Physical Function and Association with Cognitive Function in Patients in a Post-COVID-19 Clinic—A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
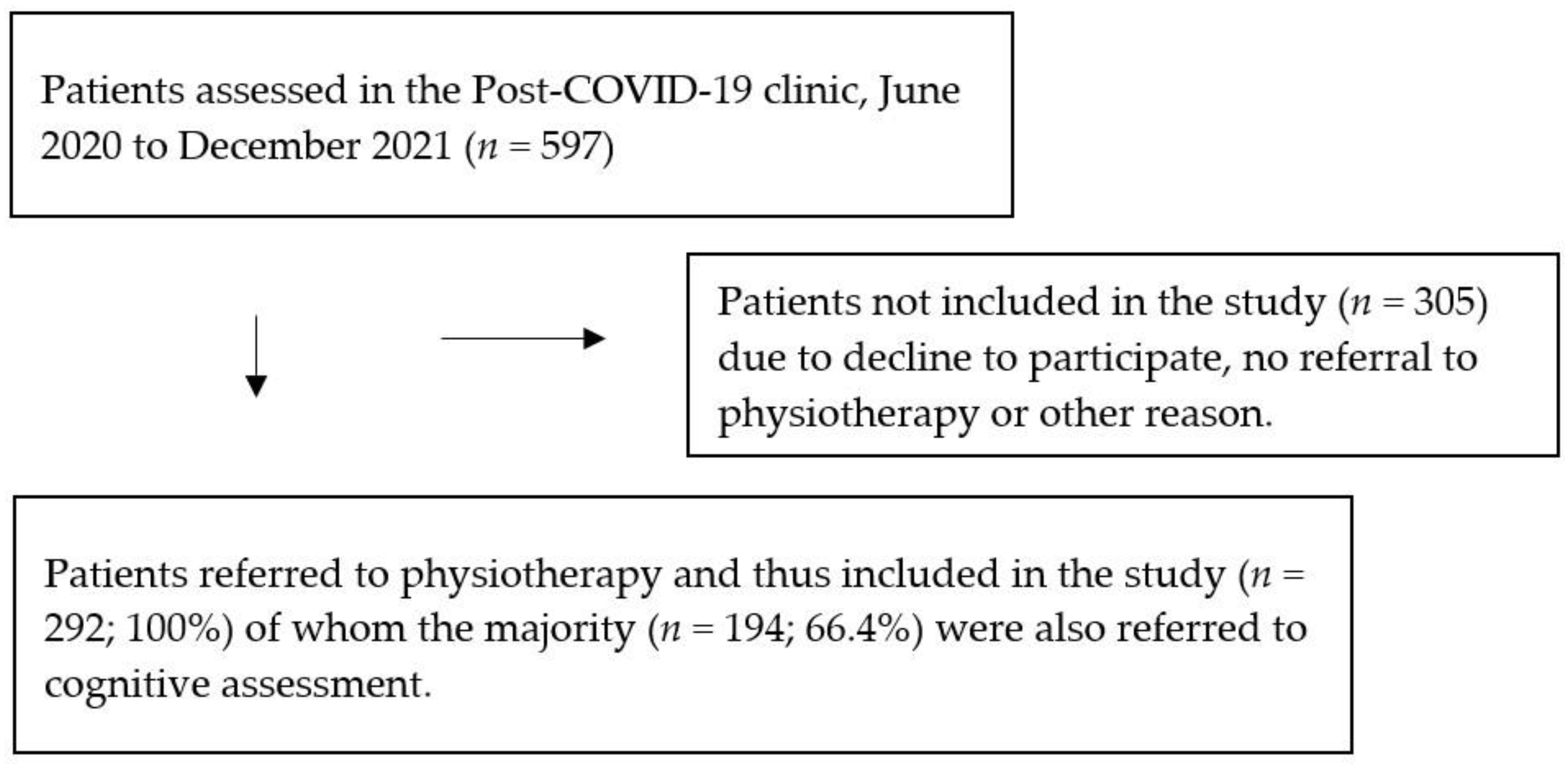
2.1. Study Design and Participants
2.2. Procedures
2.3. Assessment of Physical Function
2.4. Assessments of Cognitive Function
2.5. Patient-Reported Outcomes
2.6. Other Outcomes
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics, Sociodemographic and Patient Reported Data
3.2. Acute Severity for Hospitalised Patients
3.3. Physical and Cognitive Function
3.4. Prevalence and Risk of Physical Impairment
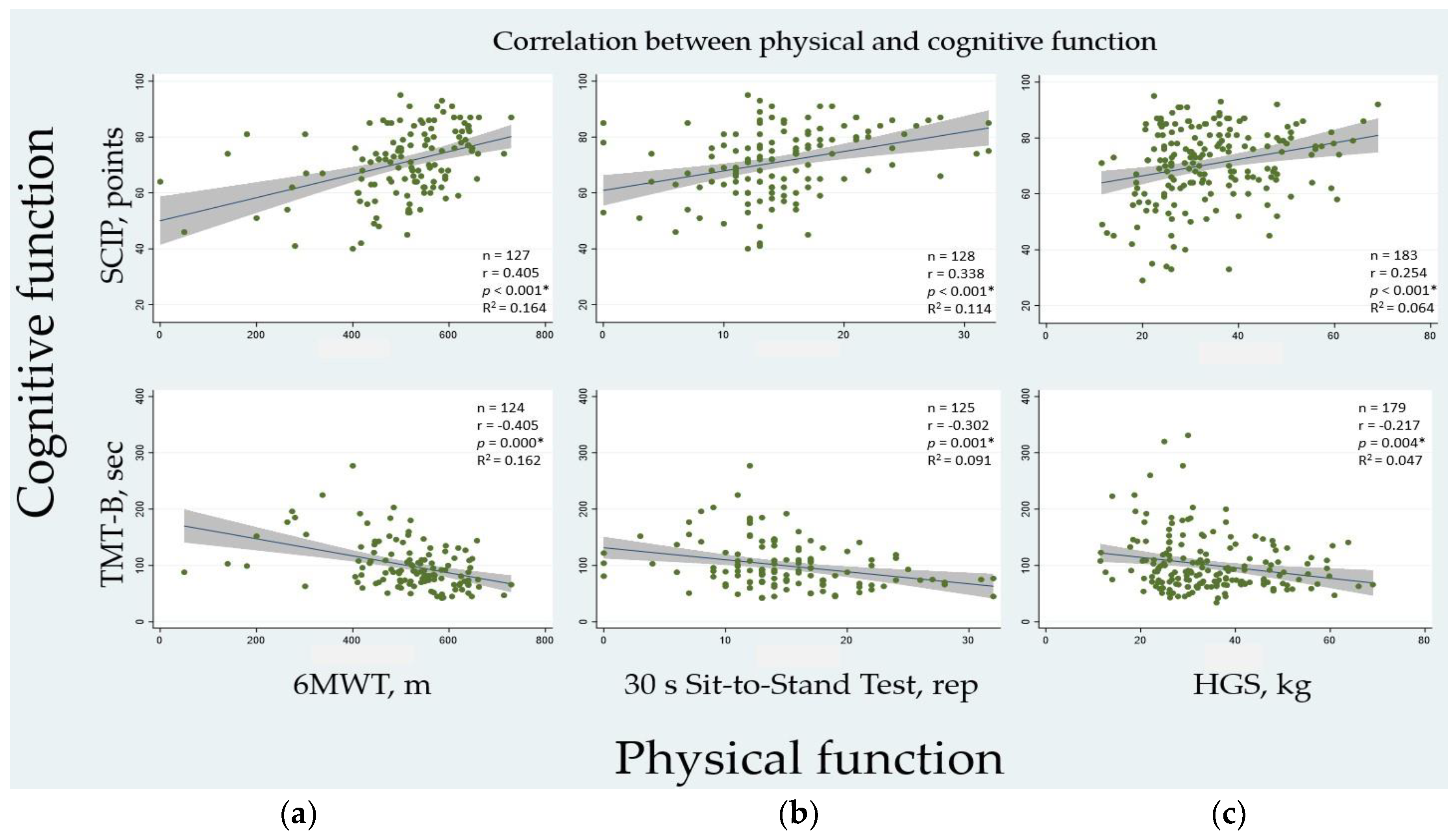
3.5. Association between Physical and Cognitive Function
3.6. Physical Function and Possible Explanatory Variables
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available online: https://coronavirus.jhu.edu/map.html (accessed on 9 March 2023).
- A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021. 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 14 December 2022).
- Domingo, F.R.; Waddell, L.A.; Cheung, A.M.; Cooper, C.L.; Belcourt, V.J.; Zuckermann, A.M.E.; Corrin, T.; Ahmad, R.; Boland, L.; Laprise, C.; et al. Prevalence of long-term effects in individuals diagnosed with COVID-19: An updated living systematic review. medRxiv 2021, 2021, 21258317. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. eClinicalMedicine 2023, 55, 101762. [Google Scholar] [CrossRef]
- Johnsen, S.; Sattler, S.M.; Miskowiak, K.W.; Kunalan, K.; Victor, A.; Pedersen, L.; Andreassen, H.F.; Jørgensen, B.J.; Heebøll, H.; Andersen, M.B.; et al. Descriptive analysis of long COVID sequelae identified in a multidisciplinary clinic serving hospitalised and non-hospitalised patients. ERJ Open Res. 2021, 7, 00205–2021. [Google Scholar] [CrossRef]
- Stavem, K.; Ghanima, W.; Olsen, M.K.; Gilboe, H.M.; Einvik, G. Prevalence and Determinants of Fatigue after COVID-19 in Non-Hospitalized Subjects: A Population-Based Study. Int. J. Environ. Res. Public Health 2021, 18, 2030. [Google Scholar] [CrossRef]
- Tabacof, L.; Tosto-Mancuso, J.; Wood, J.; Cortes, M.; Kontorovich, A.; McCarthy, D.; Rizk, D.; Rozanski, G.; Breyman, E.; Nasr, L.; et al. Post-acute COVID-19 Syndrome Negatively Impacts Physical Function, Cognitive Function, Health-Related Quality of Life, and Participation. Am. J. Phys. Med. Rehabil. 2022, 101, 48–52. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute covid-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Cristanziano, V.D.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Agergaard, J.; Ullahammer, W.M.; Gunst, J.D.; Østergaard, L.; Schiøttz-Christensen, B. Characteristics of a Danish Post-COVID Cohort Referred for Examination due to Persistent Symptoms Six Months after Mild Acute COVID-19. J. Clin. Med. 2022, 11, 7338. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Soddu, D.; Balbo, P.E.; Baricich, A.; Zeppegno, P.; Avanzi, G.C.; Baldon, G.; Bartolomei, G.; Battaglia, M.; Battistini, S.; et al. Respiratory and Psychophysical Sequelae Among Patients With COVID-19 Four Months After Hospital Discharge. JAMA Netw. Open. 2021, 4, e2036142. [Google Scholar] [CrossRef] [PubMed]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients With COVID-19: A Systematic Review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef] [PubMed]
- Van den Borst, B.; Peters, J.B.; Brink, M.; Schoon, Y.; Bleeker-Rovers, C.P.; Schers, H.; van Hees, H.W.H.; van Helvoort, H.; van den Boogaard, M.; van der Hoeven, H.; et al. Comprehensive Health Assessment 3 Months After Recovery From Acute Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 73, e1089–e1098. [Google Scholar] [CrossRef]
- Cortés-Telles, A.; López-Romero, S.; Figueroa-Hurtado, E.; Pou-Aguilar, Y.N.; Wong, A.W.; Milne, K.M.; Ryerson, C.J.; Guenette, J.A. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir. Physiol. Neurobiol. 2021, 288, 103644. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Functioning, Disability and Health. Available online: http://apps.who.int/iris/bitstream/handle/10665/42407/9241545429.pdf;jsessionid=71829FB8B8EB3F75F9447EEA6C984D53?sequence=1 (accessed on 29 June 2022).
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ’long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef]
- Davido, B.; Seang, S.; Tubiana, R.; de Truchis, P. Post-COVID-19 chronic symptoms: A postinfectious entity? Clin. Microbiol. Infect. 2020, 26, 1448–1449. [Google Scholar] [CrossRef]
- Ashton, R.; Ansdell, P.; Hume, E.; Maden-Wilkinson, T.; Ryan, D.; Tuttiett, E.; Faghy, M. COVID-19 and the long-term cardio-respiratory and metabolic health complications. Rev. Cardiovasc. Med. 2022, 23, 53. [Google Scholar] [CrossRef]
- NICE. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 29 June 2022).
- Tabbarah, M.; Crimmins, E.M.; Seeman, T.E. The relationship between cognitive and physical performance: MacArthur Studies of Successful Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M228–M235. [Google Scholar] [CrossRef]
- Clouston, S.A.; Brewster, P.; Kuh, D.; Richards, M.; Cooper, R.; Hardy, R.; Rubin, M.S.; Hofer, S.M. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol. Rev. 2013, 35, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.C.; Jones, P.W. A comparison of the visual analogue scale and modified Borg scale for the measurement of dyspnoea during exercise. Clin. Sci. 1989, 76, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Rikli, R.E.; Jones, C.J. Development and Validation of a Functional Fitness Test for Community-Residing Older Adults. J. Aging Phys. Act. 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Jensen, J.H.; Støttrup, M.M.; Nayberg, E.; Knorr, U.; Ullum, H.; Purdon, S.E.; Kessing, L.V.; Miskowiak, K.W. Optimising screening for cognitive dysfunction in bipolar disorder: Validation and evaluation of objective and subjective tools. J. Affect. Disord. 2015, 187, 10–19. [Google Scholar] [CrossRef]
- U.S. Army War Department. Battery army individual test. In Manual of Directions and Scoring; Adjutant General’s Office: Washington, DC, USA, 1944. [Google Scholar]
- Bowie, C.; Harvey, P. Administration and interpretation of Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef]
- CAT. The COPD Assessment Test (CAT) For Healthcare Professionals & Researchers. Available online: https://www.catestonline.org/hcp-homepage/academic-researcher.html (accessed on 4 April 2022).
- UK Research and Innovation. MRC Dyspnoea Scale. Available online: https://www.ukri.org/councils/mrc/facilities-and-resources/find-an-mrc-facility-or-resource/mrc-dyspnoea-scale/ (accessed on 8 April 2022).
- EuroQol Research Foundation. EQ-5D-5L. Available online: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (accessed on 4 April 2022).
- Klok, F.A.; Boon, G.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Broadbent, D.E.; Cooper, P.F.; FitzGerald, P.; Parkes, K.R. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982, 21, 1–16. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Enright, P.L.; Sherrill, D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998, 158 Pt 1, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.T.; Dall, C.H.; Aadahl, M.; Suetta, C. Systematisk måling af fysisk funktion hos voksne patienter på tværs af diagnoser. UGESKRIFT LÆGER 2022, 184, V02220134. [Google Scholar]
- Swinscow, T.D.V. Statistics at Square One; John Mark Ockerbloom: Southampton, UK, 1997. [Google Scholar]
- Lorent, N.; Vande Weygaerde, Y.; Claeys, E.; Guler Caamano Fajardo, I.; De Vos, N.; De Wever, W.; Salhi, B.; Gyselinck, I.; Bosteels, C.; Lambrecht, B.N.; et al. Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open. Res. 2022, 8, 00004-2022. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Guida, F.; Polesel, J.; Marcuzzo, A.V.; Capriotti, V.; D’Alessandro, A.; Zanelli, E.; Marzolino, R.; Lazzarin, C.; Antonucci, P.; et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19). Int. Forum Allergy Rhinol. 2021, 11, 1685–1688. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, B.; Mohn, K.G.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.B.; Leth, S.; Pedersen, M.; Harbo, H.D.; Nielsen, C.V.; Laursen, C.H.; Schiøttz-Christensen, B.; Oestergaard, L.G. Mental Fatigue, Activities of Daily Living, Sick Leave and Functional Status among Patients with Long COVID: A Cross-Sectional Study. Int. J. Environ. Res. Public. Health 2022, 19, 14739. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef]
- Troosters, T.; Gosselink, R.; Decramer, M. Six minute walking distance in healthy elderly subjects. Eur. Respir. J. 1999, 14, 270–274. [Google Scholar] [CrossRef]
- Frederiksen, H.; Hjelmborg, J.; Mortensen, J.; McGue, M.; Vaupel, J.W.; Christensen, K. Age trajectories of grip strength: Cross-sectional and longitudinal data among 8342 Danes aged 46 to 102. Ann. Epidemiol. 2006, 16, 554–562. [Google Scholar] [CrossRef]
- Aadahl, M.; Beyer, N.; Linneberg, A.; Thuesen, B.H.; Jørgensen, T. Grip strength and lower limb extension power in 19-72-year-old Danish men and women: The Health2006 study. BMJ Open 2011, 1, e000192. [Google Scholar] [CrossRef]
- Hansen, A.W.; Beyer, N.; Flensborg-Madsen, T.; Grønbæk, M.; Helge, J.W. Muscle strength and physical activity are associated with self-rated health in an adult Danish population. Prev. Med. 2013, 57, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Suetta, C.; Haddock, B.; Alcazar, J.; Noerst, T.; Hansen, O.M.; Ludvig, H.; Kamper, R.S.; Schnohr, P.; Prescott, E.; Andersen, L.L.; et al. The Copenhagen Sarcopenia Study: Lean mass, strength, power, and physical function in a Danish cohort aged 20-93 years. J. Cachexia Sarcopenia Muscle 2019, 10, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Sillen, M.J.; Groenen, M.T.; Wouters, E.F.; Franssen, F.M. New normative values for handgrip strength: Results from the UK Biobank. J. Am. Med. Dir. Assoc. 2013, 14, 775.e5-11. [Google Scholar] [CrossRef]
- Wong, S.L. Grip strength reference values for Canadians aged 6 to 79: Canadian Health Measures Survey, 2007 to 2013. Health Rep. 2016, 27, 3–10. [Google Scholar] [PubMed]
- Steiber, N. Strong or weak handgrip? Normative reference values for the German population across the life course stratified by sex, age, and body height. PLoS ONE 2016, 11, e0163917. [Google Scholar] [CrossRef]
- Bohannon, R.W. Minimal clinically important difference for grip strength: A systematic review. J. Phys. Ther. Sci. 2019, 31, 75–78. [Google Scholar] [CrossRef] [PubMed]
- ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [CrossRef] [PubMed]
- Miskowiak, K.W.; Pedersen, J.K.; Gunnarsson, D.V.; Roikjer, T.K.; Podlekareva, D.; Hansen, H.; Dall, C.H.; Johnsen, S. Cognitive impairments among patients in a long-COVID clinic: Prevalence, pattern and relation to illness severity, work function and quality of life. J. Affect. Disord. 2022, 324, 162–169. [Google Scholar] [CrossRef]
- Wang, R.; Holsinger, R.M.D. Exercise-induced brain-derived neurotrophic factor expression: Therapeutic implications for Alzheimer’s dementia. Ageing Res. Rev. 2018, 48, 109–121. [Google Scholar] [CrossRef]
- Décary, S.; Dugas, M.; Stefan, T.; Langlois, L.; Skidmore, B.; Bhéreur, A.; LeBlanc, A.; Services, A.H.; Hastings, S.; Manns, B.; et al. Care Models for Long COVID: A Rapid Systematic Review. medRxiv 2021, 2021, 21266404. [Google Scholar] [CrossRef]
- Fugazzaro, S.; Contri, A.; Esseroukh, O.; Kaleci, S.; Croci, S.; Massari, M.; Facciolongo, N.C.; Besutti, G.; Iori, M.; Salvarani, C.; et al. Rehabilitation Interventions for Post-Acute COVID-19 Syndrome: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 5185. [Google Scholar] [CrossRef] [PubMed]
- Nambi, G.; Abdelbasset, W.K.; Alrawaili, S.M.; Elsayed, S.H.; Verma, A.; Vellaiyan, A.; Eid, M.M.; Aldhafian, O.R.; Nwihadh, N.B.; Saleh, A.K. Comparative effectiveness study of low versus high-intensity aerobic training with resistance training in community-dwelling older men with post-COVID 19 sarcopenia: A randomized controlled trial. Clin. Rehabil. 2021, 36, 59–68. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Yang, Y.; Zhang, J.; Li, Y.; Chen, Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 2020, 39, 101166. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, W.; Zhan, C.; Liu, S.; Yin, Z.; Wang, J.; Chong, Y.; Zheng, C.; Fang, X.; Cheng, W.; et al. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): A randomised controlled trial. Thorax 2022, 77, 697. [Google Scholar] [CrossRef] [PubMed]
- Maley, J.H.; Alba, G.A.; Barry, J.T.; Bartels, M.N.; Fleming, T.K.; Oleson, C.V.; Rydberg, L.; Sampsel, S.; Silver, J.K.; Sipes, S.; et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of breathing discomfort and respiratory sequelae in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM&R 2022, 14, 77–95. [Google Scholar] [CrossRef]
- Miskowiak, K.W.; Fugledalen, L.; Jespersen, A.E.; Sattler, S.M.; Podlekareva, D.; Rungby, J.; Porsberg, C.M.; Johnsen, S. Trajectory of cognitive impairments over 1 year after COVID-19 hospitalisation: Pattern, severity, and functional implications. Eur. Neuropsychopharmacol. 2022, 59, 82–92. [Google Scholar] [CrossRef]
| Sociodemographic Data | Overall n = 292 | Non-Hospitalised n = 147 | Hospitalised n = 145 | p-Value |
|---|---|---|---|---|
| Age, years, mean (SD) | 51.9 (15.2) | 45.7 (14.3) | 58.2 (13.3) | <0.001 * |
| Sex | ||||
| Women, n (%) | 164 (56.2) | 112 (76.2) | 52 (35.9) | <0.001 * |
| Men, n (%) | 128 (43.8) | 35 (23.8) | 93 (64.1) | |
| Race or ethnic group (missing, n = 6) | ||||
| Caucasian, n (%) | 212 (74.1) | 120 (81.6) | 92 (63.5) | <0.001 * |
| Other, n (%) | 74 (25.9) | 22 (15.0) | 52 (35.9) | |
| BMI (missing, n = 3) | ||||
| kg x m−2, mean (SD) | 27.3 (12.1) | 25.2 (3.9) | 29.4 (5.9) | <0.001 * |
| > 30 kg x m−2, n (%) | 69 (23.9) | 14 (9.5) | 55 (37.9) | 0.181 |
| Smoking (missing, n = 41) | ||||
| Never smoker, n (%) | 143 (57.1) | 76 (51.7) | 67 (46.2) | 0.659 |
| Current smoker, n (%) | 16 (6.4) | 12 (8.2) | 4 (2.8) | |
| Previous smoker, n (%) | 92 (36.7) | 39 (26.5) | 53 (36.6) | |
| Time since cessation, median years (IQR) | 15.0 (5.1–30.0) | 10.0 (4.0–25.0) | 21.5 (11.0–40.0) | 0.002 * |
| Work status (missing, n = 53) | ||||
| Currently working, n (%) | 162 (68.6) | 101 (68.7) | 61 (42.1) | <0.022 * |
| Out of work, n (%) | 34 (14.2) | 15 (10.2) | 19 (13.1) | |
| Retired, n (%) | 43 (18.0) | 6 (4.1) | 37 (25.5) | |
| Comorbidities | ||||
| CCI, median (IQR) (missing, n = 61) | 2.0 (1.0–3.0) | 1.0 (0.0–2.0) | 3.0 (1.0–4.0) | <0.001 * |
| Asthma, n (%) (missing, n = 40) | 46 (18.3) | 15 (10.2) | 31 (21.4) | <0.001 * |
| COPD, n (%) (missing, n = 39) | 8 (3.2) | 3 (2.0) | 5 (3.5) | 0.532 |
| Time since COVID-19 infection, days, mean (SD) (missing, n = 102) | 217.2 (111.5) | 261.4 (115.6) | 166.1 (148.9) | <0.001 * |
| Patient reported measures | ||||
| Education, years, mean (SD) (missing, n = 95) | 15.3 (4.0) | 15.6 (3.9) | 14.9 (4.1) | 0.343 |
| MRC score, mean (SD) (missing, n = 59) | 2.2 (0.8) | 2.1 (0.7) | 2.2 (1.0) | 0.290 |
| CAT score, mean (SD) (missing, n = 55) | 13.9 (7.3) | 14.7 (7.1) | 13.0 (7.4) | 0.678 |
| PCFS score pre-COVID-19, median (IQR) (missing, n = 121) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.5) | 0.656 |
| PCFS score post-COVID-19, median (IQR) (missing, n = 117) | 2.0 (1.0–3.0) | 2.0 (2.0–3.0) | 2.0 (1.0–3.0) | 0.914 |
| EQ-5D-5L index, median (IQR) (missing, n = 87) | 0.84 (0.71–0.93) | 0.86 (0.65–0.95) | 0.82 (0.73–0.91) | 0.580 |
| EQ-5D-5L VAS, median (IQR) (missing, n = 87) | 70.0 (50.0–70.0) | 66.0 (50.0–80.0) | 70.0 (50.0–75.0) | 0.561 |
| CFQ total, mean (SD) (missing, n = 94) | 38.1 (17.5) | 40.6 (17.4) | 35.3 (17.4) | 0.959 |
| Rehabilitation plan (yes), n (%) | 69 (23.6) | 47 (32.0) | 22 (15.2) | 0.001 * |
| Physical Function Tests | n | Overall | n | Non-Hospitalised | n | Hospitalised | p-Value |
|---|---|---|---|---|---|---|---|
| 30 s Sit-to-Stand Test, rep, mean (SD) | 181 | 14.3 (6.0) | 100 | 15.1 (6.5) | 81 | 13.4 (5.3) | 0.035 * |
| 6MWT, m, mean (SD) | 180 | 489.5 (138.7) | 99 | 507.9 (121.5) | 81 | 467.0 (155.0) | 0.024 * |
| HGS, kg, mean (SD) | 237 | 33.0 (12.0) | 124 | 31.2 (10.2) | 113 | 34.9 (13.7) | 0.904 |
| HGS, females, kg, mean (SD) | 134 | 26.0 (7.9) | 94 | 27.2 (6.3) | 40 | 25.3 (10.8) | 0.835 |
| HGS, males, kg, mean (SD) | 103 | 41.3 (11.6) | 30 | 40.2 (12.2) | 73 | 44.0 (9.5) | 0.992 |
| Cognitive tests | |||||||
| SCIP-D total, points, mean (SD) | 196 | 70.4 (14.1) | 105 | 75.2 (10.8) | 91 | 64.8 (15.4) | <0.001 * |
| TMT-B, seconds, mean (SD) | 189 | 100.3 (50.2) | 103 | 88.8 (44.5) | 86 | 113.9 (53.3) | 0.164 |
| Overall | Non-Hospitalised | Hospitalised | p-Value | OR (95%CI) | |
|---|---|---|---|---|---|
| Functional exercise capacity (6MWT), n = 180 | |||||
| Normal, n (%) m, mean (95%CI) | 139 (77) 540.2 (526.7–553.7) | 79 (80) 546.7 (529.8–563.7) | 60 (74) 531.6 (509.2–554.1) | 0.031 * | 1.38 (0.65–2.95) |
| Impaired, n (%) m, mean (95%CI) | 41 (23) 317.6 (268.4–366.9) | 20 (20) 354.5 (285.6–423.3) | 21 (26) 282.6 (209.8–355.4) | 0.632 | |
| Muscle strength and function in the lower extremities (30 s Sit-to-Stand Test), n = 181 | |||||
| Normal, n (%) rep, mean (95%CI) | 75 (41) 18.8 (17.6–20.0) | 41 (41) 20.2 (18.5–22.0) | 34 (42) 17.1 (15.5–18.6) | 0.049 * | 0.95 (0.51–1.82) |
| Impaired, n (%) rep, mean (95%CI) | 106 (59) 11.2 (10.4–12.1) | 59 (59) 11.5 (10.4–12.6) | 47 (58) 11.0 (9.7–12.3) | 0.766 | |
| Handgrip strength (HGS), n = 237 | |||||
| Normal, n (%) kg, mean (95%CI) | 152 (64) 37.7 (35.8–39.5) | 83 (67) 34.2 (32.0–36.4) | 69 (61) 41.8 (39.1–44.5) | <0.003 * | 1.29 (0.73–2.28) |
| Impaired, n (%) kg, mean (95%CI) | 85 (36) 24.7 (22.9–26.5) | 41 (33) 25.3 (23.0–27.7) | 44 (39) 24.1 (21.3–26.9) | <0.011 * | |
| Handgrip strength (HGS)—Females, n = 134 | |||||
| Normal, n (%) kg, mean (95%CI) | 86 (64) 30.2 (45.3–49.5) | 63 (47) 29.7 (28.3–31.0) | 23 (17) 31.6 (28.0–35.1) | <0.001 * | 1.50 (0.65–3.43) |
| Impaired, n (%) kg, mean (95%CI) | 48 (36) 20.3 (18.5—22.1) | 31 (23) 22.2 (20.4–24.0) | 17 (13) 16.8 (13.4–20.2) | 0.063 | |
| Handgrip strength (HGS)—Males, n = 103 | |||||
| Normal, n (%) kg, mean (95%CI) | 66 (64) 47.4 (45.3–49.5) | 20 (67) 48.5 (45.0–52.0) | 46 (63) 46.9 (44.3–49.6) | 0.268 | 1.17 (0.44–3.24) |
| Impaired, n (%) kg, mean (95%CI) | 37 (36) 30.4 (28.0–32.9) | 10 (33) 35.0 (31.7–38.3) | 27 (37) 28.7 (25.9–31.6) | 0.070 | |
| Coefficient | 95%CI | p-Value | |
|---|---|---|---|
| Functional Exercise Capacity (6MWT) | |||
| R2 = 0.27 | |||
| SCIP | 3.08 | 1.38–4.78 | <0.001 * |
| Age | −2.15 | −3.39–−0.92 | 0.001 * |
| Sex (female) | −46.92 | −84.67–−9.15 | 0.015 * |
| Education years | −1.95 | −6.95–3.05 | 0.442 |
| Muscle strength and function in the lower extremities (30 s Sit-to-Stand Test) | |||
| R2 = 0.18 | |||
| SCIP | 0.12 | 0.04–0.21 | 0.006 * |
| Age | −0.09 | −0.16–−0.03 | 0.006 * |
| Sex (female) | −1.69 | −3.64–0.27 | 0.090 |
| Education years | −0.07 | −0.33–0.19 | 0.573 |
| Handgrip strength (HGS) | |||
| R2 = 0.56 | |||
| SCIP | 0.17 | 0.07–0.27 | 0.001 * |
| Age | −0.21 | −0.29–−0.13 | 0.000 * |
| Sex (female) | −16.48 | −18.85–−14.11 | 0.000 * |
| Education years | −0.02 | −0.35–0.30 | 0.881 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunnarsson, D.V.; Miskowiak, K.W.; Pedersen, J.K.; Hansen, H.; Podlekareva, D.; Johnsen, S.; Dall, C.H. Physical Function and Association with Cognitive Function in Patients in a Post-COVID-19 Clinic—A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 5866. https://doi.org/10.3390/ijerph20105866
Gunnarsson DV, Miskowiak KW, Pedersen JK, Hansen H, Podlekareva D, Johnsen S, Dall CH. Physical Function and Association with Cognitive Function in Patients in a Post-COVID-19 Clinic—A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2023; 20(10):5866. https://doi.org/10.3390/ijerph20105866
Chicago/Turabian StyleGunnarsson, Durita Viderø, Kamilla Woznica Miskowiak, Johanna Kølle Pedersen, Henrik Hansen, Daria Podlekareva, Stine Johnsen, and Christian Have Dall. 2023. "Physical Function and Association with Cognitive Function in Patients in a Post-COVID-19 Clinic—A Cross-Sectional Study" International Journal of Environmental Research and Public Health 20, no. 10: 5866. https://doi.org/10.3390/ijerph20105866
APA StyleGunnarsson, D. V., Miskowiak, K. W., Pedersen, J. K., Hansen, H., Podlekareva, D., Johnsen, S., & Dall, C. H. (2023). Physical Function and Association with Cognitive Function in Patients in a Post-COVID-19 Clinic—A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 20(10), 5866. https://doi.org/10.3390/ijerph20105866






