Convolutional Neural Network-Based ECG-Assisted Diagnosis for Coal Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Data Collection
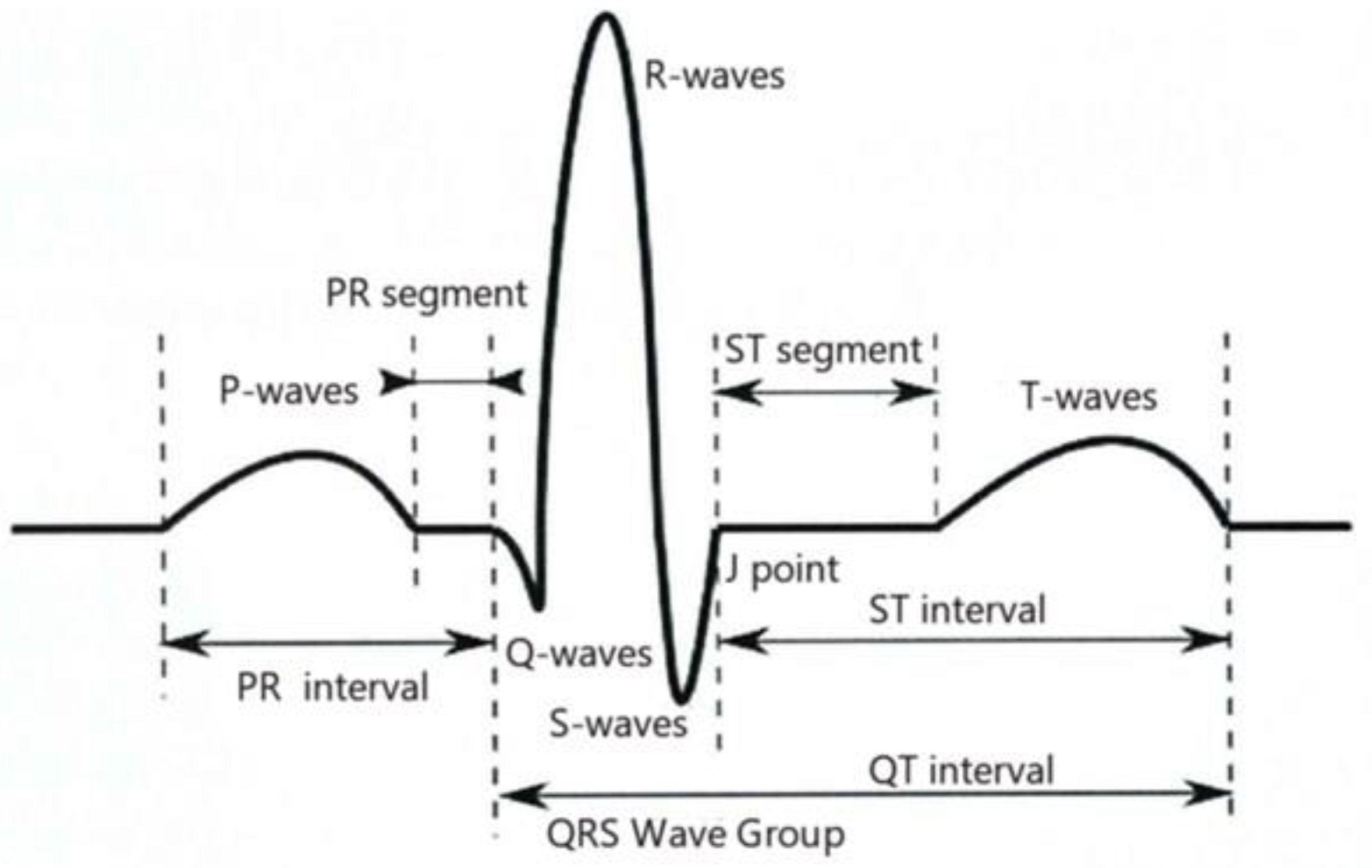
2.3. ECG Signal Pre-Processing
2.3.1. Heartbeat Cut Score
2.3.2. Whitening Treatment
- (1)
- Zero-meaning
- (2)
- Whitening transformation
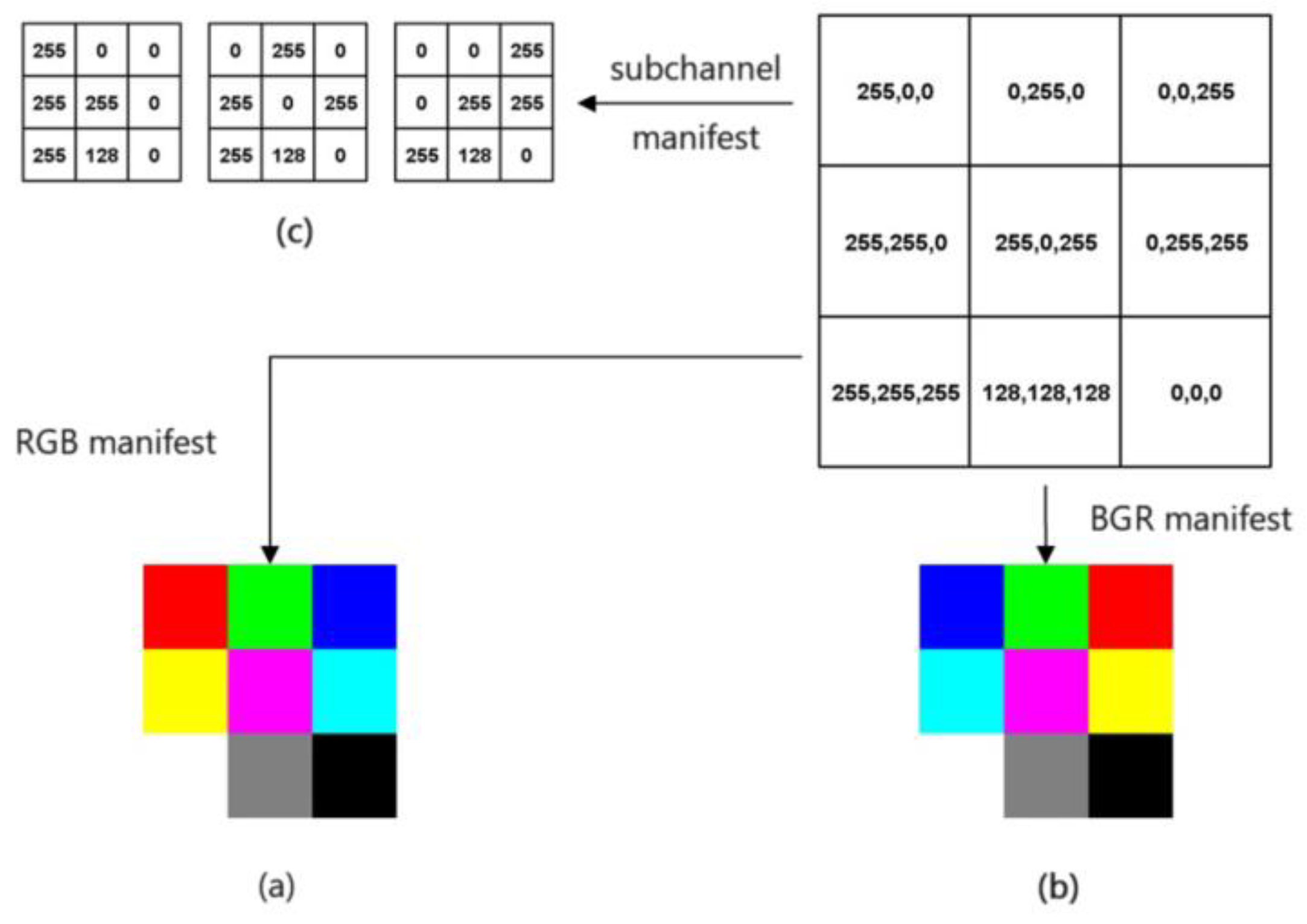
2.3.3. Image Downsampling
- —Image pixel average,
- —Original image size,
- —Downsampling multiplier.
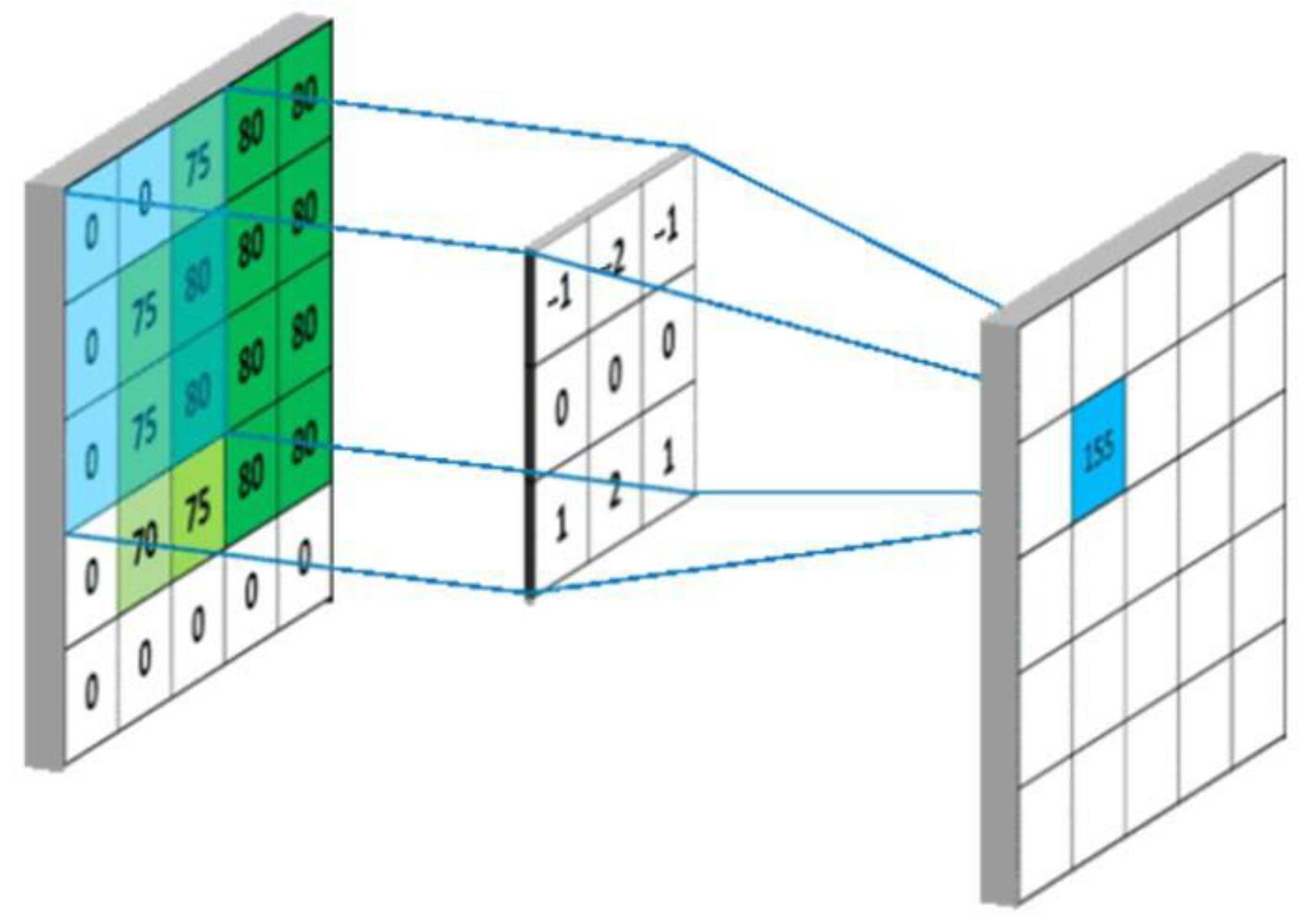
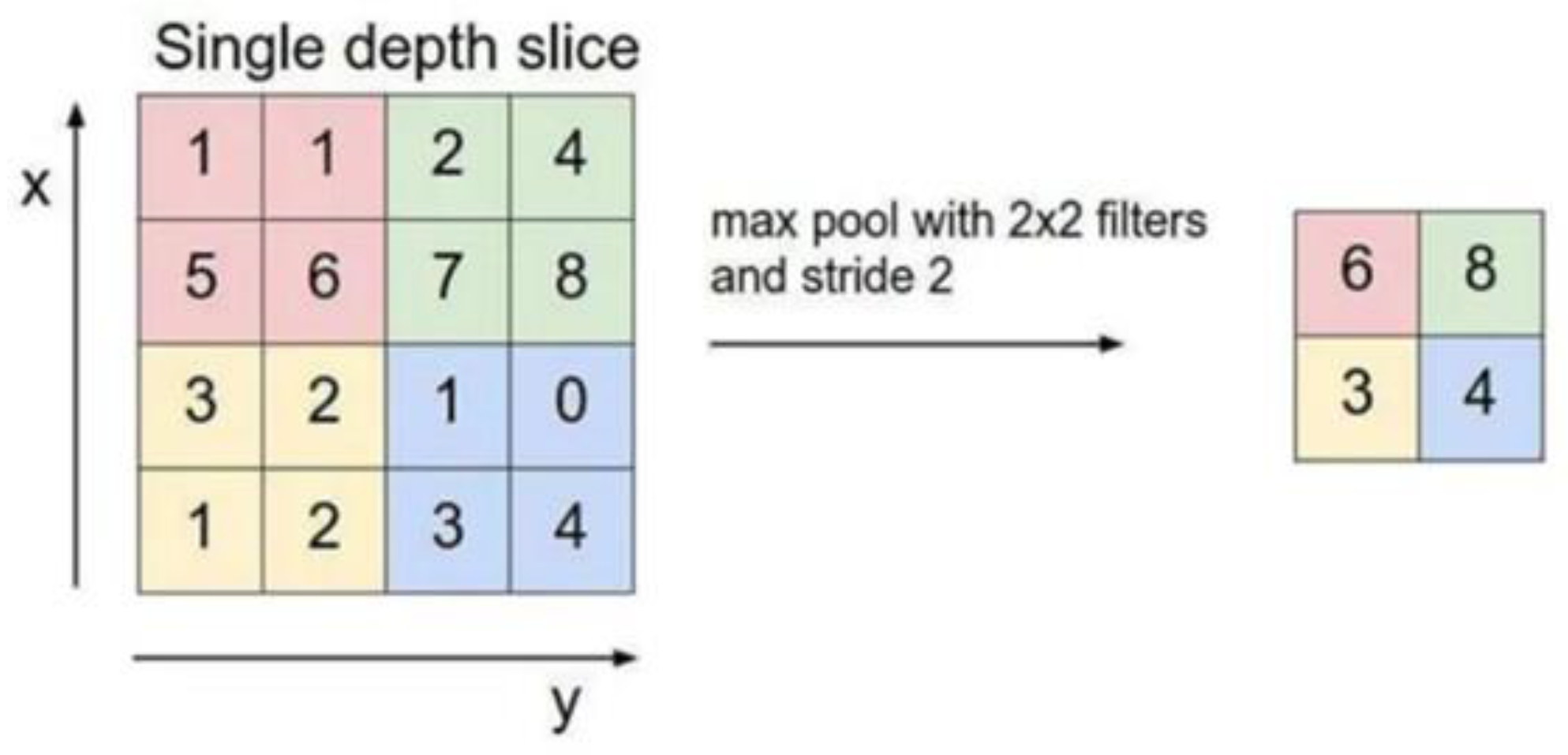
2.4. Building the Mod
- —Output image edge length,
- —Input image edge length,
- —Convolution kernel length,
- —Convolution kernel sliding step.
2.5. Statistical Methods
3. Results
3.1. Basic Information
3.2. Detection of ECG Abnormalities
- D: the depth of the network.
- l: the lth convolution layer of the neural network.
- Cl: number of convolutional kernels in this layer.
3.3. ECG Image Processing
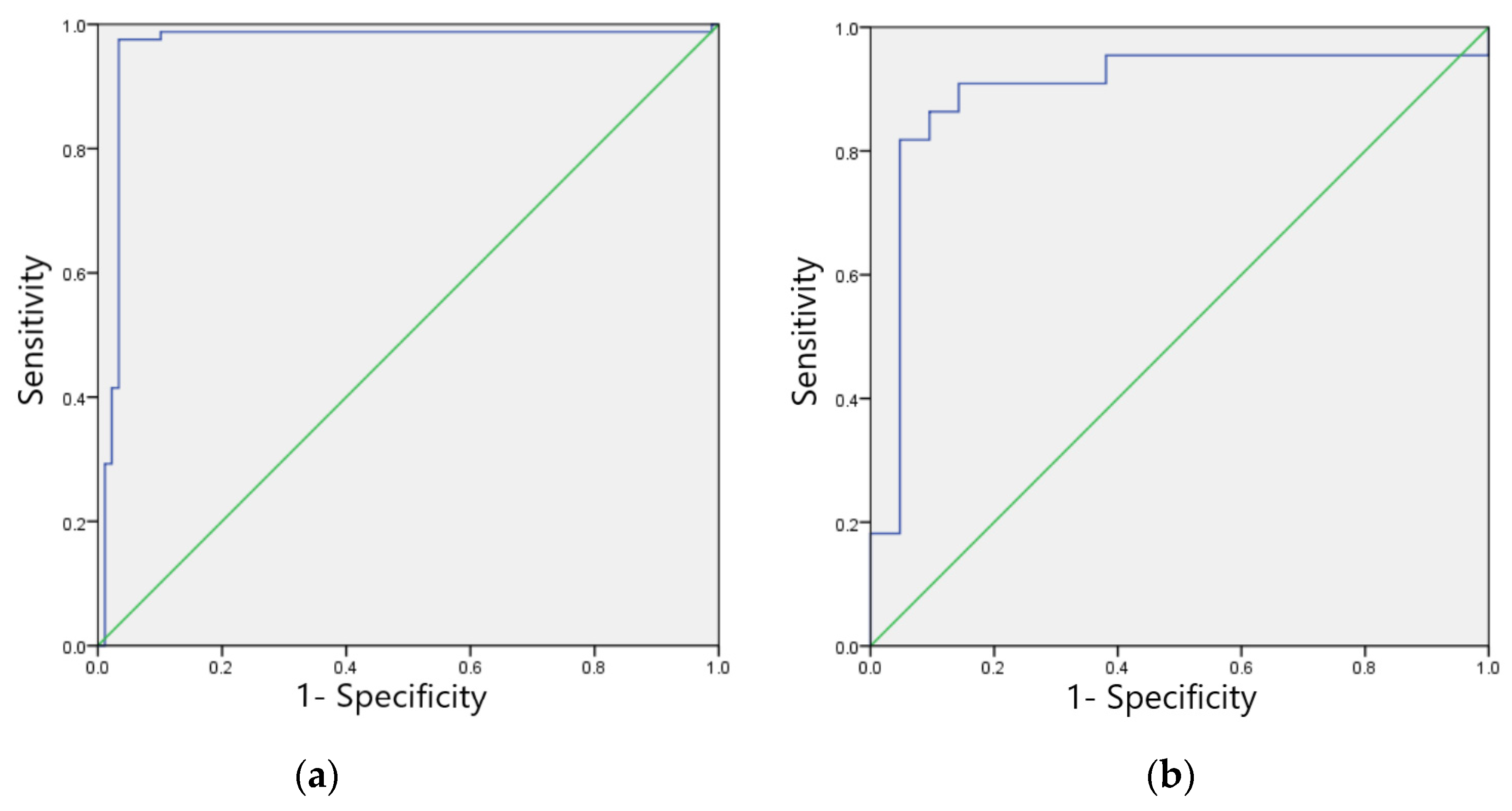
3.4. Results of Image Recognition Models for the Four Main Anomaly Types
3.4.1. Sinus Bradycardia Image Recognition Model
3.4.2. Non-Specific Indoor Conduction Delay Image Recognition Model
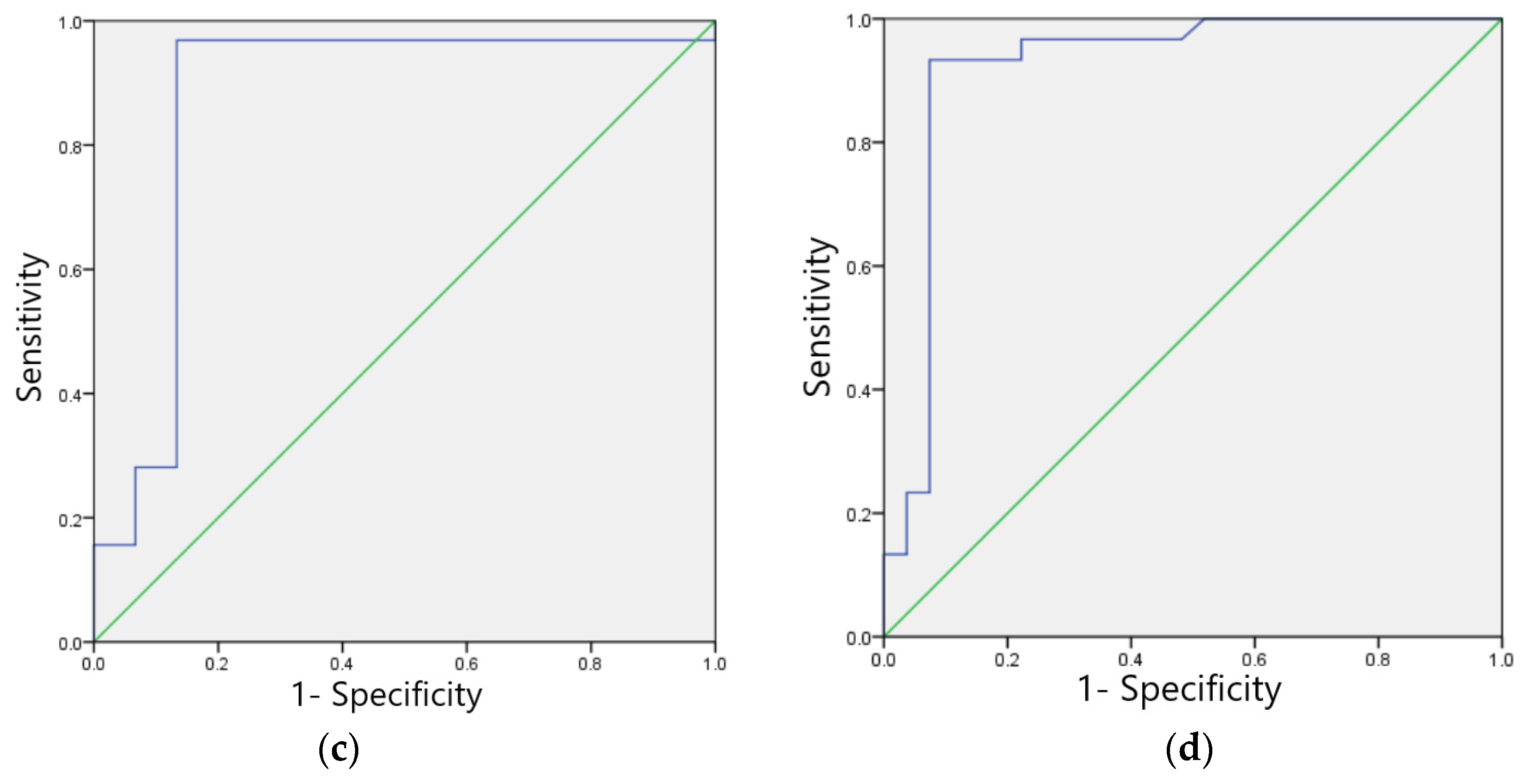
3.4.3. Myocardial Ischemia Image Recognition Model
3.4.4. Sinus Tachycardia Image Recognition Model
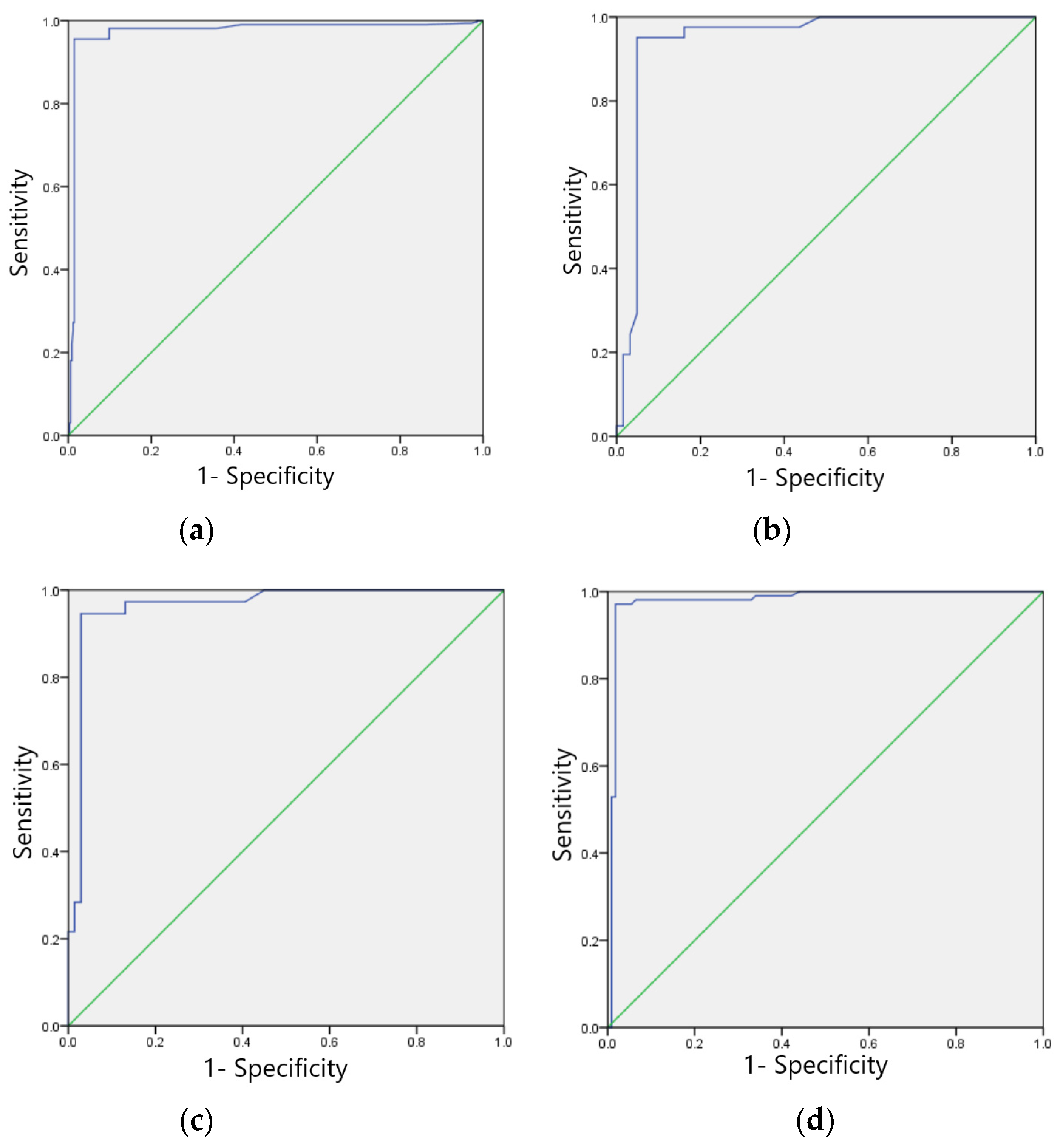
3.5. ECG Recognition Model Performance Evaluation
3.5.1. Training Set Model Effect Evaluation
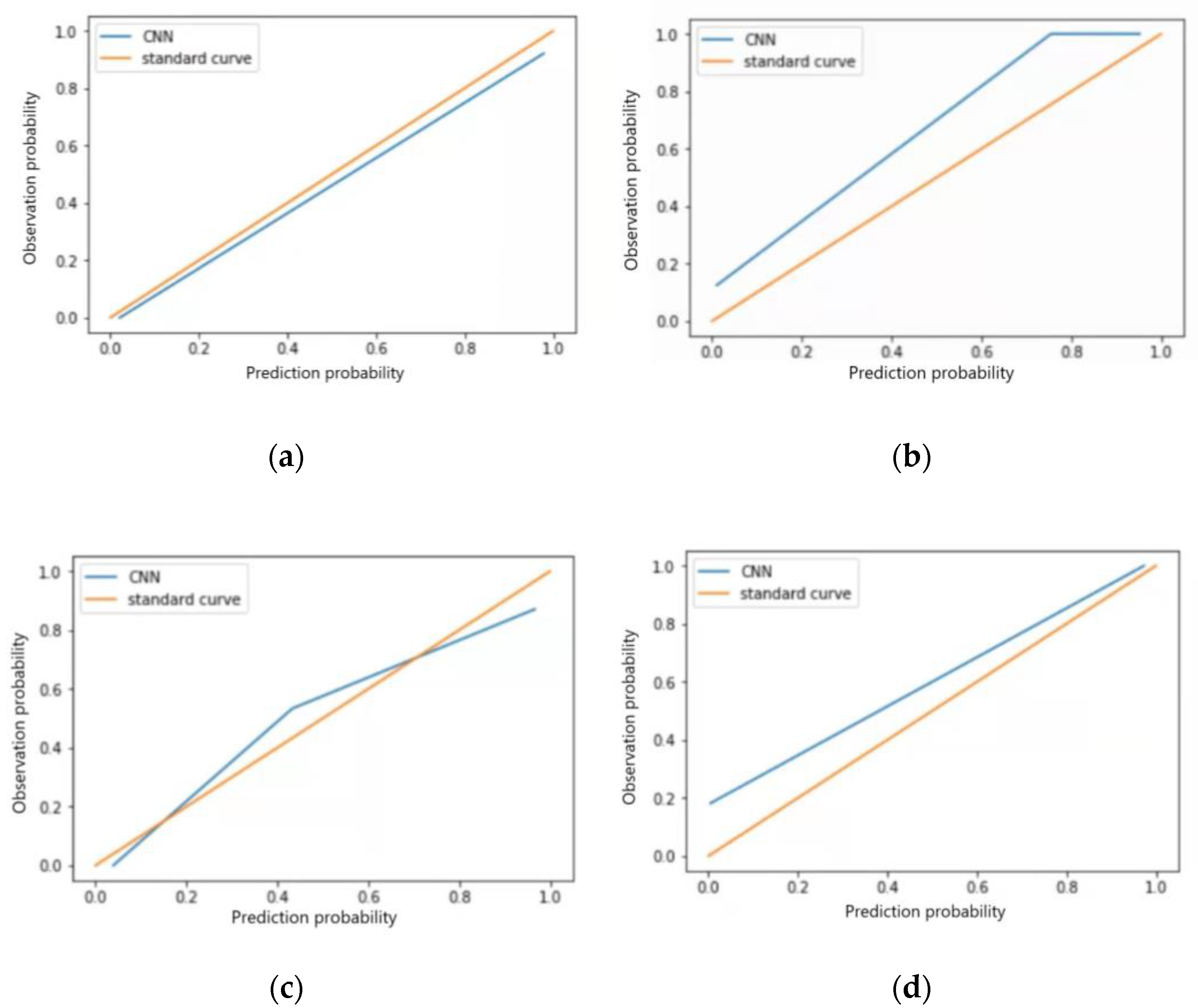
3.5.2. Test Set Model Effect Evaluation
3.5.3. Validation Set Model Effect Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.H.; Chen, S.W.; Chang, P.J.; Hua, H.T.; Lin, S.Y.; Chen, R.S. A VLSI Chip for the Abnormal Heart Beat Detection Using Convolutional Neural Network. Sensors 2022, 22, 796. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Lu, Y.; Feng, X.Y.; Wang, Y.; Wang, X.; Zhu, W.T. Pharmacoeconomic evaluation of heart stabilizing granules for cardiovascular diseases based on Meta-analysis. China Pharm. 2017, 28, 591–595. [Google Scholar]
- Vezzosi, T.; Buralli, C.; Marchesotti, F.; Porporato, F.; Tognetti, R.; Zini, E.; Domenech, O. Diagnostic accuracy of a smartphone electrocardiograph in dogs: Compari son with standard 6-lead electrocardiography. Vet. J. 2016, 216, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, F.; Vezzosi, T.; Meylan, M.; Nocera, I.; Ferrulli, V.; Buralli, C.; Meucci, V.; Tognetti, R. Comparison of smartphone-based and standard base-apex electrocardiography in healthy dairy cows. J. Vet. Intern. Med. 2019, 33, 981–986. [Google Scholar] [CrossRef]
- Ullah, A.; Rehman, S.; Tu, S.; Mehmood, R.M.; Ehatisham-ul-Haq, M.F. A Hybrid Deep CNN Model for Abnormal Arrhythmia Detection Based on Cardiac ECG Signal. Sensors 2021, 21, 951. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, W. Application of artificial intelligence in the assessment of cardiac function. Chin. Circ. J. 2021, 36, 1140–1144. [Google Scholar]
- Chang, L.; Deng, S.; Zhou, M.Q.; Wu, Z.K.; Yuan, Y.; Yang, S.H.; Wang, H. Convolutional neural networks in image understanding. J. Autom. 2016, 42, 1300–1312. [Google Scholar]
- Yao, Q.; Lin, X. Design of ETC system based on OBU and NFC recharge. Control. Eng. 2017, 24, 1004–1007. [Google Scholar]
- Xu, Q.S.; Li, L.J. ETC system scheme design is compatible with different frequency bands. J. Shanghai Inst. Shipp. Transp. Sci. 2016, 39. [Google Scholar]
- Liu, X.; Ba, J. Research and implementation of OBU power subsystem in ETC system. Electron. Des. Eng. 2016, 24, 163–165. [Google Scholar]
- Parvaneh, S.; Rubin, J.; Babaeizadeh, S.; Xu-Wilson, M. Cardiac arrhythmia detection using deep learning: A review. J. Electrocardiol. 2019, 57S, S70–S74. [Google Scholar] [CrossRef]
- Yildirim, O.; Talo, M.; Ciaccio, E.J.; Tan, R.S.; Acharya, U.R. Accurate deep neural network model to detect cardiac arrhythmia on more than 10,000 individual subject ECG records. Comput. Methods Programs Biomed. 2020, 197, 105740. [Google Scholar] [CrossRef]
- Liang, Y.; Yin, S.; Tang, Q.; Zheng, Z.; Elgendi, M.; Chen, Z. Deep Learning Algorithm Classifies Heartbeat Events Based on Electrocardiogram Signals. Front. Physiol. 2020, 11, 569050. [Google Scholar] [CrossRef]
- Wang, J.; Yan, S.; Peng, X. Rapid identification of MIT-BIH ECG signals based on MATLAB. Sci. Technol. Innov. Appl. 2018, 8, 32–33. [Google Scholar]
- Karunadas, C.P.; Mathew, C. Comparison of arrhythmia detection by conventional Holter and a novel ambulatory ECG system using a patch and Android App, over 24h period. Indian Pacing Electrophysiol. J. 2020, 20, 49–53. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambu latory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Deboleena, R.; Priyadarshini, P.; Kaushik, R. Tree-CNN: A hierarchical Deep Convolutional Neural Network for in cremental learning. Neural Netw. 2020, 121, 148–160. [Google Scholar]
- Dong, C.; Loy, C.C.; He, K.; Tang, X. Image Super-Resolution Using Deep Convolutional Networks. IEEE Trans. Pattern Anal. Mach. Intell. 2016, 38, 295–307. [Google Scholar] [CrossRef]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adam, M.; Gertych, A.; Tan, R.S. A deep convolutional neural network model to classify heartbeats. Comput. Biol. Med. 2017, 89, 389–396. [Google Scholar] [CrossRef]
- Wu, S.C.; Wei, S.Y.; Chang, C.S.; Swindlehurst, A.L.; Chiu, J.K. A Scalable Open-Set ECG Identification System Based on Compressed CNNs. IEEE Trans. Neural Netw. Learn. Syst. 2021, 3127497. [Google Scholar] [CrossRef]
- Zhou, K.; Peng, H.; Hu, J. Application of support vector machine in ECG classification diagnosis. Microcomput. Inf. 2006, 9, 237–239. [Google Scholar]
- Sun, B.; Yang, L.; Guo, X. A hybrid model based on CNN and SVM for ECG signal recognition. J. Shandong Agric. Univ. Nat. Sci. Ed. 2020, 51, 283–288. [Google Scholar]
- Feng, Y. Application of Python language in big data analysis. Comput. Knowl. Technol. 2020, 16, 72–73 + 80. [Google Scholar]
- Sun, Y. Research on Automatic Recognition Algorithm of Static ECG Based on Machine Learning; Beijing University of Technology: Beijing, China, 2020. [Google Scholar]
- Dong, M.; Huang, X.; Xu, B. Unsupervised speech recognition through spike-timingdependent plasticity in a convolutional spiking neural network. PLoS ONE 2018, 13, e020459. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Q.; Wu, G.; Yu, X. A review of convolutional neural networks. Comput. Age 2018, 22, 796. [Google Scholar]
- Zhou, C.; Mi, H. A comparative study on the performance of three commonly used activation functions in deep learning. J. Beijing Inst. Electron. Sci. Technol. 2017, 4, 27–32. [Google Scholar]
- Jia, J. A Simplified Design Method for Convolutional Neural Networks and Its Application Research; University of Chinese Academy of Sciences: Beijing, China, 2020. [Google Scholar]
- Steyerberg, E.W.; Vergouwe, Y. Towards better clinical prediction models: Seven steps for development and an ABCD for validation. Eur. Heart J. 2014, 35, 1925–1931. [Google Scholar] [CrossRef]
- Handelman, G.S.; Kok, H.K.; Chandra, R.V.; Razavi, A.H.; Huang, S.; Brooks, M.; Lee, M.J.; Asadi, H. Peering into the Black Box of Artificial Intelligence: Evaluation Metrics of Machine Learning Methods. AJR Am. J. Roentgenol. 2019, 212, 38–43. [Google Scholar] [CrossRef]
- Zhou, Y. Comparative analysis of electrocardiograms in health checkups of different age groups. China Med. Innov. 2012, 9, 93–94. [Google Scholar]
- Rim, B.; Sung, N.J.; Min, S.; Hong, M. Deep Learning in Physiological Signal Data: A Survey. Sensors 2020, 20, 969. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Sun, Z.J.; Xue, L.; Xu, Y.M.; Wang, Z. A review of deep learning research. Comput. Appl. Res. 2012, 29, 2806–2810. [Google Scholar]
- Murat, F.; Sadak, F.; Yildirim, O.; Talo, M.; Murat, E.; Karabatak, M.; Demir, Y.; Tan, R.S.; Acharya, U.R. Review of Deep Learning-Based Atrial Fibrillation Detection Studies. Int. J. Environ. Res. Public Health 2021, 18, 11302. [Google Scholar] [CrossRef]
- Wu, W. Analysis of electrocardiograms in 4112 coal miners with health checkups. J. Pract. Electrocardiol. 2006, 4, 274–275. [Google Scholar]
- Zhang, L. An Epidemiological Survey Study on Cardiovascular Diseases Among Coal Miners in Shandong Province; Shan dong University: Jinan, China, 2015. [Google Scholar]
- Goldman, A.; Hod, H.; Chetrit, A.; Dankner, R. Incidental abnormal ECG findings and long-term cardiovas cular morbidity and all-cause mortality: A population-based prospective study. Int. J. Cardiol. 2019, 295, 36–41. [Google Scholar] [CrossRef]
- Running, L. Electrocardiographic analysis of 6500 cases of occupational disease health examination in coal miners. Endem. Dis. Bull. 2010, 25, 70–73. [Google Scholar]
- Liu, C.-C. Observation and application of electrocardiogram results in employee health checkups. World Abstr. Latest Med. Inf. 2019, 19, 39–40. [Google Scholar]
- Gao, X. Analysis of clinically relevant factors for the occurrence of abnormal electrocardiograms in healthy populations. J. Clin. Ration. Drug Use 2018, 11, 151–152. [Google Scholar]
- Li, Y.; Li, B.; Du, Q.; Yang, L.; Wang, Y. Analysis of electrocardiographic findings of 9807 healthy male youths in a department of the armed police. Shandong Med. 2015, 55, 93–94. [Google Scholar]
- Zhu, L.; Zhang, C.; Tian, B.; Zheng, L. Comparative analysis of electrocardiographic performance in health checkups at different ages. World Abstr. Curr. Med. Inf. 2016, 16, 255. [Google Scholar]
- Wu, Y. Analysis of Health Status of Workers in a Textile Printing and Dyeing Enterprise in Yixing; Southeast University: Dhaka, Bangladesh, 2019. [Google Scholar]
- Ding, H. Epidemiological survey of electrocardiograms in students of Shenyang Sports College. J. Shenyang Inst. Phys. Educ. 2003, 4, 51–53. [Google Scholar]
- Li, Y.; Liu, Z.D.; Zhang, N. A review of integrated classification algorithms for imbalanced data. Comput. Appl. Res. 2014, 31, 1287–1291. [Google Scholar]
- Mei, Q. Research on ECG Diagnosis Based on Signal Processing and Machine Learning; Donghua University: Shanghai, China, 2020. [Google Scholar]
- Liu, S.; Wang, X.; Liu, Y. Deep learning-based classification algorithm for clinical electrocardiograms. Comput. Mod. 2021, 8, 52–57. [Google Scholar]
- Cui, J.F.; Wang, K.F.; Xiao, W.D. A convolutional neural network-based method for classification of cardiac arrhythmias. J. Xiamen Inst. Technol. 2021, 29, 58–66. [Google Scholar]
- Yang, C.-D.; Jia, Z.; Li, X.-W. Research on the classification of ECG signal recognition based on U-Net++. Comput. Sci. 2021, 48, 121–126. [Google Scholar]
- Yıldırım, Ö.; Pławiak, P.; Tan, R.S.; Acharya, U.R. Arrhythmia detection using a deep convolutional neural network with long duration ECG signals. Comput. Biol. Med. 2018, 102, 411–420. [Google Scholar] [CrossRef]
- Wang, E.K.; Xi, L.; Sun, R.P.; Wang, F.; Pan, L.Y.; Cheng, C.X.; Dimitrakopoulou-Srauss, A.; Zhe, N.; Li, Y.P. A new deep learning model for assisted diagnosis on electrocardiogram. MBE 2019, 16, 2481–2491. [Google Scholar] [CrossRef]
- Wang, G.; Luo, C.; Wang, L.; Song, Y.N.; Tang, Z.S.; Yang, X.J. One-dimensional convolutional neural network-based diagnosis of myocar dial infarction. China Digit. Med. 2021, 16, 55–59. [Google Scholar]
- Yu, J.; Zhang, C.; Wang, S. Convolutional neural network-based arrhythmia identification. Inf. Syst. Eng. 2021, 11, 93–96. [Google Scholar]
- Wu, Y. Research on ECG Component Classification Algorithm Based on Multi-Core and Multi-Scale Convolutional Neural Network; Chongqing University of Posts and Telecommunications: Chongqing, China, 2021. [Google Scholar]
- Wei, C.J.; Xu, S.H. A multi-channel convolutional-based ECG signal recognition method. Comput. Eng. Des. 2021, 42, 690–695. [Google Scholar]
- Chang, H.Y.; Yeh, C.Y.; Lee, C.T.; Lin, C.C. A Sleep Apnea Detection System Based on a One-Dimensional Deep Con volution Neural Network Model Using Single-Lead Electrocardiogram. Sensors 2020, 15, 4157. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F.; Huang, Q.; Chang, S.; Wang, H.; He, J. MFB-CBRNN: A Hybrid Network for MI Detection Using 12-Lead ECGs. IEEE J. Biomed. Health Inform. 2020, 24, 503–514. [Google Scholar] [CrossRef]
- Romdhane, T.F.; Pr, M.A. Electrocardiogram heartbeat classification based on a deep convolutional neural network and focal loss. Comput. Biol. Med. 2020, 123, 103866. [Google Scholar] [CrossRef]


| Basic Information | Group | Quantity | Percentage (%) |
|---|---|---|---|
| Gender | Man | 3268 | 96.34 |
| Woman | 124 | 3.66 | |
| Age | <30 | 197 | 5.81 |
| 30~ | 1573 | 46.37 | |
| 40~ | 1030 | 30.37 | |
| ≥50 | 592 | 17.45 | |
| Degree of education | Junior High School and below | 1418 | 41.81 |
| High school | 1096 | 32.31 | |
| College degree or above | 878 | 25.88 | |
| Marital status | Unmarried | 106 | 3.13 |
| Married | 3249 | 95.78 | |
| Other | 37 | 1.09 |
| Exception Category | Number of People | Percentage (%) |
|---|---|---|
| Sinus bradycardia | 441 | 51.94 |
| Non-specific intraventricular conduction delay | 147 | 17.31 |
| Myocardial ischemia | 118 | 13.90 |
| Sinus tachycardia | 73 | 8.60 |
| Left ventricular hypertrophy | 24 | 2.83 |
| Premature ventricular contractions | 14 | 1.65 |
| Supraventricular premature contractions | 10 | 1.18 |
| Ectopic atrial rhythm | 9 | 1.06 |
| Atrioventricular block | 7 | 0.82 |
| WPW syndrome (type B) | 6 | 0.71 |
| Hypertrophy of the right ventricle | 5 | 0.59 |
| Ectopic premature contractions | 3 | 0.35 |
| Intersectional tachycardia | 1 | 0.12 |
| Atrial fibrillation | 1 | 0.12 |
| WPW syndrome (type A) | 1 | 0.12 |
| Zoning | Correct | Error | Accuracy (%) |
|---|---|---|---|
| Training sets | 654 | 8 | 98.79 |
| Test Sets | 167 | 4 | 97.66 |
| Validation Sets | 94 | 3 | 96.91 |
| Zoning | Correct | Error | Accuracy (%) |
|---|---|---|---|
| Training sets | 209 | 4 | 98.12 |
| Test Sets | 55 | 2 | 96.49 |
| Validation Sets | 27 | 2 | 93.10 |
| Zoning | Correct | Error | Accuracy (%) |
|---|---|---|---|
| Training sets | 139 | 4 | 97.20 |
| Test Sets | 44 | 3 | 93.62 |
| Validation Sets | 21 | 2 | 91.30 |
| Zoning | Correct | Error | Accuracy (%) |
|---|---|---|---|
| Training sets | 99 | 4 | 96.12 |
| Test Sets | 40 | 3 | 93.02 |
| Validation Sets | 21 | 2 | 91.30 |
| Types of ECG Abnormalities | Sensitivity (%) | Specificity (%) | F1 Score | Accuracy (%) | Kappa Value | AUC | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Sinus bradycardia | 98.55 | 99.05 | 0.99 | 98.79 | 0.98 | 0.988 (0.978~0.998) | 99.13 | 98.44 |
| Non-specific intraventricular conduction delay | 98.17 | 98.08 | 0.98 | 98.12 | 0.96 | 0.981 (0.960~0.998) | 97.61 | 98.53 |
| Myocardial ischemia | 97.10 | 97.30 | 0.97 | 97.20 | 0.94 | 0.972 (0.941~0.989) | 97.30 | 97.11 |
| Sinus tachycardia | 95.16 | 97.56 | 0.96 | 96.12 | 0.92 | 0.947 (0.897~0.996) | 98.36 | 93.07 |
| Types of ECG Abnormalities | Sensitivity (%) | Specificity (%) | F1 Score | Accuracy (%) | Kappa Value | AUC | Brier Score | Calibration-in-the-Large | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Sinus bradycardia | 96.63 | 98.78 | 0.98 | 97.66 | 0.95 | 0.977 (0.951~0.995) | 0.03 | 0.026 | 99.85 | 96.42 |
| Non-specific intraventricular conduction delay | 96.30 | 96.67 | 0.97 | 96.49 | 0.93 | 0.920 (0.835~0.993) | 0.07 | 0.110 | 96.48 | 96.50 |
| Myocardial ischemia | 86.67 | 96.88 | 0.92 | 93.62 | 0.85 | 0.869 (0.810~0.987) | 0.09 | 0.041 | 92.87 | 93.94 |
| Sinus tachycardia | 95.24 | 90.90 | 0.93 | 93.02 | 0.88 | 0.894 (0.842~0.988) | 0.11 | 0.098 | 90.90 | 95.24 |
| Types of ECG Abnormalities | Sensitivity (%) | Specificity (%) | F1 Score | Accuracy (%) | Kappa Value | AUC | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Sinus bradycardia | 96.30 | 97.67 | 0.97 | 96.91 | 0.94 | 0.970 (0.931~0.992) | 97.77 | 95.49 |
| Non-specific intraventricular conduction delay | 93.75 | 92.31 | 0.93 | 93.10 | 0.86 | 0.930 (0.820~0.972) | 93.37 | 92.40 |
| Myocardial ischemia | 90.90 | 83.33 | 0.87 | 91.30 | 0.83 | 0.931 (0.777~0.958) | 87.80 | 99.20 |
| Sinus tachycardia | 92.31 | 90.00 | 0.91 | 91.30 | 0.82 | 0.912 (0.772~0.949) | 91.74 | 90.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, Z.; Tian, S.; Zhou, S.; Wang, X.; Xue, L.; Wu, J. Convolutional Neural Network-Based ECG-Assisted Diagnosis for Coal Workers. Int. J. Environ. Res. Public Health 2023, 20, 9. https://doi.org/10.3390/ijerph20010009
Wang Y, Chen Z, Tian S, Zhou S, Wang X, Xue L, Wu J. Convolutional Neural Network-Based ECG-Assisted Diagnosis for Coal Workers. International Journal of Environmental Research and Public Health. 2023; 20(1):9. https://doi.org/10.3390/ijerph20010009
Chicago/Turabian StyleWang, Yujia, Zhe Chen, Sen Tian, Shuxun Zhou, Xinbo Wang, Ling Xue, and Jianhui Wu. 2023. "Convolutional Neural Network-Based ECG-Assisted Diagnosis for Coal Workers" International Journal of Environmental Research and Public Health 20, no. 1: 9. https://doi.org/10.3390/ijerph20010009
APA StyleWang, Y., Chen, Z., Tian, S., Zhou, S., Wang, X., Xue, L., & Wu, J. (2023). Convolutional Neural Network-Based ECG-Assisted Diagnosis for Coal Workers. International Journal of Environmental Research and Public Health, 20(1), 9. https://doi.org/10.3390/ijerph20010009






