (INR) | ||||||
| None | 11.9 (10.6–13.4) | 23.3 (21.1–25.7) | 16.7 (15.1–18.4) | 11.3 (8.7 to 14.1) *** | −6.6 (−9.4 to −3.8) *** | 4.7 (2.5 to 6.9) *** |
| <3000 | 17.3 (15.2–19.7) | 35.5 (32.9–38.3) | 27.5 (25.6–29.4) | 18.2 (14.7 to 21.7) *** | −8.1 (−11.3 to −4.7) *** | 10.1 (7.2 to 13.1) *** |
| 3001–6000 | 11.6 (9.8–13.6) | 30.3 (26.8–34.1) | 22.3 (20.2–24.6) | 18.7 (14.6 to 22.9) *** | −8.0 (−12.3 to −3.7) *** | 10.7 (7.9 to 13.6) *** |
| 6001–10,000 | 12.4 (9.3–16.2) | 30.7 (25.1–36.8) | 25.3 (22.0–29.0) | 18.3 (11.5 to 25.1) *** | −5.3 (−12.1 to −1.5) NS | 12.9 (8.1 to 17.8) *** |
| >10,000 | 22.9 (14.5–34.1) | 29.4 (21.6–38.6) | 20.2 (16.6–24.4) | 6.5 (−6.5 to 20.1) NS | −9.1 (−18.6 to −0.2) NS | −2.6 (−13.2 to 7.9) NS |
| Sharing of needles/syringes | ||||||
| No | 11.5 (10.6–12.6) | 28 (26.4–29.6) | 20.3 (19.0–21.7) | 16.4 (14.5 to 18.3) *** | −7.6 (−9.7 to −5.5) *** | 8.7 (7.1 to 10.4) *** |
| Yes | 23.1 (20.1–26.4) | 39.4 (35.3–43.6) | 25.4 (23.9–27.0) | 16.2 (11.0 to 21.5) *** | −13.9 (−18.4 to −9.4) *** | 2.3 (−1.2 to 5.8) NS |
| Condom use with regular partner | ||||||
| No | 24.1 (21.7–26.7) | 32.6 (29.9–35.4) | 22.4 (20.8–24.2) | 8.5 (4.7 to 12.2) *** | −10.1 (−13.4 to −6.9) *** | −1.6 (−4.6 to 1.4) NS |
| Yes | 8.7 (7.8–9.7) | 26.6 (24.7–28.6) | 21.0 (19.7–22.3) | 17.9 (15.7 to 20.1) *** | −5.6 (−8.0 to −3.3) *** | 12.2 (10.6 to1 3.9) *** |
| Characteristics | OR (95% CI) | p-Value | AOR (95% CI) | p-Value |
|---|---|---|---|---|
| HIV status (n = 14,681) | ||||
| 2007–2011 | 1 | 1 | ||
| 2012–2016 | 2.75 (2.46–3.07) | <0.001 | 2.35 (2.07–2.66) | <0.001 |
| 2017–2021 | 1.88 (1.69–2.08) | <0.001 | 1.41 (1.24–1.59) | <0.001 |
| Gender (n = 14,680) | ||||
| Male | 1 | 1 | ||
| Female | 2.85 (2.51–3.23) | <0.001 | 2.35 (1.81–2.44) | <0.001 |
| Age (n = 14,364) | ||||
| 18–24 | 1 | 1 | ||
| 25–34 | 1.16 (1.06–1.25) | 0.001 | 1.03 (0.93–1.13) | 0.534 |
| >35 | 1.28 (1.02–1.35) | 0.025 | 0.91 (0.77–1.08) | 0.299 |
| Marital status (n = 14,639) | ||||
| Never married | 1 | 1 | ||
| Married | 1.01 (0.90–1.11) | 0.892 | 1.13 (1.00–1.27) | 0.045 |
| Separated/divorced/widowed | 1.99 (1.80–2.20) | <0.001 | 1.74 (1.54–1.96) | <0.001 |
| Education status (n = 14,655) | ||||
| Primary (0–6 years) | 1 | 1 | ||
| Middle (7–9 years) | 1.30 (1.14–1.49) | <0.001 | 1.24 (1.06–1.44) | 0.005 |
| Higher (10–12 years) | 1.29 (1.14–1.47) | <0.001 | 1.13 (0.98–1.32) | 0.081 |
| Graduate and above | 1.26 (1.00–1.59) | 0.048 | 1.03 (0.79–1.35) | 0.798 |
| Employment status (14,677) | ||||
| Unemployed | 1 | 1 | ||
| Employed | 1.43 (1.32–1.56) | <0.001 | 1.14 (1.03–1.27) | 0.011 |
| Self-employed | 1.13 (0.99–1.29) | 0.052 | 0.96 (0.82–1.12) | 0.649 |
| Average monthly income (INR) (n = 14,585) | ||||
| None | 1 | 1 | ||
| <3000 | 1.88 (1.70–2.07) | <0.001 | 2.15 (1.91–2.42) | <0.001 |
| 3001–6000 | 1.27 (1.13–1.42) | <0.001 | 1.42 (1.23–1.64) | <0.001 |
| 6001–10,000 | 1.46 (1.25–1.71) | <0.001 | 1.56 (1.29–1.89) | <0.001 |
| >10,000 | 1.44 (1.17–1.76) | 0.001 | 1.43 (1.11–1.83) | 0.004 |
| Sharing of needles/syringes (n = 14,610) | ||||
| No | 1 | 1 | ||
| Yes | 1.53 (1.41–1.67) | <0.001 | 1.78 (1.61–1.98) | <0.001 |
| Condom use with a regular partner (n = 13,448) | ||||
| No | 1 | 1 | ||
| Yes | 0.63 (0.58–0.69) | <0.001 | 0.77 (0.70–0.85) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pachuau, L.N.; Tannous, C.; Chawngthu, R.L.; Agho, K.E. Changes in and Predictors of HIV among People Who Inject Drugs in Mizoram, Northeast India, from 2007 to 2021. Int. J. Environ. Res. Public Health 2023, 20, 5871. https://doi.org/10.3390/ijerph20105871
Pachuau LN, Tannous C, Chawngthu RL, Agho KE. Changes in and Predictors of HIV among People Who Inject Drugs in Mizoram, Northeast India, from 2007 to 2021. International Journal of Environmental Research and Public Health. 2023; 20(10):5871. https://doi.org/10.3390/ijerph20105871
Chicago/Turabian StylePachuau, Lucy Ngaihbanglovi, Caterina Tannous, Richard Lalramhluna Chawngthu, and Kingsley Emwinyore Agho. 2023. "Changes in and Predictors of HIV among People Who Inject Drugs in Mizoram, Northeast India, from 2007 to 2021" International Journal of Environmental Research and Public Health 20, no. 10: 5871. https://doi.org/10.3390/ijerph20105871
APA StylePachuau, L. N., Tannous, C., Chawngthu, R. L., & Agho, K. E. (2023). Changes in and Predictors of HIV among People Who Inject Drugs in Mizoram, Northeast India, from 2007 to 2021. International Journal of Environmental Research and Public Health, 20(10), 5871. https://doi.org/10.3390/ijerph20105871







