Evaluation of External Trigeminal Nerve Stimulation to Prevent Cerebral Vasospasm after Subarachnoid Hemorrhage Due to Aneurysmal Rupture: A Randomized, Double-Blind Proof-of-Concept Pilot Trial (TRIVASOSTIM Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants: Inclusion and Non-Inclusion Criteria
- -
- Patient had to be aged between 18 and 75 years;
- -
- Patient was admitted within 48 h after the onset of SAH;
- -
- Patient had a ruptured cerebral aneurysm confirmed by computerized tomography angiography (CTA) or cerebral angiography;
- -
- Patient had no progressive psychosis or serious psychotic history requiring hospitalization;
- -
- Patient had no progressive cancerous pathology;
- -
- Patient was a candidate for management of his/her ruptured aneurysm, either by endovascular occlusion or microsurgical exclusion;
- -
- Patient had a grade between I and IV according to the classification of the World Federation of Neurosurgical Societies (WFNS);
- -
- Patient or a trusted person was able to understand and accept the constraints of the study (patient or trusted person, depending on the vigilance and cooperation of the patient);
- -
- Patient was affiliated with a health insurance plan;
- -
- Patient or a trusted person provided written consent to the study after receiving clear information.
- -
- Patient was receiving reinforced protection (i.e., minors, pregnant or breast-feeding women, persons deprived of their liberty by a legal or administrative authority, persons staying in a health or social institution, and adults under legal protection);
- -
- Patient had proven dementia or a neurological or psychiatric history that might affect their cognitive or motor skills;
- -
- Patient had a clinical severity of grade V according to the WFNS classification (very high risk of mortality);
- -
- Patient had an intracerebral or intraventricular hemorrhage, without a subarachnoid component;
- -
- Patient had SAH without evidence of an aneurysm;
- -
- Patient had an unruptured brain aneurysm;
- -
- Patient had a contraindication for the placement of a TENS device, including patients with an electronic pacemaker;
- -
- Patient had dermatological problems in the stimulation area that contraindicated the use of TENS patches.
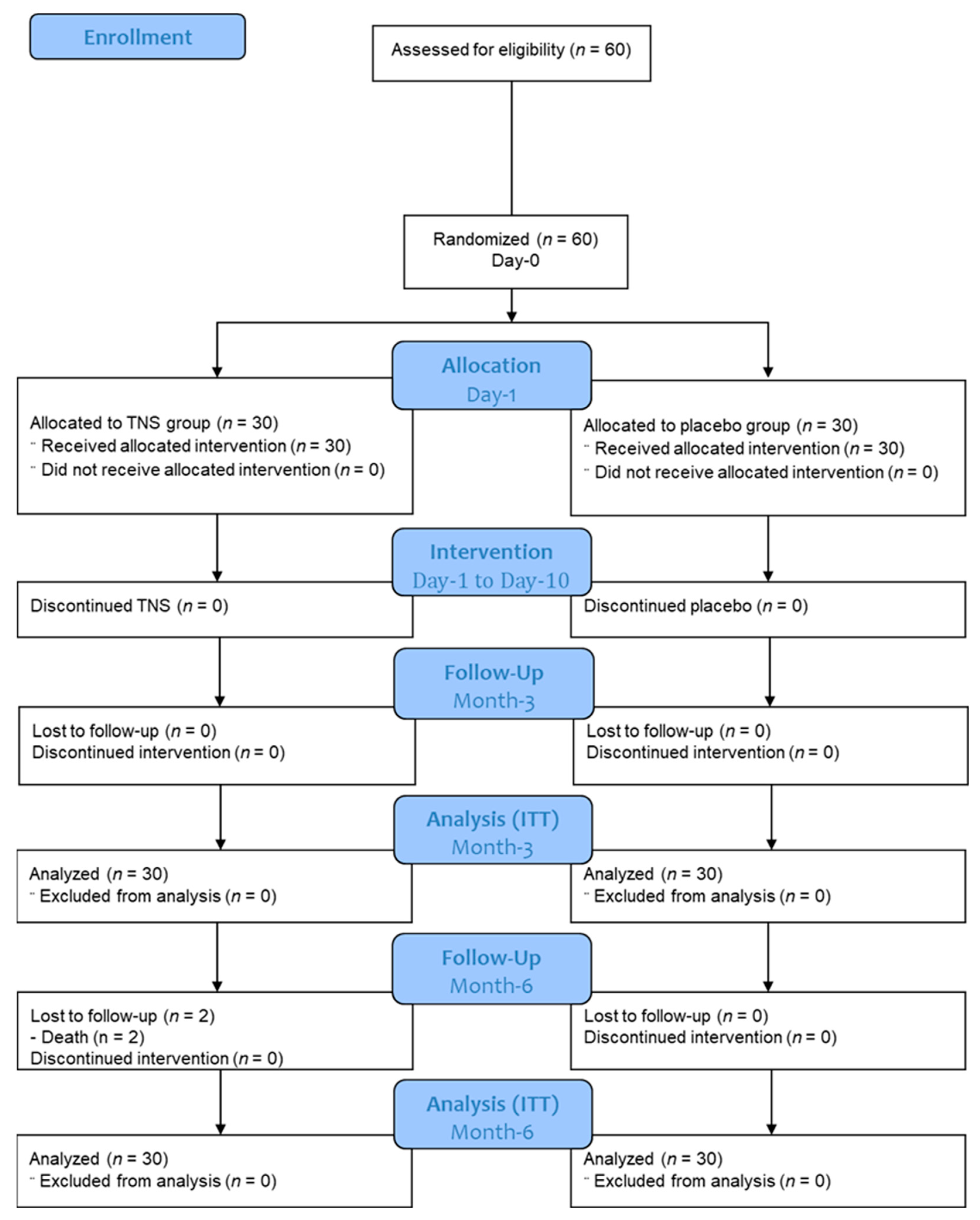
2.3. Randomization Procedure and Groups Description
- -
- For non-sedated patients, the stimulation intensity threshold was adjusted according to the sub-clinical threshold of the feeling of paresthesia for each patient. Before using any medical device in the study, for the activity which was not known by the clinician (active or sham), the clinician determined the maximum threshold of each patient before they felt paresthesia using a regular TENS eco 2 (schwa-medico, Rouffach, France) available at the center. This threshold allowed direct stimulation of the trigeminal nerve without causing paresthesia in the patients, thereby guaranteeing that both the patient and clinician would remain blinded. Sham therapy was delivered using a specific device that looks and works similarly by displaying the current intensity, but this device does not deliver any electrical stimulation. The determined maximum threshold was not significantly different between the TENS (9.6 ± 2.7 mA) and the sham groups (11.4 ± 1.5 mA, p = 0.16).
- -
- For sedated patients, the stimulation threshold was maintained at 20 mA, except for patients who were scheduled to stop sedation before the 10th day of the TENS/sham application. For these specific patients, lower stimulation thresholds were used in order to maintain double blinding after waking up the patient. The determined maximum threshold was not significantly different between the TENS (4.9 ± 2.9 mA) and sham groups (4.3 ± 2.2 mA, p = 0.49).
- -
- Aside from this specific external neurostimulation procedure, both the TNS and the sham groups received standardized and comparable aneurismal SAH treatment.
2.4. Study Protocol and Data Collection
2.5. Study Endpoints
2.6. Sample Size
2.7. Statistical Analysis
2.7.1. Description of the Population
2.7.2. Efficacy Analysis
2.7.3. Considerations for Missing Data
3. Results
3.1. Patient Characteristics
3.2. Primary and Secondary Endpoint Comparisons
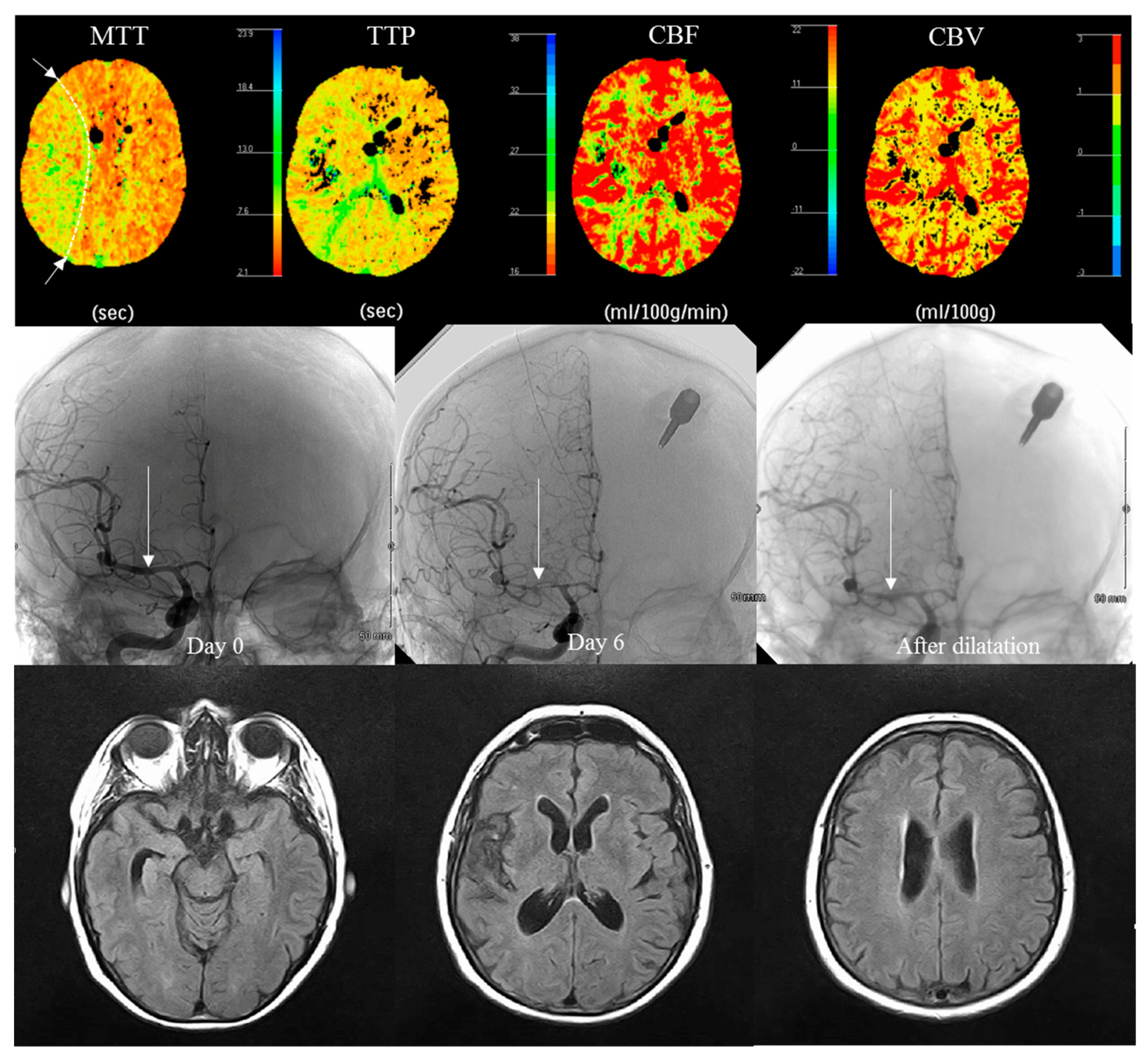
3.3. Radiological Evaluation at Day 6
3.4. Relationship between Vasospasm Occurrence and WFSN Grade and PCT Abnormality
3.5. Safety Analysis
4. Discussion
4.1. TNS Efficacy on Cerebral Vasospasm
4.2. Can Radiological Vasospasm Be Correlated to DCI Incidence?
4.3. Imaging Strategy Efficacy
4.4. External TNS Efficacy Remains Disappointing Compared to Previous Results, i.e., Cervical SCS
4.5. Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sudlow, C.L.; Warlow, C.P. Comparable Studies of the Incidence of Stroke and Its Pathological Types. Stroke 1997, 28, 491–499. [Google Scholar] [CrossRef]
- van Gijn, J.; Rinkel, G.J. Subarachnoid Haemorrhage: Diagnosis, Causes and Management. Brain 2001, 124, 249–278. [Google Scholar] [CrossRef]
- de Rooij, N.K.; Linn, F.H.H.; van der Plas, J.A.; Algra, A.; Rinkel, G.J.E. Incidence of Subarachnoid Haemorrhage: A Systematic Review with Emphasis on Region, Age, Gender and Time Trends. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Al-Khindi, T.; Macdonald, R.L.; Schweizer, T.A. Cognitive and Functional Outcome after Aneurysmal Subarachnoid Hemorrhage. Stroke 2010, 41, e519–e536. [Google Scholar] [CrossRef]
- le Roux, A.A.; Wallace, M.C. Outcome and Cost of Aneurysmal Subarachnoid Hemorrhage. Neurosurg. Clin. N. Am. 2010, 21, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.I.; Tarr, R.W.; Selman, W.R. Aneurysmal Subarachnoid Hemorrhage. N. Engl. J. Med. 2006, 354, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Biller, J.; Godersky, J.C.; Jr, H.P.A. Management of Aneurysmal Subarachnoid Hemorrhage. Stroke 1988, 19, 1300–1305. [Google Scholar] [CrossRef]
- Treggiari-Venzi, M.M.; Suter, P.M.; Romand, J.-A. Review of Medical Prevention of Vasospasm after Aneurysmal Subarachnoid Hemorrhage: A Problem of Neurointensive Care. Neurosurgery 2001, 48, 249–262. [Google Scholar] [CrossRef]
- Prevedello, D.M.-S.; Cordeiro, J.G.; de Morais, A.L.; Saucedo, N.S.; Chen, I.B.; Araújo, J.C. Magnesium Sulfate: Role as Possible Attenuating Factor in Vasospasm Morbidity. Surg. Neurol. 2006, 65, S14–S20. [Google Scholar] [CrossRef]
- Boulouis, G.; Labeyrie, M.A.; Raymond, J.; Rodriguez-Régent, C.; Lukaszewicz, A.C.; Bresson, D.; Ben Hassen, W.; Trystram, D.; Meder, J.F.; Oppenheim, C.; et al. Treatment of Cerebral Vasospasm Following Aneurysmal Subarachnoid Haemorrhage: A Systematic Review and Meta-Analysis. Eur. Radiol. 2017, 27, 3333–3342. [Google Scholar] [CrossRef]
- Hao, G.; Chu, G.; Pan, P.; Han, Y.; Ai, Y.; Shi, Z.; Liang, G. Clinical Effectiveness of Nimodipine for the Prevention of Poor Outcome after Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 13, 982498. [Google Scholar] [CrossRef]
- Geraldini, F.; De Cassai, A.; Diana, P.; Correale, C.; Boscolo, A.; Zampirollo, S.; Disarò, L.; Carere, A.; Cacco, N.; Navalesi, P.; et al. A Comparison between Enteral and Intravenous Nimodipine in Subarachnoid Hemorrhage: A Systematic Review and Network Meta-Analysis. Neurocritical Care 2022, 36, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Jennett, B.; Bond, M. Assessment of Outcome after Severe Brain Damage. Lancet 1975, 1, 480–484. [Google Scholar] [CrossRef]
- Etminan, N.; Vergouwen, M.D.I.; Ilodigwe, D.; Macdonald, R.L. Effect of Pharmaceutical Treatment on Vasospasm, Delayed Cerebral Ischemia, and Clinical Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis. J. Cereb. Blood Flow. Metab. 2011, 31, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Weyer, G.W.; Nolan, C.P.; Macdonald, R.L. Evidence-Based Cerebral Vasospasm Management. Neurosurg. Focus 2006, 21, E8. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.; Zipfel, G.; Milner, E.; Cross, D.T.; Moran, C.J.; Diringer, M.N.; Dacey, R.G.; Derdeyn, C.P. Safety and Technical Efficacy of Over-the-Wire Balloons for the Treatment of Subarachnoid Hemorrhage-Induced Cerebral Vasospasm. Neurosurg. Focus 2006, 21, E14. [Google Scholar] [CrossRef]
- Jun, P.; Ko, N.U.; English, J.D.; Dowd, C.F.; Halbach, V.V.; Higashida, R.T.; Lawton, M.T.; Hetts, S.W. Endovascular Treatment of Medically Refractory Cerebral Vasospasm Following Aneurysmal Subarachnoid Hemorrhage. AJNR Am. J. Neuroradiol. 2010, 31, 1911–1916. [Google Scholar] [CrossRef]
- Isono, M.; Kaga, A.; Fujiki, M.; Mori, T.; Hori, S. Effect of Spinal Cord Stimulation on Cerebral Blood Flow in Cats. Stereotact. Funct. Neurosurg. 1995, 64, 40–46. [Google Scholar] [CrossRef]
- Patel, S.; Huang, D.-L.; Sagher, O. Sympathetic Mechanisms in Cerebral Blood Flow Alterations Induced by Spinal Cord Stimulation. J. Neurosurg. 2003, 99, 754–761. [Google Scholar] [CrossRef]
- Deogaonkar, M.; Zibly, Z.; Slavin, K.V. Spinal Cord Stimulation for the Treatment of Vascular Pathology. Neurosurg. Clin. N. Am. 2014, 25, 25–31. [Google Scholar] [CrossRef]
- Risson, E.G.; Serpa, A.P.; Berger, J.J.; Koerbel, R.F.H.; Koerbel, A. Spinal Cord Stimulation in the Treatment of Complex Regional Pain Syndrome Type 1: Is Trial Truly Required? Clin. Neurol. Neurosurg. 2018, 171, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Horsch, S.; Schulte, S.; Hess, S. Spinal Cord Stimulation in the Treatment of Peripheral Vascular Disease: Results of a Single-Center Study of 258 Patients. Angiology 2004, 55, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Linderoth, B. Spinal Cord Stimulation in Ischemia and Ischemic Pain Possible Mechanisms of Action. In Spinal Cord Stimulation II; Horsch, S., Claeys, L., Eds.; Steinkopff: Heidelberg, Germany, 1995; pp. 19–35. [Google Scholar]
- Amann, W.; Berg, P.; Gersbach, P.; Gamain, J.; Raphael, J.H.; Ubbink, D.T.; European Peripheral Vascular Disease Outcome Study SCS-EPOS. Spinal Cord Stimulation in the Treatment of Non-Reconstructable Stable Critical Leg Ischaemia: Results of the European Peripheral Vascular Disease Outcome Study (SCS-EPOS). Eur. J. Vasc. Endovasc. Surg. 2003, 26, 280–286. [Google Scholar] [CrossRef]
- Waxenbaum, J.A.; Reddy, V.; Varacallo, M. Anatomy, Autonomic Nervous System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Johansson, S.E.; Abdolalizadeh, B.; Sheykhzade, M.; Edvinsson, L.; Sams, A. Vascular Pathology of Large Cerebral Arteries in Experimental Subarachnoid Hemorrhage: Vasoconstriction, Functional CGRP Depletion and Maintained CGRP Sensitivity. Eur. J. Pharmacol. 2019, 846, 109–118. [Google Scholar] [CrossRef] [PubMed]
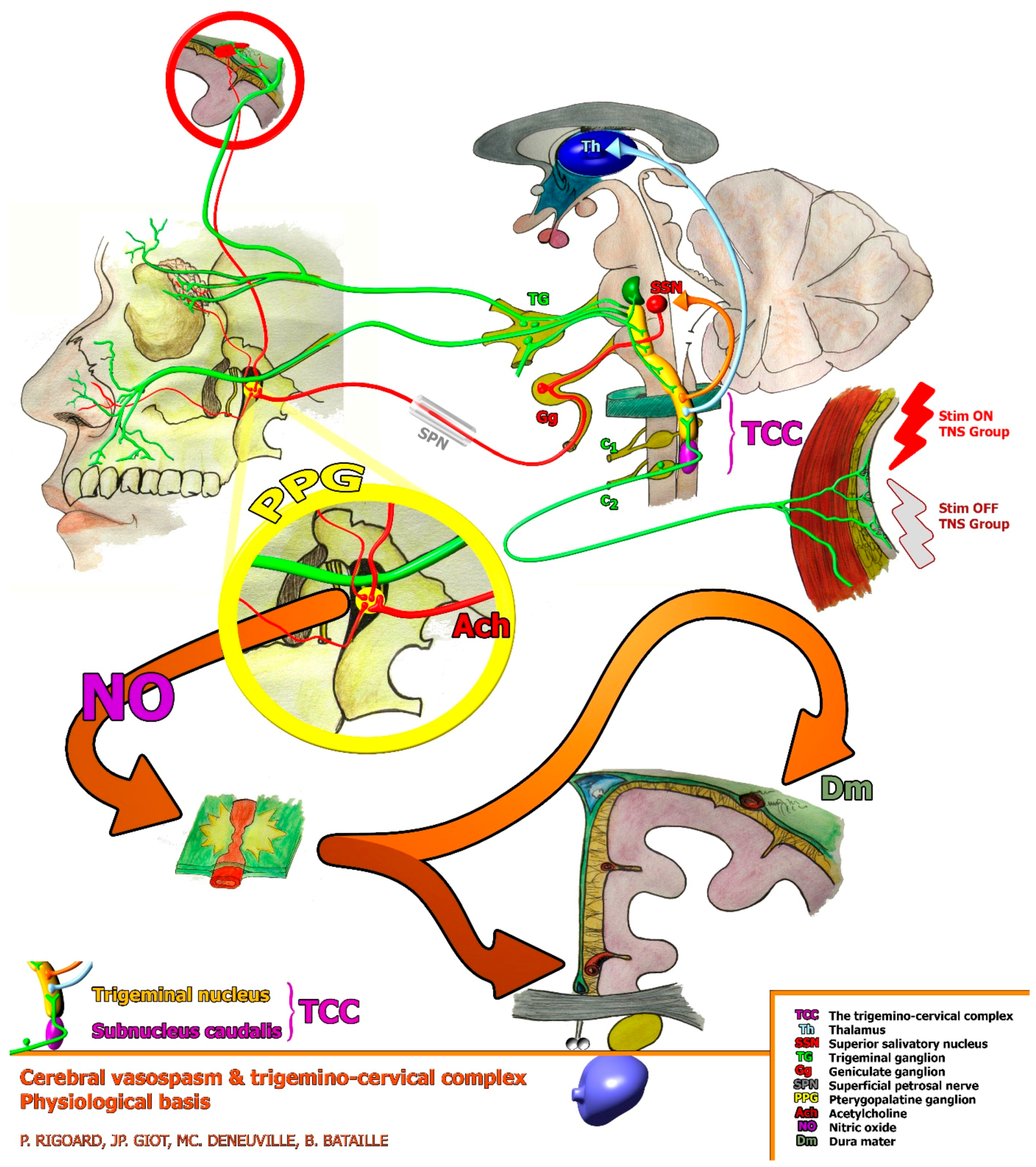
- Noseda, R.; Burstein, R. Migraine Pathophysiology: Anatomy of the Trigeminovascular Pathway and Associated Neurological Symptoms, Cortical Spreading Depression, Sensitization, and Modulation of Pain. Pain 2013, 154 (Suppl. 1), S44–S53. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Hoskin, K.L. The Distribution of Trigeminovascular Afferents in the Nonhuman Primate Brain Macaca Nemestrina: A c-Fos Immunocytochemical Study. J. Anat. 1997, 190 Pt 3, 367–375. [Google Scholar] [CrossRef]
- Kaube, H.; Keay, K.A.; Hoskin, K.L.; Bandler, R.; Goadsby, P.J. Expression of C-Fos-like Immunoreactivity in the Caudal Medulla and Upper Cervical Spinal Cord Following Stimulation of the Superior Sagittal Sinus in the Cat. Brain Res. 1993, 629, 95–102. [Google Scholar] [CrossRef]
- Hoskin, K.L.; Bulmer, D.C.; Goadsby, P.J. Fos Expression in the Trigeminocervical Complex of the Cat after Stimulation of the Superior Sagittal Sinus Is Reduced by L-NAME. Neurosci. Lett. 1999, 266, 173–176. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Release of Vasoactive Peptides in the Extracerebral Circulation of Humans and the Cat during Activation of the Trigeminovascular System. Ann. Neurol. 1988, 23, 193–196. [Google Scholar] [CrossRef]
- Uddman, R.; Edvinsson, L.; Ekman, R.; Kingman, T.; McCulloch, J. Innervation of the Feline Cerebral Vasculature by Nerve Fibers Containing Calcitonin Gene-Related Peptide: Trigeminal Origin and Co-Existence with Substance P. Neurosci. Lett. 1985, 62, 131–136. [Google Scholar] [CrossRef]
- Edvinsson, L.; Delgado-Zygmunt, T.; Ekman, R.; Jansen, I.; Svendgaard, N.A.; Uddman, R. Involvement of Perivascular Sensory Fibers in the Pathophysiology of Cerebral Vasospasm Following Subarachnoid Hemorrhage. J. Cereb. Blood Flow. Metab. 1990, 10, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.A.; White, T.G.; Powell, K.; Woo, H.H.; Narayan, R.K.; Li, C. Trigeminal Nerve Stimulation Improves Cerebral Macrocirculation and Microcirculation after Subarachnoid Hemorrhage: An Exploratory Study. Neurosurgery 2022, 90, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.E.; Tomlinson, B.; Lau, M.S.; So, K.W.; Cheung, A.H.; Critchley, J.A.; Woo, K.S. The Effect of Transcutaneous Electrical Nerve Stimulation (TENS) on Autonomic Cardiovascular Reflexes. Clin. Auton. Res. 1995, 5, 81–84. [Google Scholar] [CrossRef]
- Schlaeppi, J.-A.; Affentranger, L.; Bervini, D.; Z’Graggen, W.J.; Raabe, A.; Pollo, C. Electrical Stimulation for Cerebral Vasospasm after Subarachnoid Hemorrhage: A Systematic Review. Neuromodulation 2022, 25, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Peppucci, E.; Di Bonaventura, R.; Esposito, V.; Zhong, J.; Iacopino, G.; Barbagallo, G.; Visocchi, M. Update on Mechanism and Therapeutic Implications of Spinal Cord Stimulation and Cerebral Hemodynamics: A Narrative Review. Acta Neurochir. Suppl. 2017, 124, 27–36. [Google Scholar] [CrossRef]
- Yin, D.; Slavin, K.V. A Hypothesis on Possible Neurochemical Mechanisms of Action of Cervical Spinal Cord Stimulation in Prevention and Treatment of Cerebral Arterial Vasospasm after Aneurysmal Subarachnoid Hemorrhage. Med. Hypotheses 2015, 85, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.; White, T.G.; Nash, C.; Rebeiz, T.; Woo, H.H.; Narayan, R.K.; Li, C. The Potential Role of Neuromodulation in Subarachnoid Hemorrhage. Neuromodulation 2022, 25, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, M.; Schuhmann, M.U.; Soehle, M.; Nagel, C.; Meixensberger, J. Continuous Monitoring of Cerebrovascular Autoregulation after Subarachnoid Hemorrhage by Brain Tissue Oxygen Pressure Reactivity and Its Relation to Delayed Cerebral Infarction. Stroke 2007, 38, 981–986. [Google Scholar] [CrossRef]
- Li, C.; White, T.G.; Shah, K.A.; Chaung, W.; Powell, K.; Wang, P.; Woo, H.H.; Narayan, R.K. Percutaneous Trigeminal Nerve Stimulation Induces Cerebral Vasodilation in a Dose-Dependent Manner. Neurosurgery 2021, 88, E529–E536. [Google Scholar] [CrossRef]
- ter Laan, M.; van Dijk, J.M.C.; Stewart, R.; Staal, M.J.; Elting, J.-W.J. Modulation of Cerebral Blood Flow with Transcutaneous Electrical Neurostimulation (TENS) in Patients with Cerebral Vasospasm after Subarachnoid Hemorrhage. Neuromodulation 2014, 17, 431–436. [Google Scholar] [CrossRef]
- Westermaier, T.; Stetter, C.; Vince, G.H.; Pham, M.; Tejon, J.P.; Eriskat, J.; Kunze, E.; Matthies, C.; Ernestus, R.-I.; Solymosi, L.; et al. Prophylactic Intravenous Magnesium Sulfate for Treatment of Aneurysmal Subarachnoid Hemorrhage: A Randomized, Placebo-Controlled, Clinical Study. Crit. Care Med. 2010, 38, 1284–1290. [Google Scholar] [CrossRef]
- Doerksen, K.; Naimark, B.J.; Tate, R.B. Comparison of a Standard Neurological Tool with a Stroke Scale for Detecting Symptomatic Cerebral Vasospasm. J. Neurosci. Nurs. 2002, 34, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.R.; Ko, N.; Dillon, W.P.; Yu, M.B.; Liu, S.; Criqui, G.I.; Higashida, R.T.; Smith, W.S.; Wintermark, M. Prospective Evaluation of Multidetector-Row CT Angiography for the Diagnosis of Vasospasm Following Subarachnoid Hemorrhage: A Comparison with Digital Subtraction Angiography. Cerebrovasc. Dis 2008, 25, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.Y.; Choi, C.S.; Kim, K.H.; Cho, B.-M. Multidetector-Row CT Angiography of Cerebral Vasospasm after Aneurysmal Subarachnoid Hemorrhage: Comparison of Volume-Rendered Images and Digital Subtraction Angiography. AJNR Am. J. Neuroradiol. 2006, 27, 370–377. [Google Scholar] [PubMed]
- Binaghi, S.; Colleoni, M.L.; Maeder, P.; Uské, A.; Regli, L.; Dehdashti, A.R.; Schnyder, P.; Meuli, R. CT Angiography and Perfusion CT in Cerebral Vasospasm after Subarachnoid Hemorrhage. AJNR Am. J. Neuroradiol. 2007, 28, 750–758. [Google Scholar]
- Wintermark, M.; Ko, N.U.; Smith, W.S.; Liu, S.; Higashida, R.T.; Dillon, W.P. Vasospasm after Subarachnoid Hemorrhage: Utility of Perfusion CT and CT Angiography on Diagnosis and Management. AJNR Am. J. Neuroradiol. 2006, 27, 26–34. [Google Scholar] [PubMed]
- Nabavi, D.G.; LeBlanc, L.M.; Baxter, B.; Lee, D.H.; Fox, A.J.; Lownie, S.P.; Ferguson, G.G.; Craen, R.A.; Gelb, A.W.; Lee, T.Y. Monitoring Cerebral Perfusion after Subarachnoid Hemorrhage Using CT. Neuroradiology 2001, 43, 7–16. [Google Scholar] [CrossRef]
- Aralasmak, A.; Akyuz, M.; Ozkaynak, C.; Sindel, T.; Tuncer, R. CT Angiography and Perfusion Imaging in Patients with Subarachnoid Hemorrhage: Correlation of Vasospasm to Perfusion Abnormality. Neuroradiology 2009, 51, 85–93. [Google Scholar] [CrossRef]
- Pham, M.; Johnson, A.; Bartsch, A.J.; Lindner, C.; Müllges, W.; Roosen, K.; Solymosi, L.; Bendszus, M. CT Perfusion Predicts Secondary Cerebral Infarction after Aneurysmal Subarachnoid Hemorrhage. Neurology 2007, 69, 762–765. [Google Scholar] [CrossRef]
- Washington, C.W.; Zipfel, G.J.; Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Detection and Monitoring of Vasospasm and Delayed Cerebral Ischemia: A Review and Assessment of the Literature. Neurocritical Care 2011, 15, 312–317. [Google Scholar] [CrossRef]
- Suarez, J.I.; Qureshi, A.I.; Yahia, A.B.; Parekh, P.D.; Tamargo, R.J.; Williams, M.A.; Ulatowski, J.A.; Hanley, D.F.; Razumovsky, A.Y. Symptomatic Vasospasm Diagnosis after Subarachnoid Hemorrhage: Evaluation of Transcranial Doppler Ultrasound and Cerebral Angiography as Related to Compromised Vascular Distribution. Crit. Care Med. 2002, 30, 1348–1355. [Google Scholar] [CrossRef]
- Majewska, P.; Hara, S.; Gulati, S.; Solheim, O. Association between Transcranial Doppler Vasospasm and Functional Outcome after Subarachnoid Hemorrhage. Brain Circ. 2021, 7, 271–276. [Google Scholar] [CrossRef]
- Lysakowski, C.; Walder, B.; Costanza, M.C.; Tramèr, M.R. Transcranial Doppler versus Angiography in Patients with Vasospasm Due to a Ruptured Cerebral Aneurysm: A Systematic Review. Stroke 2001, 32, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Konstas, A.A.; Goldmakher, G.V.; Lee, T.-Y.; Lev, M.H. Theoretic Basis and Technical Implementations of CT Perfusion in Acute Ischemic Stroke, Part 2: Technical Implementations. AJNR Am. J. Neuroradiol. 2009, 30, 885–892. [Google Scholar] [CrossRef]
- Velat, G.J.; Kimball, M.M.; Mocco, J.D.; Hoh, B.L. Vasospasm after Aneurysmal Subarachnoid Hemorrhage: Review of Randomized Controlled Trials and Meta-Analyses in the Literature. World Neurosurg. 2011, 76, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Rabinstein, A.A.; Friedman, J.A.; Weigand, S.D.; McClelland, R.L.; Fulgham, J.R.; Manno, E.M.; Atkinson, J.L.D.; Wijdicks, E.F.M. Predictors of Cerebral Infarction in Aneurysmal Subarachnoid Hemorrhage. Stroke 2004, 35, 1862–1866. [Google Scholar] [CrossRef]
- Ohkuma, H.; Manabe, H.; Tanaka, M.; Suzuki, S. Impact of Cerebral Microcirculatory Changes on Cerebral Blood Flow during Cerebral Vasospasm after Aneurysmal Subarachnoid Hemorrhage. Stroke 2000, 31, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.J.P.; Raju, P.P.J.; Gholkar, A. Does the Method of Treatment of Acutely Ruptured Intracranial Aneurysms Influence the Incidence and Duration of Cerebral Vasospasm and Clinical Outcome? J. Neurol. Neurosurg. Psychiatry 2004, 75, 868–872. [Google Scholar] [CrossRef]
- Goudman, L.; Brouns, R.; Linderoth, B.; Moens, M. Effects of Spinal Cord Stimulation on Heart Rate Variability in Patients with Failed Back Surgery Syndrome. PLoS ONE 2019, 14, e0219076. [Google Scholar] [CrossRef]
- Goudman, L.; De Smedt, A.; Louis, F.; Stalmans, V.; Linderoth, B.; Rigoard, P.; Moens, M. The Link between Spinal Cord Stimulation and the Parasympathetic Nervous System in Patients with Failed Back Surgery Syndrome. Neuromodulation 2022, 25, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Barchini, J.; Tchachaghian, S.; Shamaa, F.; Jabbur, S.J.; Meyerson, B.A.; Song, Z.; Linderoth, B.; Saadé, N.E. Spinal Segmental and Supraspinal Mechanisms Underlying the Pain-Relieving Effects of Spinal Cord Stimulation: An Experimental Study in a Rat Model of Neuropathy. Neuroscience 2012, 215, 196–208. [Google Scholar] [CrossRef]
- Cui, J.G.; O’Connor, W.T.; Ungerstedt, U.; Linderoth, B.; Meyerson, B.A. Spinal Cord Stimulation Attenuates Augmented Dorsal Horn Release of Excitatory Amino Acids in Mononeuropathy via a GABAergic Mechanism. Pain 1997, 73, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Schechtmann, G.; Song, Z.; Ultenius, C.; Meyerson, B.A.; Linderoth, B. Cholinergic Mechanisms Involved in the Pain Relieving Effect of Spinal Cord Stimulation in a Model of Neuropathy. Pain 2008, 139, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Linderoth, B.; Foreman, R.D. Conventional and Novel Spinal Stimulation Algorithms: Hypothetical Mechanisms of Action and Comments on Outcomes. Neuromodulation 2017, 20, 525–533. [Google Scholar] [CrossRef] [PubMed]
| Variables | TNS n = 30 | Sham n = 30 | p-Value |
|---|---|---|---|
| Mean ± SD (min–max) | Mean ± SD (min–max) | ||
| Age (years) | 55.3 ± 9.3 (36–73) | 59.6 ± 11.5 (27–79) | 0.12 # |
| Height (m) | 165.4 ± 9.5 (145–180) | 168.8 ± 7.7 (153–185) | 0.14 # |
| Weight (kg) | 72.0 ± 19.8 (45–130) | 68.8 ± 11.8 (46–92) | 0.45 # |
| Body mass index (kg/m2) | 26.1 ± 5.9 (18.4–45.0) | 24.2 ± 4.0 (18.1–36.9) | 0.15 # |
| Median (min–max) | Median (min–max) | ||
| Admission time (h) | 6 (1–32) | 7 (1–25) | 0.36 £ |
| n (%) | n (%) | ||
| Sex | 0.18 $ | ||
| Female | 22 (73) | 17 (57) | |
| Male | 8 (27) | 13 (43) | |
| Background | |||
| Alcohol abuse | 5 (17) | 4 (13) | 0.99 & |
| Smoking | 18 (60) | 13 (43) | 0.20 $ |
| High blood pressure | 8 (27) | 11 (37) | 0.41 $ |
| WFNS grade at admission | 0.36 & | ||
| I | 14 (47) | 16 (54) | |
| II | 7 (23) | 9 (30) | |
| III | 0 | 1 (3) | |
| IV | 9 (30) | 4 (13) |
| Variables | TNS n = 30 | Sham n = 30 | p-Value |
|---|---|---|---|
| Number (%) | Number (%) | ||
| Subarachnoid compartment | 0.72 & | ||
| Fisher Grade 1 | 1 (3) | 0 | |
| Fisher Grade 2 | 4 (13) | 2 (7) | |
| Fisher Grade 3 | 6 (20) | 6 (20) | |
| Fisher Grade 4 | 19 (63) | 22 (73) | |
| Intraventricular compartment | 0.23 & | ||
| No visible blood | 15 (50) | 9 (30) | |
| Posterior sedimentation | 7 (23) | 14 (47) | |
| Complete filling | 2 (7) | 1 (3) | |
| Partial filling | 6 (20) | 6 (20) | |
| Intra-parenchymal hematoma | 4 (13) | 3 (10) | 0.99 & |
| Lobar topography | 4 (13) | 3 (10) | 0.99 & |
| Deep topography | 3 (10) | 0 | 0.24 & |
| Hydrocephalus | 8 (27) | 9 (30) | 0.99 & |
| Topography | 0.85 & | ||
| Horizontal portion of the ACA | 2 (7) | 1 (3) | |
| ACA | 7 (23) | 6 (20) | |
| PCA | 2 (7) | 2 (7) | |
| Distal portion ACA | 2 (7) | 3 (10) | |
| Horizontal segment (M1) MCA | 1 (3) | 0 | |
| Major bifurcation MCA | 4 (13) | 11 (37) | |
| Distal segment (M2) MCA | 0 | 1 (3) | |
| Carotido-ophthalmic ICA | 1 (3) | 1 (3) | |
| Posterior communicating ICA | 6 (20) | 3 (10) | |
| Anterior choroidal | 1 (3) | 0 | |
| ICA termination | 2 (7) | 2 (7) | |
| Basilar artery termination | 1 (3) | 1 (3) | |
| Basilar artery trunk | 1 (3) | 0 | |
| Posterior ICA | 0 | 1 (3) | |
| Multiple aneurysms | 4 (13) | 4 (13) | |
| Laterality | 0.65 $ | ||
| Right | 12 (40) | 15 (50) | |
| Left | 10 (33) | 7 (23) | |
| Not applicable | 8 (27) | 8 (27) |
| Variables | TNS n = 30 | Sham n = 30 | p-Value |
|---|---|---|---|
| Count (%) | Count (%) | ||
| Immediate preoperative WFNS grade | 0.95 & | ||
| I | 15 (50) | 15 (50) | |
| II | 6 (20) | 6 (20) | |
| III | 0 | 1 (3) | |
| IV | 8 (27) | 6 (20) | |
| V | 1 (3) | 2 (7) | |
| Treatment of the aneurysm | 0.99 & | ||
| Surgical | 2 (7) | 3 (10) | |
| Endovascular | 28 (93) | 27 (90) | |
| Including complications | 4 (14) | 3 (11) | |
| Result of the treatment | 0.76 & | ||
| Complete obliteration | 24 (80) | 22 (73) | |
| Incomplete obliteration | 6 (20) | 8 (27) |
| Variables | TNS n = 30 | Sham n = 30 | p-Value |
|---|---|---|---|
| Count (%) | Count (%) | ||
| Primary endpoint at 3-month follow-up | |||
| Infarction present | 7 (23) | 8 (27) | 0.99 & |
| Early infarction, persistent | 4/7 (57) | 2/8 (25) | |
| Late infarction, not seen at D6 | 3/7 (43) | 6/8 (75) | 0.47 & |
| Cortical/Deep/Mixed | 2/4/1 | 2/5/1 | |
| Unilateral/bilateral | 6/1 | 7/1 | |
| Carrier artery/other | 2/5 | 2/6 | |
| Adjacent to the aneurysm | 5 | 5 | |
| Rankin Scale (MRS) at 6-month follow-up | 0.65 & | ||
| 0 | 14 (47) | 14 (47) | |
| 1 | 10 (33) | 9 (30) | |
| 2 | 3 (10) | 6 (20) | |
| 3 | 1 (3) | 1 (3) | |
| 4 | 0 | 0 | |
| 5 | 0 | 0 | |
| 6 (deceased patients) | 2 (7) | 0 | |
| GOS at 6-month follow-up | 0.53 & | ||
| I: recovery | 23 (82) | 22 (73) | |
| II: moderate disability | 5 (18) | 8 (27) | |
| III: severe disability | 0 | 0 | |
| EQ5D-3L at 6-month follow-up | Mean ± SD [min–max] (n) | Mean ± SD [min–max] (n) | |
| EQ5D Index | 0.79 ± 0.23 [0.25–1.00] (n = 28) | 0.79 ± 0.22 [0.27–1.00] (n = 30) | 0.99 £ |
| EQ5D Visual Analog Scale | 82 ± 12 [50–100] (n = 28) | 86 ± 11 [65–100] (n = 30) | 0.26 £ |
| Variables | TNS n = 30 | Sham n = 30 | p-Value | |
|---|---|---|---|---|
| Count (%) | Count (%) | |||
| CT scan of the head | ||||
| Infarct-related hypodensity | 3 (10) | 4 (13) | 0.99 & | |
| Type of infarction | Cortical | 1 | 1 | |
| Deep structures | 2 | 3 | ||
| Vascular territory | ACA | 1 | 0 | |
| MCA | 2 | 4 | ||
| Uni/bilateral | 3/0 | 4/0 | ||
| Carrier artery/other arteries | 0/3 | 4/0 | ||
| Intraparenchymal hematoma | 6 (20) | 4 (13) | 0.73 & | |
| Lobar | 6 | 4 | ||
| Deep | 2 | 0 | ||
| Hydrocephalus | 8 (27) | 8 (27) | 0.99 & | |
| Cerebrospinal fluid drainage | 8 | 8 | ||
| External ventricular drain | 7 | 8 | ||
| Perfusion scanner (PCT) | ||||
| Perfusion abnormality | 12 (40) | 5 (17) | 0.084 & | |
| Anterior cerebral | 1 | 0 | ||
| Sylvian | 9 | 5 | ||
| Junctional | 2 | 0 | ||
| Angiography (CTA) | ||||
| Vasospasm: yes | 14 (47) | 10 (33) | 0.43 & | |
| Arterial diameter reduction Mild: 0 to 25% | 1 | 2 | ||
| Moderate: 26 to 50% | 6 | 7 | ||
| Severe: 51 to 100% | 7 | 1 | ||
| Distal/proximal | 2/12 | 4/6 | ||
| Uni/bilateral | 8/6 | 8/2 | ||
| Symptomatic | 7 (50%) | 2 (20%) | ||
| Mean ± SD (min–max) (n) | Mean ± SD (min–max) (n) | |||
| Perfusion scanner (PCT) | ||||
| ATT (s) | 8.9 ± 14.6 (2–70) (n = 28) | 5.9 ± 7.8 (2–45) (n = 28) | 0.72 £ | |
| ATT ratio: pathological/healthy | 1.39 ± 1.41 (0.25–8.0) (n = 28) | 1.23 ± 0.68 (0.10–3.0) (n = 27) | 0.40 £ | |
| Blood volume CBV (mL/100 g) | 4.0 ± 6.2 (1–35) (n = 28) | 5.5 ± 7.7 (1–30) (n = 28) | 0.33 £ | |
| Blood flow (mL/100 g/min) | 38.0 ± 17.5 (4.5–70) (n = 28) | 37.3 ± 12.5 (15–60) (n = 27) | 0.80 £ | |
| p < 0.0001 & | No/Mild Vasospasm (n = 39) | Moderate Vasospasm (n = 13) | Severe Vasospasm (n = 8) |
|---|---|---|---|
| Perfusion abnormality: None | 37 (94.9%) | 6 (46.2%) | 0 (0%) |
| Perfusion abnormality: Yes | 2 (5.1%) | 7 (53.8%) | 8 (100%) |
| p = 0.016 & | No Vasospasm (n = 36) | With Vasospasm (n = 24) |
|---|---|---|
| WFNS grade | ||
| Grade 1 | 22 (61.1%) | 8 (33.3%) |
| Grade 2 | 6 (16.7%) | 6 (25.0%) |
| Grade 3 | 1 (2.8%) | 0 (0%) |
| Grade 4 | 4 (11.1%) | 10 (41.7%) |
| Grade 5 | 3 (8.3%) | 0 (0%) |
| Variables | TNS n = 30 | Sham n = 30 | p-Value |
|---|---|---|---|
| Count (%) | Count (%) | ||
| Secondary complications | 11 (37) | 10 (33) | 0.99 & |
| Procedural ischemia | 0 | 1 | |
| Non-procedural ischemia | 2 | 1 | |
| Re-bleeding | 1 | 1 | |
| Hydrocephalus | 10 | 9 | |
| Cerebrospinal fluid Drainage | 9 | 9 | |
| External ventricular drains | 9 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigoard, P.; Billot, M.; Moens, M.; Goudman, L.; El-Hajj, H.; Ingrand, P.; Ounajim, A.; Roulaud, M.; Page, P.; Babin, E.; et al. Evaluation of External Trigeminal Nerve Stimulation to Prevent Cerebral Vasospasm after Subarachnoid Hemorrhage Due to Aneurysmal Rupture: A Randomized, Double-Blind Proof-of-Concept Pilot Trial (TRIVASOSTIM Study). Int. J. Environ. Res. Public Health 2023, 20, 5836. https://doi.org/10.3390/ijerph20105836
Rigoard P, Billot M, Moens M, Goudman L, El-Hajj H, Ingrand P, Ounajim A, Roulaud M, Page P, Babin E, et al. Evaluation of External Trigeminal Nerve Stimulation to Prevent Cerebral Vasospasm after Subarachnoid Hemorrhage Due to Aneurysmal Rupture: A Randomized, Double-Blind Proof-of-Concept Pilot Trial (TRIVASOSTIM Study). International Journal of Environmental Research and Public Health. 2023; 20(10):5836. https://doi.org/10.3390/ijerph20105836
Chicago/Turabian StyleRigoard, Philippe, Maxime Billot, Maarten Moens, Lisa Goudman, Hassan El-Hajj, Pierre Ingrand, Amine Ounajim, Manuel Roulaud, Philippe Page, Etienne Babin, and et al. 2023. "Evaluation of External Trigeminal Nerve Stimulation to Prevent Cerebral Vasospasm after Subarachnoid Hemorrhage Due to Aneurysmal Rupture: A Randomized, Double-Blind Proof-of-Concept Pilot Trial (TRIVASOSTIM Study)" International Journal of Environmental Research and Public Health 20, no. 10: 5836. https://doi.org/10.3390/ijerph20105836
APA StyleRigoard, P., Billot, M., Moens, M., Goudman, L., El-Hajj, H., Ingrand, P., Ounajim, A., Roulaud, M., Page, P., Babin, E., Et Talby, M., Dany, J., Johnson, S., Bataille, B., David, R., & Slavin, K. V. (2023). Evaluation of External Trigeminal Nerve Stimulation to Prevent Cerebral Vasospasm after Subarachnoid Hemorrhage Due to Aneurysmal Rupture: A Randomized, Double-Blind Proof-of-Concept Pilot Trial (TRIVASOSTIM Study). International Journal of Environmental Research and Public Health, 20(10), 5836. https://doi.org/10.3390/ijerph20105836









