Non-Pharmacological Therapies for Post-Viral Syndromes, Including Long COVID: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
- Population: The population of interest consisted of adults and children with a PVS (including Long COVID). There is no universally agreed definition of PVS, as there is heterogeneity in the temporal description of PVS onset following the initial viral exposure and additional overlap with the definition of post-viral fatigue syndrome (PVFS; ICD-10 G93.3) [18,19,20]. In line with the minimum timeframe for Long COVID used by the World Health Organization (WHO), where the temporal criteria were described, we included only those studies where post-viral symptoms lasted beyond 12 weeks [21]. However, we also included publications that provided no firm timeframe but indicated an aspect of chronicity or prolonged persistence of symptoms, as it should be noted conditions, such as PVFS, are usually denoted as a condition lasting for more than 6 months [20].
- Intervention and comparator: We included studies that assessed the effectiveness of non-pharmacological interventions designed to improve the symptoms of PVS against standard care, an alternative non-pharmacological therapy, or a placebo.
- Outcomes: The outcomes were changes in symptoms, exercise capacity, quality of life (including changes in mental and physical wellbeing) and work capability.
- Study type: Only randomised controlled trials (RCT) were included for patients with PVS, for conditions other than COVID-19. However, as we anticipated a lack of RCTs for SARS-CoV-2, we also included observational studies where the viral pathogen was SARS-CoV-2.
2.1. Information Sources and Search Strategy
2.2. Study Selection
- Randomised controlled trials of non-pharmacological treatments/interventions for those with PVS.
- Randomised controlled trials and non-randomised observational studies report non-pharmacological treatments/interventions specifically designed for Long COVID.
- Pharmacological interventions;
- In vitro and animal studies;
- Case reports/series;
- Systematic reviews;
- Non-pharmacological treatments/interventions in non-viral conditions;
- Protocols of trials.
2.3. Data Extraction and Quality Appraisal
2.4. Narrative Synthesis
2.5. Patient and Public Involvement
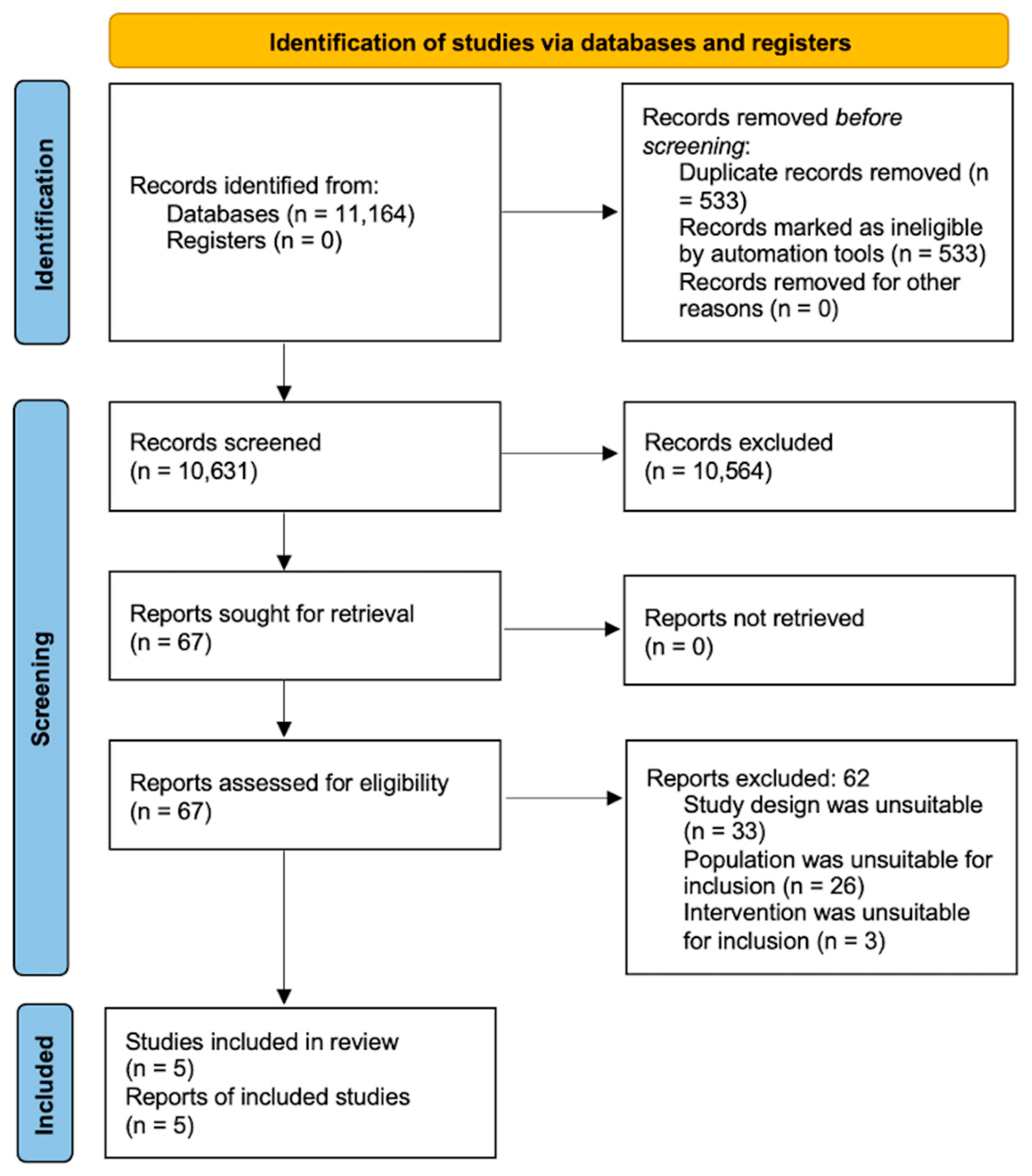
3. Results
3.1. Description of Studies
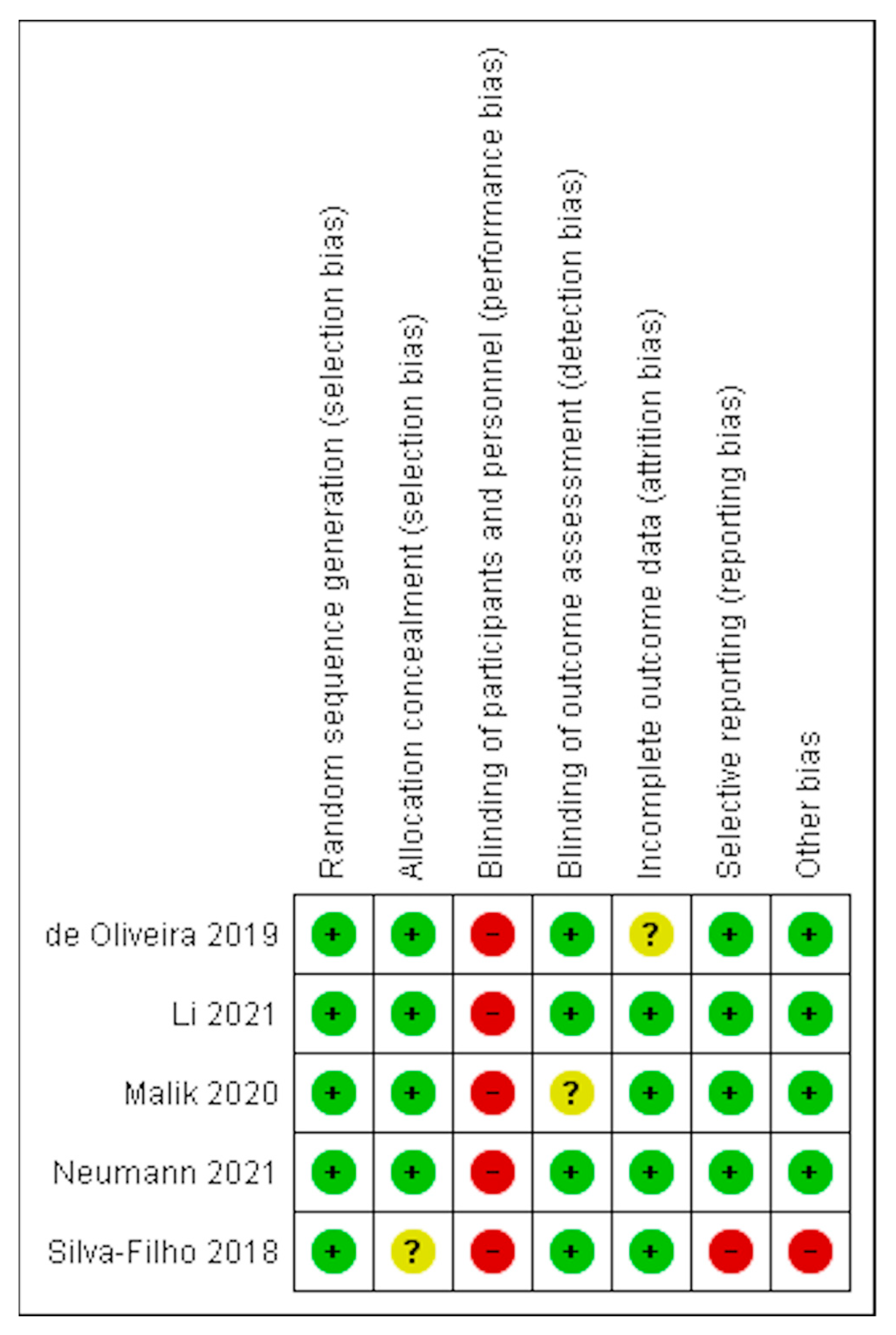
3.2. Risk of Bias in Included Studies
3.3. Effects of Interventions
3.4. Tele-Rehabilitation
3.5. Music Therapy in Combination with Cognitive Behavioural Therapy
3.6. Resistance Exercises
3.7. Pilates
3.8. Neuromodulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johns Hopkins University. COVID-19 Map—Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 13 May 2022).
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and Effectiveness of MRNA BNT162b2 Vaccine against SARS-CoV-2 Infections and COVID-19 Cases, Hospitalisations, and Deaths Following a Nationwide Vaccination Campaign in Israel: An Observational Study Using National Surveillance Data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Direct and Indirect Impact of the Vaccination Programme on COVID-19 Infections and Mortality. 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/997495/Impact_of_COVID-19_vaccine_on_infection_and_mortality.pdf (accessed on 13 May 2022).
- Gupta, S.; Cantor, J.; Simon, K.I.; Bento, A.I.; Wing, C.; Whaley, C.M. Vaccinations Against COVID-19 May Have Averted Up To 140,000 Deaths in The United States. Health Aff. 2021, 40, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Office for National Statistics. Prevalence of Ongoing Symptoms Following Coronavirus (COVID-19) Infection in the UK. 2022. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/7april2022 (accessed on 10 June 2022).
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. Symptoms, Complications and Management of Long COVID: A Review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Perego, E.; Callard, F.; Stras, L.; Melville-Jóhannesson, B.; Pope, R.; Alwan, N.A. Why We Need to Keep Using the Patient Made Term “Long COVID”. BMJ 2020.
- Long COVID: Let Patients Help Define Long-Lasting COVID Symptoms. Nature 2020, 586, 170. [CrossRef]
- National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. 2022. Available online: https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742 (accessed on 10 June 2022).
- Aucott, J.N.; Rebman, A.W. Long-Haul COVID: Heed the Lessons from Other Infection-Triggered Illnesses. Lancet 2021, 397, 967–968. [Google Scholar] [CrossRef]
- Salamanna, F.; Veronesi, F.; Martini, L.; Landini, M.P.; Fini, M. Post-COVID-19 Syndrome: The Persistent Symptoms at the Post-Viral Stage of the Disease. A Systematic Review of the Current Data. Front. Med. 2021, 8, 392. [Google Scholar] [CrossRef]
- White, P.D. What Causes Prolonged Fatigue after Infectious Mononucleosis—And Does It Tell Us Anything about Chronic Fatigue Syndrome? J. Infect. Dis. 2007, 196, 4–5. [Google Scholar] [CrossRef]
- Malik, S.; Asprusten, T.T.; Pedersen, M.; Mangersnes, J.; Trondalen, G.; Van Roy, B.; Skovlund, E.; Wyller, V.B. Cognitive–Behavioural Therapy Combined with Music Therapy for Chronic Fatigue Following Epstein-Barr Virus Infection in Adolescents: A Randomised Controlled Trial. BMJ Paediatr. Open 2020, 4, e000797. [Google Scholar] [CrossRef]
- Fowler-Davis, S.; Platts, K.; Thelwell, M.; Woodward, A.; Harrop, D. A Mixed-Methods Systematic Review of Post-Viral Fatigue Interventions: Are There Lessons for Long COVID? PLoS ONE 2021, 16, e0259533. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Chandan, J.S.; Brown, K.; Simms-Williams, N.; Camaradou, J.; Bashir, N.; Heining, D.; Lee Aiyegbusi, O.; Turner, G.; Cruz Rivera, S.; Hotham, R.; et al. Non-Pharmacological Therapies for Post-Viral Syndromes, Including Long COVID: A Systematic Review and Meta-Analysis Protocol. BMJ Open 2022, 12, e057885. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Collingwood, J. An Overview of Post-Viral Syndrome. 2021. Available online: https://sma.org/post-viral-syndrome/ (accessed on 13 May 2022).
- Breakspear Medical. Post-Viral Fatigue & Post-Viral Syndrome. Available online: https://www.breakspearmedical.com/treatments/post-viral/ (accessed on 8 January 2023).
- ICD-10-Data. 2023 ICD-10-CM Diagnosis Code G93. 2023. Available online: https://www.icd10data.com/ICD10CM/Codes/G00-G99/G89-G99/G93-/G93.3 (accessed on 8 January 2023).
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 13 May 2022).
- Centers for Disease Control and Prevention. Middle East Respiratory Syndrome (MERS). 2019. Available online: https://www.cdc.gov/coronavirus/mers/about/index.html (accessed on 9 February 2023).
- LeDuc, J.W.; Barry, M.A. SARS, the First Pandemic of the 21st Century. Emerg. Infect. Dis. 2004, 10, e26. [Google Scholar] [CrossRef]
- Boutron, I.; Chaimani, A.; Meerpohl, J.J.; Hróbjartsson, A.; Devane, D.; Rada, G.; Tovey, D.; Grasselli, G.; Ravaud, P.; Ala-Wadhi, S.; et al. The COVID-NMA Project: Building an Evidence Ecosystem for the COVID-19 Pandemic. Ann. Intern. Med. 2020, 173, 1015–1017. [Google Scholar] [CrossRef]
- Counotte, M.; Imeri, H.; Heron, L.; Ipekci, A.M.; Low, N. Living Evidence on COVID-19. 2020. Available online: https://ispmbern.github.io/covid-19/living-review/ (accessed on 29 October 2021).
- Veritas Health Innovation. Covidence Systematic Review Software; Covidence: Melbourne, Australia, 2022. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Haroon, S.; Nirantharakumar, K.; Hughes, S.E.; Subramanian, A.; Aiyegbusi, O.L.; Davies, E.H.; Myles, P.; Williams, T.; Turner, G.; Chandan, J.S.; et al. Therapies for Long COVID in Non-Hospitalised Individuals: From Symptoms, Patient-Reported Outcomes and Immunology to Targeted Therapies (The TLC Study). BMJ Open 2022, 12, e060413. [Google Scholar] [CrossRef]
- Staniszewska, S.; Brett, J.; Simera, I.; Seers, K.; Mockford, C.; Goodlad, S.; Altman, D.G.; Moher, D.; Barber, R.; Denegri, S.; et al. GRIPP2 reporting checklists: Tools to improve reporting of patient and public involvement in research. Res. Involv. Engagem. 2017, 3, 13. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, M.H.; Do, B.N.; Tran, C.Q.; Nguyen, T.T.P.; Pham, K.M.; Pham, L.V.; Tran, K.V.; Duong, T.T.; Tran, T.V.; et al. People with Suspected COVID-19 Symptoms Were More Likely Depressed and Had Lower Health-Related Quality of Life: The Potential Benefit of Health Literacy. J. Clin. Med. 2020, 9, 965. [Google Scholar] [CrossRef]
- Joshi, R.; Rathi, M.; Thakur, J. Post-COVID-19 Physiotherapy Rehabilitation: A Case Report. J. Clin. Diagnostic Res. 2021, 15, YD01–YD02. [Google Scholar] [CrossRef]
- Vlake, J.H.; Van Bommel, J.; Wils, E.-J.; Bienvenu, J.; Hellemons, M.E.; Korevaar, T.I.M.; Schut, A.F.C.; Labout, J.A.M.; Schreuder, L.L.H.; van Bavel, M.P.; et al. Intensive Care Unit–Specific Virtual Reality for Critically Ill Patients With COVID-19: Multicenter Randomized Controlled Trial. J. Med. Internet Res. 2022, 24, e32368. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-M.; Ko, H.-J.; Lee, G.H.; Kim, A.-S.; Lee, D.-W. A Well-Structured Follow-Up Program Is Required after Recovery from Coronavirus Disease 2019 (COVID-19); Release from Quarantine Is Not the End of Treatment. J. Clin. Med. 2021, 10, 2329. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.C.; Limbach, M.; Schuler, M.; Merkl, S.; Schwarzl, G.; Jakab, K.; Nowak, D.; Schultz, K. Effectiveness of a Three-Week Inpatient Pulmonary Rehabilitation Program for Patients after COVID-19: A Prospective Observational Study. Int. J. Environ. Res. Public Health 2021, 18, 9001. [Google Scholar] [CrossRef] [PubMed]
- Khah, A.S.; Mortazavian, S.; Kateb, M.Y.; Najafabadi, M.G.; Niyazi, S. The Effect of Online Mindfulness Program on Physical Pain, Stress and Depression in the COVID-19 Patients: A Randomized Control Trail. J. Pain Manag. 2021, 14, 57–63. [Google Scholar]
- Parizad, N.; Goli, R.; Faraji, N.; Mam-Qaderi, M.; Mirzaee, R.; Gharebaghi, N.; Baghaie, R.; Feizipour, H.; Haghighi, M.-M. Effect of Guided Imagery on Anxiety, Muscle Pain, and Vital Signs in Patients with COVID-19: A Randomized Controlled Trial. Complement. Ther. Clin. Pract. 2021, 43, 101335. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Jiang, J.; Xu, X.; Wu, J.; Xu, Y.; Lin, X.; Hall, J.; Xu, H.; Xu, J.; et al. The Effect of Cognitive Behavioral Therapy on Depression, Anxiety, and Stress in Patients With COVID-19: A Randomized Controlled Trial. Front. Psychiatry 2020, 11, 1096. [Google Scholar] [CrossRef]
- Liu, Z.; Qiao, D.; Xu, Y.; Zhao, W.; Yang, Y.; Wen, D.; Li, X.; Nie, X.; Dong, Y.; Tang, S.; et al. The Efficacy of Computerized Cognitive Behavioral Therapy for Depressive and Anxiety Symptoms in Patients With COVID-19: Randomized Controlled Trial. J. Med. Internet Res. 2021, 23, e26883. [Google Scholar] [CrossRef]
- Sun, J.; Liu, J.; Li, H.; Shang, C.; Li, T.; Ji, W.; Wu, J.; Han, X.; Shi, Z. Pulmonary Rehabilitation Focusing on the Regulation of Respiratory Movement Can Improve Prognosis of Severe Patients with COVID-19. Ann. Palliat. Med. 2021, 10, 4262272–4264272. [Google Scholar] [CrossRef]
- Gonzalez-Gerez, J.J.; Saavedra-Hernandez, M.; Anarte-Lazo, E.; Bernal-Utrera, C.; Perez-Ale, M.; Rodriguez-Blanco, C. Short-Term Effects of a Respiratory Telerehabilitation Program in Confined COVID-19 Patients in the Acute Phase: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 7511. [Google Scholar] [CrossRef]
- Kong, X.; Kong, F.; Zheng, K.; Tang, M.; Chen, Y.; Zhou, J.; Li, Y.; Diao, L.; Wu, S.; Jiao, P.; et al. Effect of Psychological–Behavioral Intervention on the Depression and Anxiety of COVID-19 Patients. Front. Psychiatry 2020, 11, 1241. [Google Scholar] [CrossRef]
- Daynes, E.; Gerlis, C.; Chaplin, E.; Gardiner, N.; Singh, S.J. Early Experiences of Rehabilitation for Individuals Post-COVID to Improve Fatigue, Breathlessness Exercise Capacity and Cognition—A Cohort Study. Chron. Respir. Dis. 2021, 18, 147997312110156. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh, H.G.; Alavi, S.S.; Akbari, Z.; Jannatifard, F.; Artounian, V. The Effect of Brief Crisis Intervention Package on Improving Quality of Life and Mental Health in Patients with COVID-19. Iran. J. Psychiatry 2020, 15, 205. [Google Scholar] [CrossRef]
- Champion, T.; Bauer, N. Effects of a Structured Cardiopulmonary Rehabilitation Program for Individuals Recovering from COVID-19. Cardiopulm. Phys. Ther. J. 2021, 32, e5–e6. [Google Scholar]
- Polsky, M.B.; Moraveji, N. Post-Acute Care Management of a Patient with COVID-19 Using Remote Cardiorespiratory Monitoring. Respir. Med. Case Rep. 2021, 33, 101436. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Wang, Z.Y.; Chen, Y.J.; Ni, J. The Application of Eight-Segment Pulmonary Rehabilitation Exercise in People With Coronavirus Disease 2019. Front. Physiol. 2020, 11, 646. [Google Scholar] [CrossRef]
- Hui, F.; Boyle, E.; Vayda, E.; Glazier, R.H. A Randomized Controlled Trial of a Multifaceted Integrated Complementary-Alternative Therapy for Chronic Herpes Zoster-Related Pain. Altern. Med. Rev. 2012, 17, 57–68. [Google Scholar]
- Ye, L.; Sun, P.; Wang, T. Acupuncture Strategies to Tackle Post COVID-19 Psychological and Neuropsychiatric Disorders. J. Chinese Med. 2020, 2020, 11–21. [Google Scholar]
- Keijmel, S.P.; Delsing, C.E.; Bleijenberg, G.; van der Meer, J.W.M.; Donders, R.T.; Leclercq, M.; Kampschreur, L.M.; Van Den Berg, M.; Sprong, T.; Nabuurs-Franssen, M.H.; et al. Effectiveness of Long-Term Doxycycline Treatment and Cognitive-Behavioral Therapy on Fatigue Severity in Patients with Q Fever Fatigue Syndrome (Qure Study): A Randomized Controlled Trial. Clin. Infect. Dis. 2017, 64, 998–1005. [Google Scholar] [CrossRef]
- Mayer, K.P.; Steele, A.K.; Soper, M.K.; Branton, J.D.; Lusby, M.L.; Kalema, A.G.; Dupont-Versteegden, E.E.; Montgomery-Yates, A.A. Physical Therapy Management of an Individual with Post-COVID Syndrome: A Case Report. Phys. Ther. 2021, 101, pzab098. [Google Scholar] [CrossRef]
- Dalbosco-Salas, M.; Torres-Castro, R.; Rojas Leyton, A.; Morales Zapata, F.; Henríquez Salazar, E.; Espinoza Bastías, G.; Beltrán Díaz, M.E.; Tapia Allers, K.; Mornhinweg Fonseca, D.; Vilaró, J. Effectiveness of a Primary Care Telerehabilitation Program for Post-COVID-19 Patients: A Feasibility Study. J. Clin. Med. 2021, 10, 4428. [Google Scholar] [CrossRef]
- Tsiskarishvili, N.; Tsiskarishvili, N.; Tsiskarishvili, T. Local Phototherapy and Pulse Current in Complex Treatment of Relapsing Herpes Simplex. Georg. Med News 2007, 152, 31–34. [Google Scholar]
- Vlake, J.H.; Van Bommel, J.; Wils, E.-J.; Korevaar, T.I.M.; Hellemons, M.E.; Schut, A.F.C.; Labout, J.A.M.; Schreuder, L.L.H.; Gommers, D.; Van Genderen, M.E. Effect of Intensive Care Unit-Specific Virtual Reality (ICU-VR) to Improve Psychological Well-Being and Quality of Life in COVID-19 ICU Survivors: A Study Protocol for a Multicentre, Randomized Controlled Trial. Trials 2021, 22, 328. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, W.; Yang, Y.; Zhang, J.; Li, Y.; Chen, Y. Respiratory Rehabilitation in Elderly Patients with COVID-19: A Randomized Controlled Study. Complement. Ther. Clin. Pract. 2020, 39, 101166. [Google Scholar] [CrossRef] [PubMed]
- Szlamka, Z.; Kiss, M.; Bernáth, S.; Kámán, P.; Lubani, A.; Karner, O.; Demetrovics, Z. Mental Health Support in the Time of Crisis: Are We Prepared? Experiences With the COVID-19 Counselling Programme in Hungary. Front. Psychiatry 2021, 12, 655211. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, I.S.; Leal, F.S.; Tateno, R.Y.; Palma, L.F.; Campos, L. Photobiomodulation Therapy and Antimicrobial Photodynamic Therapy for Orofacial Lesions in Patients with COVID-19: A Case Series. Photodiagnosis Photodyn. Ther. 2021, 34, 102281. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Jiang, J.; Shen, P.; Li, M.; You, H.; Liu, C.; Chen, L.; Wang, Z.; Zhou, C.; Feng, Z. Liuzijue Is a Promising Exercise Option for Rehabilitating Discharged COVID-19 Patients. Medicine 2021, 100, e24564. [Google Scholar] [CrossRef]
- Cineka, A.; Raj, J. Dance and Music as a Therapy to Heal Physical and Psychological Pain: An Analytical Study of COVID-19 Patients during Quarantine. Eur. J. Mol. Clin. Med. 2020, 7, 99–109. [Google Scholar]
- Imamura, M.; Mirisola, A.R.; Ribeiro, F.D.Q.; De Pretto, L.R.; Alfieri, F.M.; Delgado, V.R.; Battistella, L.R. Rehabilitation of Patients after COVID-19 Recovery: An Experience at the Physical and Rehabilitation Medicine Institute and Lucy Montoro Rehabilitation Institute. Clinics 2021, 76, e2804. [Google Scholar] [CrossRef]
- Pan, H.J.; Bao, X.H.; Chen, J.Y.; Feng, Y.; Kang, B.Y.; Wang, J.X.; Xie, Q. Respiratory Rehabilitation Assisted by Respiratory Trainers in Patients with Coronavirus Disease 2019: An Analysis of Efficacy. Acad. J. Second Mil. Med. Univ. 2021, 42, 255–260. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Fang, J.-Q. Analysis on Therapeutic Effect of Variable-Frequency Electroacupuncture Combined with Herbal-Moxa Moxibustion for Post-Zoster Neuralgia. Zhen Ci Yan Jiu Acupunct. Res. 2012, 37, 64–66. [Google Scholar]
- Zhang, X.B.; Zhang, J.L.; Li, M.X.; Yuan, Y.P.; Sun, J. Baduanjin Exercise Can Alleviate Anxiety and Depression of Patients with COVID-19 in Square Cabin Hospital: A Cross-Sectional Survey. Medicine 2021, 100, e26898. [Google Scholar] [CrossRef]
- Wootton, S.L.; King, M.; Alison, J.A.; Mahadev, S.; Chan, A.S.L. COVID-19 Rehabilitation Delivered via a Telehealth Pulmonary Rehabilitation Model: A Case Series. Respirol. Case Rep. 2020, 8, 669. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Xu, X.; Wang, D.; Qiao, G.; Zhuang, W.; Huang, S. Modified Rehabilitation Exercises for Mild Cases of COVID-19. Ann. Palliat. Med. 2020, 9, 3100–3106. [Google Scholar] [CrossRef] [PubMed]
- Biondi Situmorang, D.D. Music Therapy for the Treatment of Patients with COVID-19: Psychopathological Problems Intervention and Well-Being Improvement. Infect. Dis. Clin. Pract. 2021, 29, E198. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.K.; Nanda, N.; Pal, P.; Renugasundari, M.; Hariprasad, R.; Rajesh, D.R.; Pachegaonkar, U. Asanas in Prone Posture and Slow Pranayamic Breathing Promote Early Recoveryand Prevent Post-Recovery Complications in COVID-19 Patients: A Preliminary Study. Biomedicine 2021, 41, 390–396. [Google Scholar]
- Voshaar, T.; Stais, P.; Köhler, D.; Dellweg, D. Conservative Management of COVID-19 Associated Hypoxaemia. ERJ Open Res. 2021, 7, 00292–02021. [Google Scholar] [CrossRef]
- Sanger, N.S.; Kankakee, A.; Singh, R.; Gupta, A.; Shrikhande, B.; Ganu, G. A Randomized Controlled Clinical Trial to Evaluate the Potential of Ayurvedic Medicines in Patients with Mild Symptoms of COVID-19. Eur. J. Mol. Clin. Med. 2021, 8, 3017–3027. [Google Scholar]
- Olenskaya, T.L.; Nikolayeva, A.H.; Piatsko, V.V.; Azaronak, M.K.; Yukhno, Y.S. Application of Altitude Chamber Adaptation as a Component of the Medical Rehabilitation Patients after COVID-19. Profil. Meditsina 2021, 24, 76. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, R.; Yang, X.; Zhang, S.; Li, D.; Zhang, Y.; Liu, J.; Pakhale, S.; Krewski, D.; Wen, S.W. Feasibility and Preliminary Results of Effectiveness of Social Media-Based Intervention on the Psychological Well-Being of Suspected COVID-19 Cases during Quarantine. Can. J. Psychiatry 2020, 65, 736–738. [Google Scholar] [CrossRef]
- Schindl, A.; Neumann, R. Low-Intensity Laser Therapy Is an Effective Treatment for Recurrent Herpes Simplex Infection. Results from a Randomized Double-Blind Placebo-Controlled Study. J. Invest. Dermatol. 1999, 113, 221–223. [Google Scholar] [CrossRef]
- Shakerian, N.; Mofateh, R.; Saghazadeh, A.; Rezaei, N.; Rezaei, N. Potential Prophylactic and Therapeutic Effects of Respiratory Physiotherapy for COVID-19. Acta Biomed. 2020, 92, e2021020. [Google Scholar] [CrossRef] [PubMed]
- Kurtaiş Aytür, Y. Pulmonary Rehabilitation Principles in SARS-CoV-2 Infection (COVID-19): A Guideline for the Acute and Subacute Rehabilitation. Turkish J. Phys. Med. Rehabil. 2020, 66, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Hewson-Bower, B.; Drummond, P.D. Psychological Treatment for Recurrent Symptoms of Colds and Flu in Children. J. Psychosom. Res. 2001, 51, 369–377. [Google Scholar] [CrossRef]
- Zampogna, E.; Paneroni, M.; Belli, S.; Aliani, M.; Gandolfo, A.; Visca, D.; Bellanti, M.T.; Ambrosino, N.; Vitacca, M. Pulmonary Rehabilitation in Patients Recovering from COVID-19. Respiration 2021, 100, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Blanco, C.; Gonzalez-Gerez, J.J.; Bernal-Utrera, C.; Anarte-Lazo, E.; Perez-Ale, M.; Saavedra-Hernandez, M. Short-Term Effects of a Conditioning Telerehabilitation Program in Confined Patients Affected by COVID-19 in the Acute Phase. A Pilot Randomized Controlled Trial. Medicina 2021, 57, 684. [Google Scholar] [CrossRef]
- Jenefer Jerrin, R.; Theebika, S.; Panneerselvam, P.; Venkateswaran, S.; Manavalan, N.; Maheshkumar, K. Yoga and Naturopathy Intervention for Reducing Anxiety and Depression of COVID-19 Patients–A Pilot Study. Clin. Epidemiol. Glob. Heal. 2021, 11, 100800. [Google Scholar] [CrossRef] [PubMed]
- Bickton, F.M.; Chisati, E.; Rylance, J.; Morton, B. An Improvised Pulmonary Telerehabilitation Program for Postacute COVID-19 Patients Would Be Feasible and Acceptable in a Low-Resource Setting. Am. J. Phys. Med. Rehabil. 2021, 100, 209–212. [Google Scholar] [CrossRef]
- Cheng, S.I. Medical Acupuncture as a Treatment for Novel COVID-19-Related Respiratory Distress: Personal Experience from a Frontline Anesthesiologist. Med. Acupunct. 2021, 33, 83–85. [Google Scholar] [CrossRef]
- Pal, G.K.; Nanda, N.; Renugasundari, M.; Pal, P.; Pachegaonkar, U. Acute Effects of Prone Asanas and Pal’s Pranayama on Myalgia, Headache, Psychological Stress and Respiratory Problems in the COVID-19 Patients in the Recovery Phase. Biomedicine 2020, 40, 526–530. [Google Scholar] [CrossRef]
- Karthikeyan, T. Therapeutic Effectiveness of Diaphragmatic with Costal Breathing Exercises on C-19 PEFR Patients. Intensive Care Med. Exp. 2021, 9, 196. [Google Scholar]
- Song, J.; Jiang, R.; Chen, N.; Qu, W.; Liu, D.; Zhang, M.; Fan, H.; Zhao, Y.; Tan, S. Self-Help Cognitive Behavioral Therapy Application for COVID-19-Related Mental Health Problems: A Longitudinal Trial. Asian J. Psychiatr. 2021, 60, 102656. [Google Scholar] [CrossRef]
- Gilmutdinova, I.R.; Kolyshenkov, V.A.; Lapickaya, K.A.; Trepova, A.S.; Vasileva, V.A.; Prosvirnin, A.N.; Marchenkova, L.A.; Terentev, K.V.; Yakovlev, M.Y.; Rachin, A.P.; et al. Telemedicine Platform COVIDREHAB for Remote Rehabilitation of Patients after COVID-19. Eur. J. Transl. Myol. 2021, 31, 9783. [Google Scholar] [CrossRef]
- Lerner, D.; Garvey, K.; Arrighi-Allisan, A.; Filimonov, A.; Filip, P.; Liu, K.; Ninan, S.; Schaberg, M.; Colley, P.; Del Signore, A.; et al. Letter to the Editor: Study Summary—Randomized Control Trial of Omega-3 Fatty Acid Supplementation for the Treatment of COVID-19 Related Olfactory Dysfunction. Trials 2020, 21, 942. [Google Scholar] [CrossRef]
- Reid, M.R.; Mackinnon, L.T.; Drummond, P.D. The Effects of Stress Management on Symptoms of Upper Respiratory Tract Infection, Secretory Immunoglobulin A, and Mood in Young Adults. J. Psychosom. Res. 2001, 51, 721–728. [Google Scholar] [CrossRef]
- De Lima Thomas, J.; Leiter, R.E.; Abrahm, J.L.; Shameklis, J.C.; Kiser, S.B.; Gelfand, S.L.; Sciacca, K.R.; Reville, B.; Siegert, C.A.; Zhang, H.; et al. Development of a Palliative Care Toolkit for the COVID-19 Pandemic. J. Pain Symptom Manag. 2020, 60, e22–e25. [Google Scholar] [CrossRef]
- Meeus, M.; Nijs, J.; Van Oosterwijck, J.; Van Alsenoy, V.; Truijen, S. Pain Physiology Education Improves Pain Beliefs in Patients with Chronic Fatigue Syndrome Compared With Pacing and Self-Management Education: A Double-Blind Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2010, 91, 1153–1159. [Google Scholar] [CrossRef]
- Curci, C.; Pisano, F.; Bonacci, E.; Camozzi, D.M.; Ceravolo, C.; Bergonzi, R.; De Franceschi, S.; Moro, P.; Guarnieri, R.; Ferrillo, M.; et al. Early Rehabilitation in Post-Acute COVID-19 Patients: Data from an Italian COVID-19 Rehabilitation Unit and Proposal of a Treatment Protocol. Eur. J. Phys. Rehabil. Med. 2020, 56, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, W.; Zhan, C.; Liu, S.; Yin, Z.; Wang, J.; Chong, Y.; Zheng, C.; Fang, X.; Cheng, W.; et al. A Telerehabilitation Programme in Post-Discharge COVID-19 Patients (TERECO): A Randomised Controlled Trial. Thorax 2021, 77, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.L.; De Oliveira, D.A.; De Barros, E.L.; Santos, G.D.S.; De Oliveira, L.S.; Duarte, A.L.; Marques, C.D.; Dantas, A.T.; Dantas, D.; De Siqueira, G.R.; et al. Resistance Exercises Improve Physical Function in Chronic Chikungunya Fever Patients: A Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2021, 57, 620–629. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, B.F.A.; Carvalho, P.R.C.; De Souza Holanda, A.S.; Dos Santos, R.I.S.B.; Da Silva, F.A.X.; Barros, G.W.P.; De Albuquerque, E.C.; Dantas, A.T.; Cavalcanti, N.G.; Ranzolin, A.; et al. Pilates Method in the Treatment of Patients with Chikungunya Fever: A Randomized Controlled Trial. Clin. Rehabil. 2019, 33, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, E.; Okano, A.H.; Morya, E.; Albuquerque, J.; Cacho, E.; Unal, G.; Bikson, M.; Pegado, R. Neuromodulation Treats Chikungunya Arthralgia: A Randomized Controlled Trial. Sci. Rep. 2018, 8, 16010. [Google Scholar] [CrossRef] [PubMed]
- RAND Corporation. 36-Item Short Form Survey (SF-36). Available online: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html (accessed on 13 May 2022).
- RAND Corporation. 12-Item Short Form Survey (SF-12). Available online: https://www.rand.org/health-care/surveys_tools/mos/12-item-short-form.html (accessed on 13 May 2022).
- Pedersen, M.; Asprusten, T.T.; Godang, K.; Leegaard, T.M.; Osnes, L.T.; Skovlund, E.; Tjade, T.; Øie, M.G.; Wyller, V.B.B. Predictors of Chronic Fatigue in Adolescents Six Months after Acute Epstein-Barr Virus Infection: A Prospective Cohort Study. Brain. Behav. Immun. 2019, 75, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Boggio, P.S.; Zaghi, S.; Fregni, F. Modulation of Emotions Associated with Images of Human Pain Using Anodal Transcranial Direct Current Stimulation (TDCS). Neuropsychologia 2009, 47, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Seron, P.; Oliveros, M.J.; Gutierrez-Arias, R.; Fuentes-Aspe, R.; Torres-Castro, R.C.; Merino-Osorio, C.; Nahuelhual, P.; Inostroza, J.; Jalil, Y.; Solano, R.; et al. Effectiveness of Telerehabilitation in Physical Therapy: A Rapid Overview. Phys. Ther. 2021, 101, pzab053. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, B.; Casey, D.; Devane, D.; Murphy, K.; Murphy, E.; Lacasse, Y. Pulmonary Rehabilitation for Chronic Obstructive Pulmonary Disease. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef]
- Gloeckl, R.; Leitl, D.; Jarosch, I.; Schneeberger, T.; Nell, C.; Stenzel, N.; Vogelmeier, C.F.; Kenn, K.; Koczulla, A.R. Benefits of Pulmonary Rehabilitation in COVID-19: A Prospective Observational Cohort Study. ERJ Open Res. 2021, 7, 00108–02021. [Google Scholar] [CrossRef]
- Ahmed, I.; Inam, A.B.; Belli, S.; Ahmad, J.; Khalil, W.; Jafar, M.M. Effectiveness of Aerobic Exercise Training Program on Cardio-Respiratory Fitness and Quality of Life in Patients Recovered from COVID-19. Eur. J. Physiother. 2021, 33, 1–6. [Google Scholar] [CrossRef]
- McGregor, G.; Sandhu, H.; Bruce, J.; Sheehan, B.; McWilliams, D.; Yeung, J.; Jones, C.; Lara, B.; Smith, J.; Ji, C.; et al. Rehabilitation Exercise and PsycholoGical Support After COVID-19 InfectioN’ (REGAIN): A Structured Summary of a Study Protocol for a Randomised Controlled Trial. Trials 2021, 22, 1–3. [Google Scholar] [CrossRef]
- Pilloni, G.; Bikson, M.; Badran, B.W.; George, M.S.; Kautz, S.A.; Okano, A.H.; Baptista, A.F.; Charvet, L.E. Update on the Use of Transcranial Electrical Brain Stimulation to Manage Acute and Chronic COVID-19 Symptoms. Front. Hum. Neurosci. 2020, 14, 493. [Google Scholar] [CrossRef]
- Silva-Filho, E.; Moura, S.; Da Cruz Santos, A.; Do Socorro Brasileiro-Santos, M.; De Albuquerque, J.A. Transcranial Direct Current Stimulation as a Strategy to Manage COVID-19 Pain and Fatigue. Rev. Assoc. Med. Bras. 2021, 67, 26–28. [Google Scholar] [CrossRef]
- Gómez, L.; Vidal, B.; Cabrera, Y.; Hernández, L.; Rondón, Y. Successful Treatment of Post-COVID Symptoms With Transcranial Direct Current Stimulation. Prim. Care Companion CNS Disord. 2021, 23, 38522. [Google Scholar] [CrossRef] [PubMed]
- Transcranial Direct Stimulation for Persistent Fatigue Treatment Post-COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT05252481 (accessed on 13 May 2022).
- Home-Based Brain Stimulation Treatment for Post-Acute Sequelae of COVID-19 (PASC). Available online: https://clinicaltrials.gov/ct2/show/NCT05092516 (accessed on 13 May 2022).
- National Institute for Health and Care Excellence. NICE ME/CFS Guideline Outlines Steps for Better Diagnosis and Management. 2021. Available online: https://www.nice.org.uk/news/article/nice-me-cfs-guideline-outlines-steps-for-better-diagnosis-and-management (accessed on 13 May 2022).
- Hughes, S.E.; Haroon, S.; Subramanian, A.; McMullan, C.; Aiyegbusi, O.L.; Turner, G.M.; Jackson, L.; Davies, E.H.; Frost, C.; McNamara, G.; et al. Development and Validation of the Symptom Burden Questionnaire for Long COVID (SBQ-LC): Rasch Analysis. BMJ 2022, 377, e070230. [Google Scholar] [CrossRef] [PubMed]
| Study Characteristics | Viral Definitions | Inclusion/Exclusion Criteria | Baseline Characteristics | ||||
|---|---|---|---|---|---|---|---|
| Authors | Virus Being Studied | Definition of Post-Viral Syndrome and Symptoms Reported by Patient Groups: | Inclusion | Exclusion | Intervention Group | Comparator Group | Overall |
| Authors: Li et al. [90] 2021 Country: China Setting: Hospital Study period: 2020 | SARS-CoV-2 | Definition: No time length specified but average time from hospital discharge to baseline was 70.07 days Symptoms: Dyspnoea | 18–75 years old; Discharged from one of the three participating hospitals (three major hospitals in Jiangsu and Hubei provinces in China: Jiangsu Province Hospital/Nanjing Medical University First Affiliated Hospital, Hubei Province Hospital of Integrated Chinese and Western Medicine, and Hubei Huangshi Hospital of Chinese Medicine) after having inpatient treatment for COVID-19; and had a modified British Medical Research Council (mMRC) dyspnoea score of 2–3. | Patients with an mMRC score of 4–5 were excluded for safety reasons; Other exclusion: resting heart rate over 100 bpm, uncontrolled hypertension, uncontrolled chronic disease (e.g., diabetes with random blood glucose >16.7mmol/L, haemoglobin A1C >7.0%), cerebrovascular disease within 6 months, intra-articular drug injection or surgical treatment of lower extremities within 6 months, taking medication affecting cardiopulmonary function such as bronchodilators or beta-blockers, unable to walk independently with assistive device, unable or unwilling to collaborate with assessments, enrolled or participated in other trials within the past 3 months, having a history of severe cognitive or mental disorder or substance abuse, enrolment in other rehabilitation programme. | N = 59 Male: 27 (46%) Female: 32 (54%) Age: 49.2 (SD 10.8) | N = 60 Male: 26 (43%) Female: 34 (57%) Age: 52.0 (SD 11.1) | N = 119 Male: 53 (45%) Female: 66 (55%) Age: 50.6 (SD 11.0) |
| Authors: Malik et al. [13] 2020 Country: Norway Setting: Not described Study period: 2015–17 | Epstein–Barr virus (EBV) | Definition: Patients had to have chronic fatigue syndrome (CF) for at least 6 months after acute infection with EBV. Symptoms: Fatigue, post-exertional malaise and pain | Developed CF 6 months after an acute EBV infection; A serological pattern indicating acute EBV infection; Age between 12 and 20 years; Living in one of the Norwegian counties Oslo, Akershus, or Buskerud | More than 6 weeks since debut of symptoms suggesting acute EBV infection; Any chronic disease that needed regular use of medication; Pregnancy. | N = 21 Male: 4 (19%) Female: 17 (81%) Age: 17.7 (SD 1.4) | N = 22 Male: 6 (27%) Female: 16 (73%) Age: 16.9 (SD 1.7) | N = 43 Male: 10 (23%) Female: 33 (77%) Age: Overall age not provided |
| Authors: Neumann et al. [91] 2021 Country: Brazil Setting: Hospital Study period: 2018–19 | Chikungunya | Definition: Musculoskeletal symptoms lasting beyond three months Symptoms: Arthralgia | Aged 18–75 years; Serological diagnosis of Chikungunya fever and symptoms lasting 3+ months | Cognitive impairment as assessed by mini-mental state examination (MMSE); Contraindication for physical exercise (e.g., unstable angina, uncontrolled hypertension, or kidney disorder); Neurological disorders; Previous diagnosis of rheumatic disorders (except osteoarthritis); Physical impairment preventing intervention; Pregnancy; Receiving other physical modality treatments during research period; Engagement in regular physical exercise (mild- or moderate-intensity aerobic activities for 30 min five times a week or vigorous physical activity for at least 20 min three times a week) | N = 15 Male: 1 (7%) Female: 14 (93%) Age: 54.9 (SD 9.6) | N = 16 Male: 2 (12.5%) Female: 14 (87.5%) Age: 56.7 (SD 11.0) | N = 31 Male: 3 (10%) Female: 28 (90%) Age: 56.0 (SD 10.0) |
| Authors: Silva-Filho et al. [93] 2018 Country: Brazil Setting: Community Study period: 2016–17 | Chikungunya | Definition: Positive CHIK virus for at least 6 months Symptoms: Arthralgia | Positive laboratory tests for the CHIK virus for at least 6 months (chronic phase); Preserved intellectual capacity determined by the mini mental state examination (MMSE); Physical capacity to do physical evaluation; 18 and 65 years old | Pain clearly related to any other aetiology, such as dengue, zika, rheumatoid arthritis, gout, lupus, neurologic and muscular diseases, psychiatric illness, and history of drug abuse; Signs or history of dizziness or epileptic disease; Pregnancy; Signs of severity and/or indication of hospitalization and metal implants in the head | N = 10 Male: 0 (0%) Female: 10 (100%) Age: 46.1 (SD 16.0) | N = 10 Male: 1 (10%) Female: 9 (90%) Age: 44.1 (SD 13.5) | N = 20 Male: 1 (5%) Female: 19 (95%) Age: Overall age not provided |
| Authors: de Oliveria et al. [92] 2019 Country: Brazil Setting: Outpatient clinical, Hospital Study period: 2017 | Chikungunya | Definition: Symptoms lasting more than 3 months Symptoms: Arthralgia | 18+ years; Confirmed diagnosis of Chikungunya fever; Patient in clinical treatment at Chikungunya outpatient clinic; Chronic phase of the disease (symptoms lasting more than three months) | Contraindication for physical exercise according to the treating physician; A severely limiting cognitive, auditory, visual, or motor deficit confirmed by a specialist physician; History of inflammatory, rheumatic, neurological, or neoplastic disorders | N = 22 Male: 3 (14%) Female: 19 (86%) Age: 54.4 (SD 10.6) | N = 20 Male: 0 (0%) Female: 20 (100%) Age: 59.6 (SD 9.4) | N = 42 Male: 3 (7%) Female: 39 (83%) Age: 56.9 (SD 10.6) |
| Authors | Description of Intervention and Comparator | Outcome of Interest (Including Method of Measurement) | Description of Key Findings |
|---|---|---|---|
| Li et al. [90] | Intervention: Telerehabilitation programme in post-discharge COVID-19 patients (TERECO) is an unsupervised home-based 6-week exercise programme comprising breathing control and thoracic expansion, aerobic exercise, and lower limb muscle strength (LMS) exercise, delivered via smartphone, and remotely monitored with heart rate telemetry. Comparator: One-off short educational instruction at baseline | Primary outcome:
Secondary outcomes:
| Primary outcome: The mean 6MWD in the control group increased by 17.1 m (SD 63.9) from baseline to post-treatment assessment, whereas 6MWD in the TERECO group improved by 80.2 m (SD 74.7). The adjusted between-group difference in change in 6MWD from baseline (treatment effect) was 65.5 m (95% CI 43.8 to 87.1; p < 0.001). Secondary outcomes:
|
| Malik et al. [13] | Intervention: The intervention consisted of a 10-week mental training programme. The patients had one introductory session followed by nine individual therapy sessions (one per week) for 1.5 h and related homework, combining elements from CBT and music therapy. Of the nine therapy sessions, four were given by a music therapist and five were given by a cognitive therapist. Comparator: Care as usual—As reported by the authors “neither General Practitioners or paediatricians in Norway schedule appointment with postinfectious CF patients unless they have strongly reduced physical function. Thus, ‘care as usual’ implies that the relevant individuals would not receive any healthcare for their CF condition in the follow-up period apart from the follow-up visits in the present study.” | Physical activity:
Symptoms:
| There were no statistically significant differences between the two groups for any outcomes. Primary outcome: The mean number of steps per day decreased in the treatment group from 3 months post-baseline (7217) to 5680 at 15 months post-baseline. A decrease was also seen in the control group from 8515 at 3 months post-baseline to 7587 at 15 months. The difference between the two groups was not statistically significant: difference (95% CI) = −1298 (−4874 to 2278)). |
| Neumann et al. [91] | Intervention: The intervention group underwent resistance exercises with elastic bands (24 sessions over 12 weeks) supervised by a physical therapist. The exercise begins with a 5-min warm up on a stationary bike with no load, followed by resistance exercises for muscle groups that stabilize the shoulders, elbows, wrists, knees, and ankles. Comparator: Participants maintained their usual care of treatment and only received phone calls to monitor their symptoms. | Primary outcome:
Secondary outcomes:
| Primary outcomes: There was a significant improvement between the groups on the 30-s CST, with the resisted exercise group improving their performance compared to the control group (p = 0.04, d = 0.39) at 12 weeks follow-up. However, there was no significant improvement in the FPWT, 4SCPT, or DASH. Secondary outcomes:
|
| Silva-Filho et al. [93] | Intervention: Patients randomised to intervention arm were given transcranial direct current stimulation (tDCS) arm where they experienced a constant current of 2 mA for 20 min. Comparator: Sham-tDCS was performed on 5 consecutive days with electrodes placed on the same position, and a constant current of 2 mA was delivered only for 30 s (10-s ramp-up) of the 20 min. | Primary outcome:
| Primary outcome: There was a statistically significant improvement in VAS in the tDCS group. Secondary outcomes:
|
| de Oliveira et al. [92] | Intervention: The intervention group received 24 sessions of Pilates over a 12-week period. Patients had two sessions per week for 50 min per session, and of light-to-moderate intensity (increasing the number of repetitions, starting with 6 and increasing to 12 repetitions). The exercises involved coordination, strength, flexibility, and balance. Comparator: Usual follow-up at the Chikungunya outpatient clinic, with standard clinical care for the treatment of the disease. | Primary outcome:
Secondary outcomes:
| Regarding the primary and secondary outcomes, in the intragroup analysis, a significant improvement was observed in all parameters after 24 Pilates sessions (week 12) in relation to the baseline (week 0), but the same was not observed in the control group. The relative risk of an individual having been treated with Pilates and having decreased pain (measured by VAS) was 0.48 (95% CI = 0.28–0.82, p < 0.0001) with a number needed to treat two patients (95% CI 7–2, p < 0.0001). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandan, J.S.; Brown, K.R.; Simms-Williams, N.; Bashir, N.Z.; Camaradou, J.; Heining, D.; Turner, G.M.; Rivera, S.C.; Hotham, R.; Minhas, S.; et al. Non-Pharmacological Therapies for Post-Viral Syndromes, Including Long COVID: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 3477. https://doi.org/10.3390/ijerph20043477
Chandan JS, Brown KR, Simms-Williams N, Bashir NZ, Camaradou J, Heining D, Turner GM, Rivera SC, Hotham R, Minhas S, et al. Non-Pharmacological Therapies for Post-Viral Syndromes, Including Long COVID: A Systematic Review. International Journal of Environmental Research and Public Health. 2023; 20(4):3477. https://doi.org/10.3390/ijerph20043477
Chicago/Turabian StyleChandan, Joht Singh, Kirsty R. Brown, Nikita Simms-Williams, Nasir Z. Bashir, Jenny Camaradou, Dominic Heining, Grace M. Turner, Samantha Cruz Rivera, Richard Hotham, Sonica Minhas, and et al. 2023. "Non-Pharmacological Therapies for Post-Viral Syndromes, Including Long COVID: A Systematic Review" International Journal of Environmental Research and Public Health 20, no. 4: 3477. https://doi.org/10.3390/ijerph20043477
APA StyleChandan, J. S., Brown, K. R., Simms-Williams, N., Bashir, N. Z., Camaradou, J., Heining, D., Turner, G. M., Rivera, S. C., Hotham, R., Minhas, S., Nirantharakumar, K., Sivan, M., Khunti, K., Raindi, D., Marwaha, S., Hughes, S. E., McMullan, C., Marshall, T., Calvert, M. J., ... Aiyegbusi, O. L., on behalf of the TLC Study. (2023). Non-Pharmacological Therapies for Post-Viral Syndromes, Including Long COVID: A Systematic Review. International Journal of Environmental Research and Public Health, 20(4), 3477. https://doi.org/10.3390/ijerph20043477