Microplastics and Antibiotic Resistance: The Magnitude of the Problem and the Emerging Role of Hospital Wastewater
Abstract
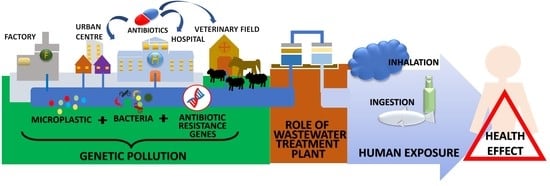
1. Introduction
2. Microplastics: Addressing the Main Problem
3. Mechanism of Interaction between MPs and ARGs
4. The Role of Wastewater in Spreading Environmental Antibiotic-Resistance
5. Potential Impact on Health of MPs and Their Contaminants
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Environmental Approach to Antimicrobial Resistance. ISS (Istituto Superiore di Sanità), ISTISAN Report 21/3, 2021. Available online: https://www.iss.it/web/iss-en/rapporti-istisan/-/asset_publisher/WCuPanLPFGdJ/content/id/5670994 (accessed on 1 February 2023).
- ECDC. Annual Epidemiological Report 2019. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/monitoring/all-annual-epidemiological-reports (accessed on 5 February 2023).
- Baquero, F.; Martínez, J.L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Word Health Organization (WHO). Global Action Plan on AMR, 2016. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 10 February 2023).
- Zhang, M.; Xu, L. Transport of micro- and nanoplastics in the environment: Trojan-Horse effect for organic contaminants. Crit. Rev. Environ. Sci. Technol. 2020, 52, 810–846. [Google Scholar] [CrossRef]
- Herrera, A.; Raymond, E.; Martínez, I.; Álvarez, S.; Canning-Clode, J.; Gestoso, I.; Pham, C.K.; Ríos, N.; Rodríguez, Y.; Gómez, M. First evaluation of neustonic microplastics in the Macaronesian region, NE Atlantic. Mar. Pollut. Bull. 2020, 153, 110999. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Ferreira, G.V.; Barrows, A.P.; Christiansen, K.S.; Treinish, G.; Toshack, M.C. Global patterns for the spatial distribution of floating microfibers: Arctic Ocean as a potential accumulation zone. J. Hazard. Mater. 2020, 403, 123796. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme. UNEP YearBook 2014: Emerging Issues Update; United Nations Environment Programme: Nairobi, Kenya, 2014. [Google Scholar]
- Rochman, C.M. Microplastics research—From sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- WHO Report-Microplastics in Drinking-Water. 2019. Available online: https://cdn.who.int/media/docs/default-source/wash-documents/microplastics-in-dw-information-sheet190822.pdf (accessed on 30 January 2023).
- Plastics-the Facts 2022 Plastic Europe. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 30 January 2023).
- Li, C.; Wang, L.; Ji, S.; Chang, M.; Wang, L.; Gan, Y.; Liu, J. The ecology of the plastisphere: Microbial composition, function, assembly, and network in the freshwater and seawater ecosystems. Water Res. 2021, 202, 117428. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- The Mediterranean: Mare Plasticum IUCN, 2020. Available online: https://portals.iucn.org/library/node/49124 (accessed on 30 January 2023).
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic–toxic chemical interaction: A review study on quantified levels, mechanism and implication. SN Appl. Sci. 2019, 1, 1400. [Google Scholar] [CrossRef]
- Fred-Ahmadu, O.H.; Bhagwat, G.; Oluyoye, I.; Benson, N.U.; Ayejuyo, O.O.; Palanisami, T. Interaction of chemical contaminants with microplastics: Principles and perspectives. Sci. Total. Environ. 2019, 706, 135978. [Google Scholar] [CrossRef] [PubMed]
- Barrows, A.; Cathey, S.; Petersen, C. Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environ. Pollut. 2018, 237, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Mincer, T.J.; Zettler, E.R.; Amaral-Zettler, L.A. Biofilms on plastic debris and their influence on marine nutrient cycling, productivity, and hazardous chemical mobility. In Hazardous Chemicals Associated with Plastics in the Marine Environment; The Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2016; Volume 78, pp. 221–233. [Google Scholar] [CrossRef]
- Krause, S.; Molari, M.; Gorb, E.V.; Gorb, S.N.; Kossel, E.; Haeckel, M. Persistence of plastic debris and its colonization by bacterial communities after two decades on the abyssal seafloor. Sci. Rep. 2020, 10, 9484. [Google Scholar] [CrossRef]
- Zhu, J.; Dong, X.; Zhao, N.; Jiang, S.; Jin, H. Microplastics in polystyrene-made food containers from China: Abundance, shape, size, and human intake. Environ. Sci. Pollut. Res. 2023, 30, 40084–40093. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Sun, R.; Yu, P.; Cheng, Y.; Wu, W.; Bao, J.; Alvarez, P.J.J. UV-aging of microplastics increases proximal ARG donor-recipient adsorption and leaching of chemicals that synergistically enhance antibiotic resistance propagation. J. Hazard. Mater. 2021, 427, 127895. [Google Scholar] [CrossRef] [PubMed]
- Ochman, H.; Lawrence, J.G.; Groisman, E.A. Lateral gene transfer and the nature of bacterial innovation. Nature 2000, 405, 299–304. [Google Scholar] [CrossRef]
- Zarei-Baygi, A.; Smith, A.L. Intracellular versus extracellular antibiotic resistance genes in the environment: Prevalence, horizontal transfer, and mitigation strategies. Bioresour. Technol. 2020, 319, 124181. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2016–2059. [Google Scholar] [CrossRef]
- Schlundt, C.; Welch, J.L.M.; Knochel, A.M.; Zettler, E.R.; Amaral-Zettler, L.A. Spatial structure in the “Plastisphere”: Molecular resources for imaging microscopic communities on plastic marine debris. Mol. Ecol. Resour. 2019, 20, 620–634. [Google Scholar] [CrossRef]
- Flach, C.-F.; Pal, C.; Svensson, C.J.; Kristiansson, E.; Östman, M.; Bengtsson-Palme, J.; Tysklind, M.; Larsson, D.J. Does antifouling paint select for antibiotic resistance? Sci. Total. Environ. 2017, 590–591, 461–468. [Google Scholar] [CrossRef]
- Imran, M.; Das, K.R.; Naik, M.M. Co-selection of multi-antibiotic resistance in bacterial pathogens in metal and microplastic contaminated environments: An emerging health threat. Chemosphere 2019, 215, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, Y.; Wu, J.; Luo, Y. Effects of microplastics on distribution of antibiotic resistance genes in recirculating aquaculture system. Ecotoxicol. Environ. Saf. 2019, 184, 109631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A Review of Microplastics in Table Salt, Drinking Water, and Air: Direct Human Exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cao, N.; Duan, C.; Wang, Q.; Ding, C.; Wang, J. Selection of antibiotic resistance genes on biodegradable and non-biodegradable microplastics. J. Hazard. Mater. 2020, 409, 124979. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef]
- Shruti, V.; Kutralam-Muniasamy, G. Bioplastics: Missing link in the era of Microplastics. Sci. Total. Environ. 2019, 697, 134139. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Allen Burton, G.; Janssen, C. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef]
- Schwarz, A.; Ligthart, T.; Boukris, E.; van Harmelen, T. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: A review study. Mar. Pollut. Bull. 2019, 143, 92–100. [Google Scholar] [CrossRef]
- Eckert, E.M.; Di Cesare, A.; Kettner, M.T.; Arias, M.D.J.; Fontaneto, D.; Grossart, H.-P.; Corno, G. Microplastics increase impact of treated wastewater on freshwater microbial community. Environ. Pollut. 2018, 234, 495–502. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, G.; Song, W.; Ye, C.; Lin, H.; Li, Z.; Liu, W. Plastics in the marine environment are reservoirs for antibiotic and metal resistance genes. Environ. Int. 2018, 123, 79–86. [Google Scholar] [CrossRef]
- Rolsky, C.; Kelkar, V.; Driver, E.; Halden, R.U. Municipal sewage sludge as a source of microplastics in the environment. Curr. Opin. Environ. Sci. Health 2019, 14, 16–22. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Pal, C.; Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genom. 2015, 16, 964. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.L.; Burgos, M.J.G.; Pérez-Pulido, R.; Gálvez, A.; Lucas, R. Resistance to Antibiotics, Biocides, Preservatives and Metals in Bacteria Isolated from Seafoods: Co-Selection of Strains Resistant or Tolerant to Different Classes of Compounds. Front. Microbiol. 2017, 8, 1650. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.L.; Guo, W.S.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Lambirth, K.; Tsilimigras, M.; Lulla, A.; Johnson, J.; Al-Shaer, A.; Wynblatt, O.; Sypolt, S.; Brouwer, C.; Clinton, S.; Keen, O.; et al. Microbial Community Composition and Antibiotic Resistance Genes within a North Carolina Urban Water System. Water 2018, 10, 1539. [Google Scholar] [CrossRef]
- Anand, U.; Reddy, B.; Singh, V.K.; Singh, A.K.; Kesari, K.K.; Tripathi, P.; Kumar, P.; Tripathi, V.; Simal-Gandara, J. Potential Environmental and Human Health Risks Caused by Antibiotic-Resistant Bacteria (ARB), Antibiotic Resistance Genes (ARGs) and Emerging Contaminants (ECs) from Municipal Solid Waste (MSW) Landfill. Antibiotics 2021, 10, 374. [Google Scholar] [CrossRef]
- Galvin, S.; Boyle, F.; Hickey, P.; Vellinga, A.; Morris, D.; Cormican, M. Enumeration and Characterization of Antimicrobial-Resistant Escherichia coli Bacteria in Effluent from Municipal, Hospital, and Secondary Treatment Facility Sources. Appl. Environ. Microbiol. 2010, 76, 4772–4779. [Google Scholar] [CrossRef]
- Chagas, T.; Seki, L.; da Silva, D.; Asensi, M. Occurrence of KPC-2-producing Klebsiella pneumoniae strains in hospital wastewater. J. Hosp. Infect. 2011, 77, 281. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Hutinel, M.; Larsson, D.J.; Flach, C.-F. Antibiotic resistance genes of emerging concern in municipal and hospital wastewater from a major Swedish city. Sci. Total. Environ. 2021, 812, 151433. [Google Scholar] [CrossRef] [PubMed]
- Gönder, Z.B.; Kara, E.M.; Celik, B.O.; Vergili, I.; Kaya, Y.; Altinkum, S.M.; Bagdatli, Y.; Yilmaz, G. Detailed characterization, antibiotic resistance and seasonal variation of hospital wastewater. Environ. Sci. Pollut. Res. 2021, 28, 16380–16393. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Ye, J.; Yang, Q.; Hu, Y.; Zhang, T.; Jiang, L.; Munezero, S.; Lin, K.; Cui, C. Occurrence and removal of antibiotics, antibiotic resistance genes, and bacterial communities in hospital wastewater. Environ. Sci. Pollut. Res. 2021, 28, 57321–57333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, P.; Yang, Q. Occurrence and diversity of antibiotic resistance in untreated hospital wastewater. Sci. Total. Environ. 2018, 621, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Kaya, Y.; Vergili, I.; Gönder, Z.B.; Özhan, G.; Celik, B.O.; Altinkum, S.M.; Bagdatli, Y.; Boergers, A.; Tuerk, J. Characterization and toxicity of hospital wastewaters in Turkey. Environ. Monit. Assess. 2017, 189, 55. [Google Scholar] [CrossRef]
- Azanu, D.; Styrishave, B.; Darko, G.; Weisser, J.J.; Abaidoo, R.C. Occurrence and risk assessment of antibiotics in water and lettuce in Ghana. Sci. Total. Environ. 2018, 622–623, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shuai, X.-Y.; Lin, Z.-J.; Sun, Y.-J.; Zhou, Z.-C.; Meng, L.-X.; Zhu, Y.-G.; Chen, H. Landscape of genes in hospital wastewater breaking through the defense line of last-resort antibiotics. Water Res. 2021, 209, 117907. [Google Scholar] [CrossRef]
- Sib, E.; Lenz-Plet, F.; Barabasch, V.; Klanke, U.; Savin, M.; Hembach, N.; Schallenberg, A.; Kehl, K.; Albert, C.; Gajdiss, M.; et al. Bacteria isolated from hospital, municipal and slaughterhouse wastewaters show characteristic, different resistance profiles. Sci. Total. Environ. 2020, 746, 140894. [Google Scholar] [CrossRef]
- Kayali, O.; Icgen, B. Untreated HWWs Emerged as Hotpots for ARGs. Bull. Environ. Contam. Toxicol. 2020, 104, 386–392. [Google Scholar] [CrossRef]
- Wang, C.; Mantilla-Calderon, D.; Xiong, Y.; Alkahtani, M.; Bashawri, Y.M.; Al Qarni, H.; Hong, P.-Y. Investigation of Antibiotic Resistome in Hospital Wastewater during the COVID-19 Pandemic: Is the Initial Phase of the Pandemic Contributing to Antimicrobial Resistance? Environ. Sci. Technol. 2022, 56, 15007–15018. [Google Scholar] [CrossRef]
- Kosma, C.I.; Kapsi, M.G.; Konstas, P.-S.G.; Trantopoulos, E.P.; Boti, V.I.; Konstantinou, I.K.; Albanis, T.A. Assessment of multiclass pharmaceutical active compounds (PhACs) in hospital WWTP influent and effluent samples by UHPLC-Orbitrap MS: Temporal variation, removals and environmental risk assessment. Environ. Res. 2020, 191, 110152. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, L.; Llompart, M.; Dagnac, T. Complementarity of two approaches based on the use of high-resolution mass spectrometry for the determination of multi-class antibiotics in water. Photodegradation studies and non-target screenings. Environ. Sci. Pollut. Res. 2023, 30, 1871–1888. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.; Moreau-Guigon, E.; Labadie, P.; Alliot, F.; Teil, M.-J.; Blanchard, M.; Eurin, J.; Chevreuil, M. Fate of antibiotics from hospital and domestic sources in a sewage network. Sci. Total. Environ. 2017, 575, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-H.; Ng, C.; Chen, H.; Yi, X.Z.; Koh, T.H.; Barkham, T.M.S.; Zhou, Z.; Gin, K.Y.-H. Occurrences and Characterization of Antibiotic-Resistant Bacteria and Genetic Determinants of Hospital Wastewater in a Tropical Country. Antimicrob. Agents Chemother. 2016, 60, 7449–7456. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, E.; Baricz, A.; Chiriac, C.M.; Farkas, A.; Opris, O.; Soran, M.-L.; Andrei, A.-S.; Rudi, K.; Balcázar, J.L.; Dragos, N.; et al. Abundance of antibiotics, antibiotic resistance genes and bacterial community composition in wastewater effluents from different Romanian hospitals. Environ. Pollut. 2017, 225, 304–315. [Google Scholar] [CrossRef]
- Hassoun-Kheir, N.; Stabholz, Y.; Kreft, J.-U.; de la Cruz, R.; Romalde, J.L.; Nesme, J.; Sørensen, S.J.; Smets, B.F.; Graham, D.; Paul, M. Comparison of antibiotic-resistant bacteria and antibiotic resistance genes abundance in hospital and community wastewater: A systematic review. Sci. Total. Environ. 2020, 743, 140804. [Google Scholar] [CrossRef]
- Mao, D.; Yu, S.; Rysz, M.; Luo, Y.; Yang, F.; Li, F.; Hou, J.; Mu, Q.; Alvarez, P.J.J. Prevalence and proliferation of antibiotic resistance genes in two municipal wastewater treatment plants. Water Res. 2015, 85, 458–466. [Google Scholar] [CrossRef]
- Uluseker, C.; Kaster, K.M.; Thorsen, K.; Basiry, D.; Shobana, S.; Jain, M.; Kumar, G.; Kommedal, R.; Pala-Ozkok, I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021, 12, 717809. [Google Scholar] [CrossRef]
- Castiglioni, S.; Davoli, E.; Riva, F.; Palmiotto, M.; Camporini, P.; Manenti, A.; Zuccato, E. Data on occurrence and fate of emerging contaminants in a urbanised area. Data Brief 2018, 17, 533–543. [Google Scholar] [CrossRef]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P. Antibiotic-Resistance Genes in Waste Water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options. J. Hydrol. 2010, 389, 416–428. [Google Scholar] [CrossRef]
- Mammo, F.; Amoah, I.; Gani, K.; Pillay, L.; Ratha, S.; Bux, F.; Kumari, S. Microplastics in the environment: Interactions with microbes and chemical contaminants. Sci. Total. Environ. 2020, 743, 140518. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic-an emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007, 3, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Rist, S.; Almroth, B.C.; Hartmann, N.B.; Karlsson, T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total. Environ. 2018, 626, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO Fisheries and Aquaculture Technical Paper No. 615 - T615; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Sun, T.; Zhan, J.; Li, F.; Ji, C.; Wu, H. Evidence-based meta-analysis of the genotoxicity induced by microplastics in aquatic organisms at environmentally relevant concentrations. Sci. Total. Environ. 2021, 783, 147076. [Google Scholar] [CrossRef]
- Beer, S.; Garm, A.; Huwer, B.; Dierking, J.; Nielsen, T.G. No increase in marine microplastic concentration over the last three decades—A case study from the Baltic Sea. Sci. Total. Environ. 2018, 621, 1272–1279. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Leslie, H.A.; Quinn, B. Microplastics in drinking water: A review and assessment. Curr. Opin. Environ. Sci. Health 2018, 7, 69–75. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef]
- Schymanski, D.; Goldbeck, C.; Humpf, H.-U.; Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2017, 129, 154–162. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (Contam). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Galloway, T.S. Micro-and nano-plastics and human health. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(nano)plastics: A threat to human health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Lv, W.; Wang, H.; Chen, H.; Xu, Q.; Cai, H.; Dai, J. Intratracheal administration of polystyrene microplastics induces pulmonary fibrosis by activating oxidative stress and Wnt/β-catenin signaling pathway in mice. Ecotoxicol. Environ. Saf. 2022, 232, 113238. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, S.; Liu, Q.; Wei, J.; Jin, Y.; Wang, X.; Zhang, L. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/β-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ. Pollut. 2020, 265, 115025. [Google Scholar] [CrossRef]
- Jin, Y.; Xia, J.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. Pollut. 2018, 235, 322–329. [Google Scholar] [CrossRef]
- Kesy, K.; Hentzsch, A.; Klaeger, F.; Oberbeckmann, S.; Mothes, S.; Labrenz, M. Fate and stability of polyamide-associated bacterial assemblages after their passage through the digestive tract of the blue mussel Mytilus edulis. Mar. Pollut. Bull. 2017, 125, 132–138. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Tan, W.; Zhang, Z. Microplastics as an Emerging Environmental Pollutant in Agricultural Soils: Effects on Ecosystems and Human Health. Front. Environ. Sci. 2022, 10, 855292. [Google Scholar] [CrossRef]
- Huang, Z.; Weng, Y.; Shen, Q.; Zhao, Y.; Jin, Y. Microplastic: A potential threat to human and animal health by interfering with the intestinal barrier function and changing the intestinal microenvironment. Sci. Total. Environ. 2021, 785, 147365. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef]
- Perković, S.; Paul, C.; Vasić, F.; Helming, K. Human Health and Soil Health Risks from Heavy Metals, Micro(nano)plastics, and Antibiotic Resistant Bacteria in Agricultural Soils. Agronomy 2022, 12, 2945. [Google Scholar] [CrossRef]
- Sathicq, M.B.; Sabatino, R.; Corno, G.; Di Cesare, A. Are microplastic particles a hotspot for the spread and the persistence of antibiotic resistance in aquatic systems? Environ. Pollut. 2021, 279, 116896. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Liu, W.; Juhasz, A.; Chen, J.; Ma, L. Emerging contaminants antibiotic resistance genes and microplastics in the environment: Introduction to 21 review articles published in CREST during 2018–2022. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4135–4146. [Google Scholar] [CrossRef]
| Author | Location | Sampling Years | Hospital Capacity | Average ARGs Absolute Abundance, Minimum and Maximum Value (Gene Copies/L) | Antibiotics Abundance Average, Minimum and Maximum Value (µg/L) |
|---|---|---|---|---|---|
| Rodriguez-Mozaz et al., 2015 [48] | Girona, Spain | 2011–2012 | Main hospital, 400 beds | blaTEM: ~107; qnrS: ~107; ermB: ~109; sulI: ~108; tetW: ~109 | CIP: 8.305–13.779; OFX: 4.750–14.378; CFZ: 0.045–0.083; CTX: 0.144–0.240; AZM: 0.020–0.059; CLAR: 0.167–0.941; SMX: 4.817–0.190; TMP: 3.787–0.136; MTR: 1.793–0.524 |
| Hutinel et al., 2022 [49] | Gothenburg, Sweden | 2015–2019 | Sahlgrenska University Hospital, 2000 beds | * mcr-1: 9.96 × 10−7–2.25 × 10−5; mcr-3: 4.34 × 10−5–1.45 × 10−3; mcr-4: 4.88 × 10−6–3.20 × 10−5; mcr-5: 4.07 × 10−5–1.19 × 10−4; sul4: 7.53 × 10−6–7.14 × 10−5; gar: 8.53 × 10−7–5.09 × 10−4; optrA: 2.60 × 10−5–4.74 × 10−5; cfr(A): 3.20 × 10−7–7.61 × 10−6 | Data not available |
| Gönder et al., 2021 [50] | Istanbul, Turkey | 2 years | Medical faculty hospital, 1358 beds | Data not available | AMP: 0.41; AZM: 1.06; CIP: 3.48 CLAR: 5.34; CLM: 0.1; ERY: 0.28; MTR: 0.86; NOR: 0.1; OFX: 0.96; SDZ: 0.24; SMX: 15.68; SPR: 1.93; TMP: 3.6 |
| Yao et al., 2021 [51] | East China | 2019 | Primary Hospital, 80 beds Secondary Hospital, 150 beds Tertiary Hospital, 964 beds | qeqA: 5.73 × 105; qnrA: 1.44 × 108; qnrD: 2.02 × 107; qnrS: 1.82 × 108; blaOXA-1: 2.22 × 1010; blaTEM-1: 7.17 × 109; blaGES-1: 1.33 × 1010; blaOXA-10: 3.50 × 109; blaSHV-1: 1.16 × 107; blaDHA-1: 2.02 × 108 | CN: 0.03–0.88; CEF: 0.02–0.11; CED: 0.37–2.38; CAZ: 0.14–31.21; AMX: 0.04–1.43; AMP: 0.14–0.67; OFX: 1.39–49.47; NOR: 0.05–0.61; TMP: 0.02–0.50; FOX: 0.36–8.96; CFZ: 0.45–5.01; CEP: 106.76–540.39; MER: 0.02–0.2 |
| Wang et al., 2018 [52] | XinXiang city, Central China | 2016 | Tertiary Hospital 1, 1740 beds Tertiary Hospital 2, 1200 beds Tertiary Hospital 3, 700 beds | tetX: 1.30 × 109–4.14 × 109; tetM: 4.10 × 107–4.15 × 108; tetO: 1.38 × 109–8.34 × 1010; ereA: 6.81 × 107–3.50 × 108; ermA: 1.88; ermB: 8.28 × 108–3.20 × 109; sul1: 1.30 × 1010–7.24 × 1010; sul2: 1.29 × 109–7.83 × 109; sul3: 6.40 × 105–4.84 × 107; qnrA: 1.01× 107–4.79 × 107; qnrD: 1.73 × 106–3.72 × 106; oqxB: 3.34 × 106–1.85 × 107 | SDZ: 0.123; SMX: 0.533; OFX: 2.329; NOR:1.629; CIP: 1.334; TET: 1.411; OTC: 1.479; ERY: 0.479; LN: 0.132; TMP: 0.268; CN: 2.392 |
| Yilmaz et al., 2017 [53] | Istanbul, Turkey | 2014 | Medical Faculty Hospital 1, 1358 beds Medical Faculty Hospital 2, 1285 beds Training and Research Hospital, 612 beds | Data not available | SMX: 0.55–8.5; CLN: <0.01–4.1; TMP: <0.01–2.2; CIP: 1.9–24; CAZ: 1.6; AZM: <0.01–0.4; CLAR: 0.063–15; SDZ: <0.01–0.16; OFX: 0.082–200; CLM: <0.01–0.036; MTR: <0.03–3; SPR: <0.01–0.047 |
| Azanu et al., 2018 [54] | Kumasi, Ghana | 2014 | Komfo Anokye Teaching Hospital, 1200 beds University Hospital, 120 beds | Data not available | CIP: 0.247–0.420; ERY: 11.352–15.733; TMP: 7.944–10.613; SMX: 0.094–4.826; AMX: 0.058–0.116; AMP: 0.075–0.252; CFX: 0.016–0.024; MTR: 0.024–0.120; DOX: 0.002–0.006; TET: 0.107–0.324; CTC: 1.052–1.557; OTC: 2.315–3.590 |
| Zhu et al., 2021 [55] | Eastern China | 2018–2019 | Three public hospitals | blaNDM1–15: 3.13 × 107–1.72 × 1010; blaNDM16: 3.13 × 107–1.72 × 1010; mcr-1–mcr-5: 2.03 × 106–1.32 × 109; tet(X1)–tet(X5): 5.62 × 105–3.69 × 109 | Data not available |
| Sib et al., 2020 [56] | Germany | 2016–2018 | Maximum care hospital, 1274 beds | blaNDM: 108–109; blaVIM: 108–109; blaCTX-15-M: ~107; sul1: 1010–1011; mcr-1: ~103–105 | Data not available |
| Kayali and Icgen, 2020 [57] | Ankara, Turkey | 2017 | Six major hospitals H1, 160 beds H2, 270 beds H3, 468 beds H4, 484 beds H5, 730 beds H6, 1140 beds | aadA: 9.5 × 107; tetA: 2.5 × 107; cmlA: 8.3 × 106; sul1: 3.7 × 106; qnrS: 1.2 × 106; ermB: 2.9 × 105; blaCTX-M: 1.1 × 103 | Data not available |
| Wang et al., 2022 [58] | Jeddah, Saudi Arabia | 2020 | Infection Disease Hospital (Hospital A) Psychiatric hospital (Hospital B) | Data not available | SMX: 0.013–0.330; ERY: 0.00006–0.006; CIP: 0.015–0.068; MFX: 0.008–0.009; PG: 0.008–0.017; MER: 0.013; TET: 0.005–0.006 |
| Kosma et al., 2020 [59] | Ioannina, Greece | 1 year | Ioannina University Hospital: 800 beds | Data not available | SDZ: 0.348; SMX: 0.525; SPR: 0.262; STZ: 0.109; TMP: 0.295 |
| Vazquez et al., 2023 [60] | Galicia, Spain Porto, Portugal | Data not available | Hospital 1 Hospital 2 | Data not available | AZM: 1.9–3.4; CIP: 0.18–0.66; OFX: 2.5–3.5; SDZ: 0.35; SMX: 0.073–0.10; TMP: 0.13–0.20; CLAR: 0.034; ERY: 0.063; NOR: 0.21 |
| Dinh et al., 2017 [61] | Fontenay-les-Briis, France | 2009–2014 | Polyvalent medical centre: 360 beds | Data not available | ERY: 0.80; TET: 0.03; AMX: 0.11; TMP: 0.94; ORM: 0.04; SMX: 2.1; PI: 0.02; ENO: 0.76; LOM: 0.25; NOR: 12.1; CIP: 5.8; OFX: 17.9; VAN: 3.6 |
| Le et al., 2016 [62] | Singapore | 2014 | 2 Hospital Blocks, 1597 beds Block A, clinical isolation wards Block B, general wards Hospital 2, 1500 beds | blaNDM: 2.29 × 109; blaKPC: 4.08 × 1010; blaCTX-M: 1.25 × 109; blaSHV: 6.19 × 108 | AZM: 0.11–1.51; CIP: 1.72–76.44 CHL: 0.65; CLAR: 0.76–72.87; ERY: 0.3–17.63; MER: 0.19–1.01; SMX: 0.94–28.36; TMP: 0,78–71.8; CLM: 1.43; LN: 0.015; TET: 0.82 |
| Szekeres et al., 2017 [63] | Cluj County, Romania | 2015 | Oncological Hospital, 535 beds General Hospital, 113 beds General Hospital, 453 beds | aacC2: 4.41 × 108–1.08 × 109; blaVIM: 1.43 × 105–3.19 × 106; catA1: 5.31 × 106–1.44 × 108; ermA: 2.16 × 105; floR: 1.58 × 104–1.73 × 108; tnpA: 1.41 × 109–2.76 × 109; mefA: 8.01 × 107–1.91 × 108; qacEΔ1: 2.22 × 109–3.80 × 106; blaSHV: 1.94 × 108–3.41 × 105; sulI: 6.11 × 109–1.51 × 1010; sulII: 4.29 × 107–5.75 × 108; tetA: 2.69 × 108–5.94 × 108; tetB: 1.13 × 106–1.66 × 106; tetC: 1.33 × 105–1.92 × 105; tetO: 4.91 × 107–1.09 × 109; tetW: 6.79 × 106–1.69 × 108 | AMP: 8.07–53.05; CAZ: 3.66–10.46; CEP: 5.18–8.52; TMP: 13.06–30.38; IPM: 14.42; TAZ: 10.26; VAN: 5.03–13.98; PIP: 7.81; ERY: 7.52; SMX: 6.06; TET: 1.34; GM: 7.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuvo, B.; Scarpaci, M.; Bracaloni, S.; Esposito, E.; Costa, A.L.; Ioppolo, M.; Casini, B. Microplastics and Antibiotic Resistance: The Magnitude of the Problem and the Emerging Role of Hospital Wastewater. Int. J. Environ. Res. Public Health 2023, 20, 5868. https://doi.org/10.3390/ijerph20105868
Tuvo B, Scarpaci M, Bracaloni S, Esposito E, Costa AL, Ioppolo M, Casini B. Microplastics and Antibiotic Resistance: The Magnitude of the Problem and the Emerging Role of Hospital Wastewater. International Journal of Environmental Research and Public Health. 2023; 20(10):5868. https://doi.org/10.3390/ijerph20105868
Chicago/Turabian StyleTuvo, Benedetta, Michela Scarpaci, Sara Bracaloni, Enrica Esposito, Anna Laura Costa, Martina Ioppolo, and Beatrice Casini. 2023. "Microplastics and Antibiotic Resistance: The Magnitude of the Problem and the Emerging Role of Hospital Wastewater" International Journal of Environmental Research and Public Health 20, no. 10: 5868. https://doi.org/10.3390/ijerph20105868
APA StyleTuvo, B., Scarpaci, M., Bracaloni, S., Esposito, E., Costa, A. L., Ioppolo, M., & Casini, B. (2023). Microplastics and Antibiotic Resistance: The Magnitude of the Problem and the Emerging Role of Hospital Wastewater. International Journal of Environmental Research and Public Health, 20(10), 5868. https://doi.org/10.3390/ijerph20105868