Chitin-Based Magnesium Oxide Biocomposite for the Removal of Methyl Orange from Water
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Preparation of CHt@MgO
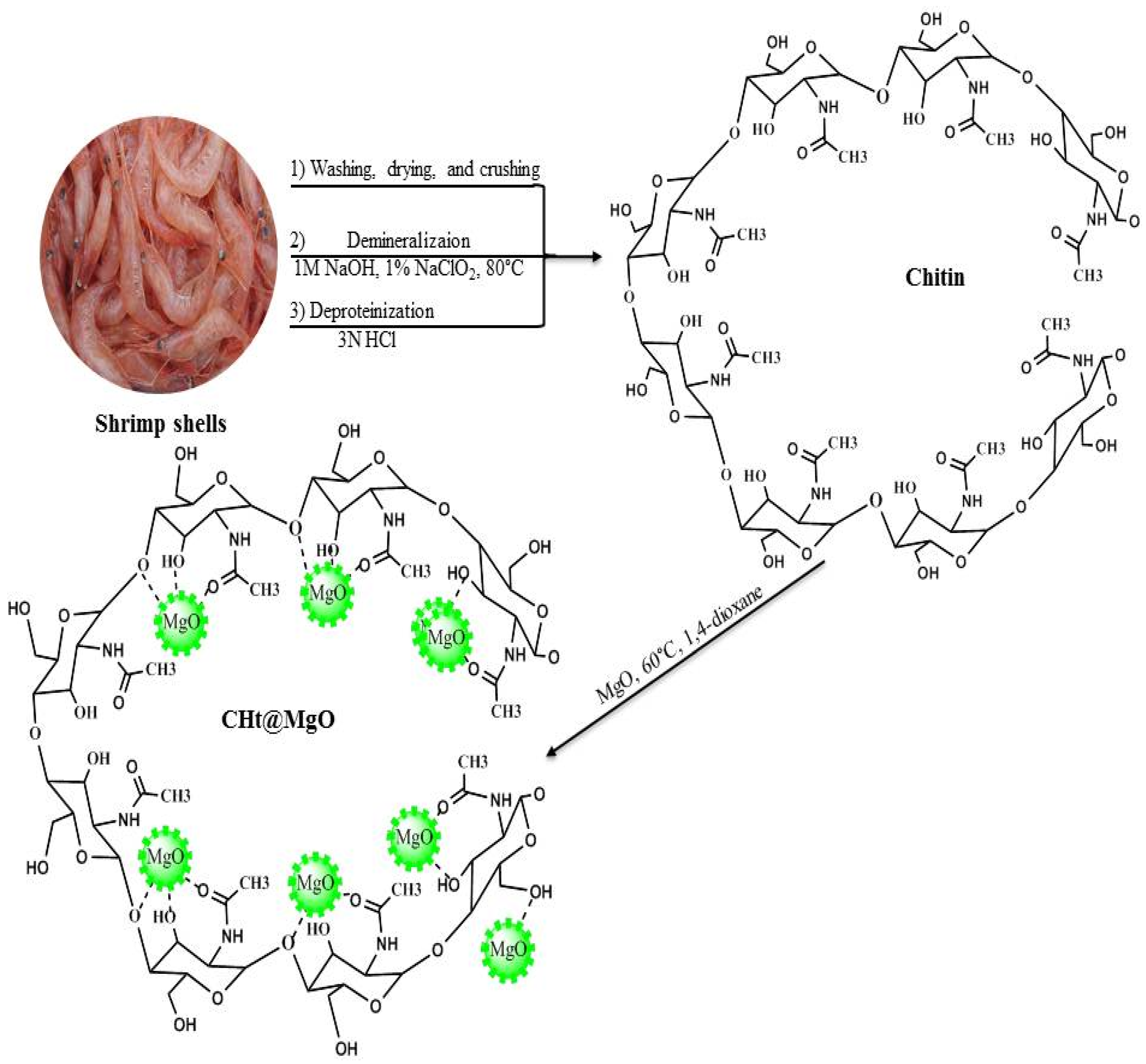
2.2.1. Extraction of Chitin
2.2.2. Synthesis of CHt@MgO
2.3. Characterization of CHt@MgO
2.4. MO Adsorption and Regeneration Studies
3. Results and Discussion
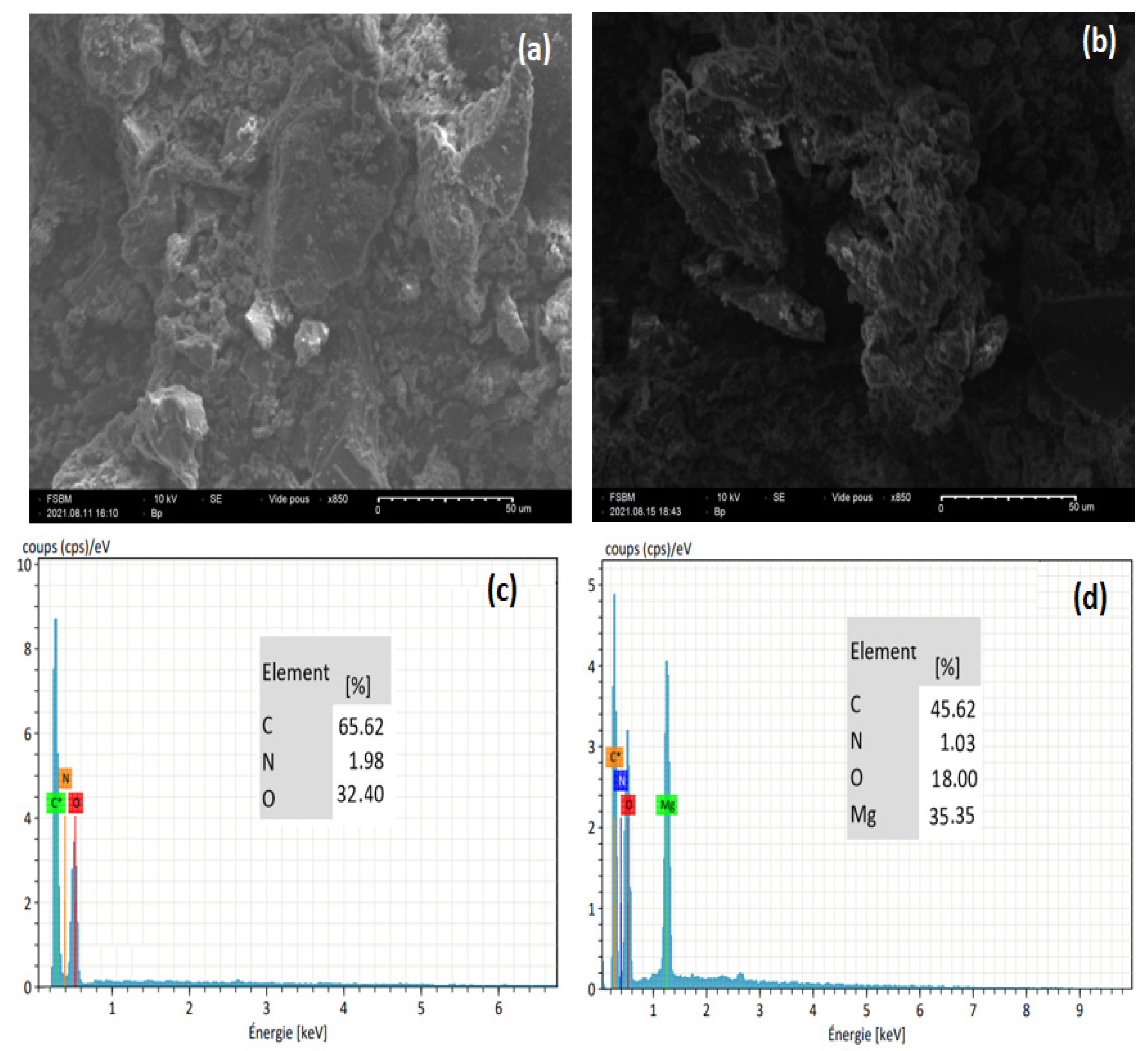
3.1. Characterization of CHt@MgO
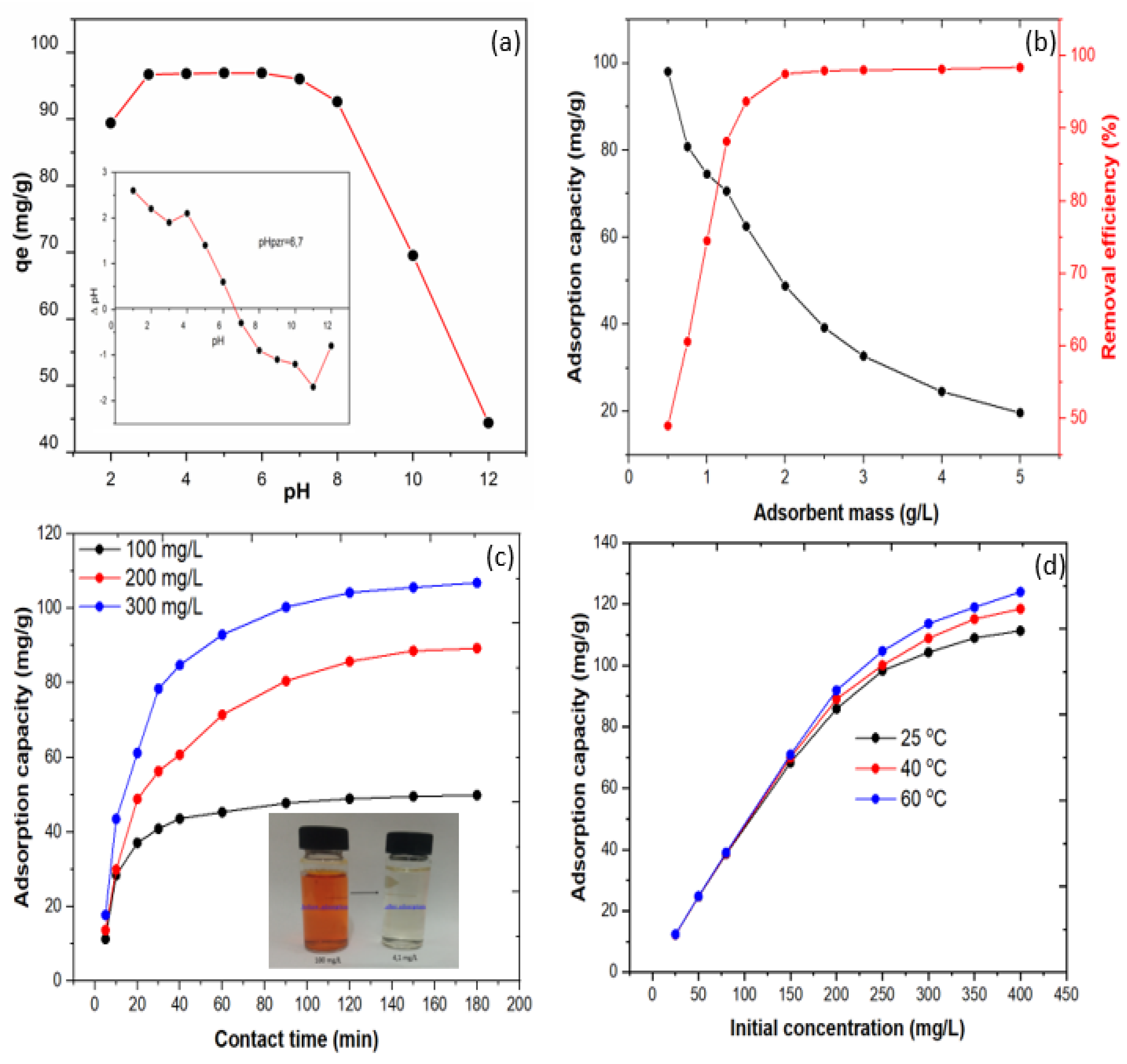
3.2. MO Adsorption Experiments on CHt@MgO Biocomposite
3.3. MO Adsorption Modelling
3.3.1. Adsorption Isotherm Modeling
3.3.2. Kinetic Modeling
3.3.3. Thermodynamic Modeling
3.4. Comparative Performance of CHt@MgO with Other Adsorbents
3.5. Regeneration of CHt@MgO Biocomposite
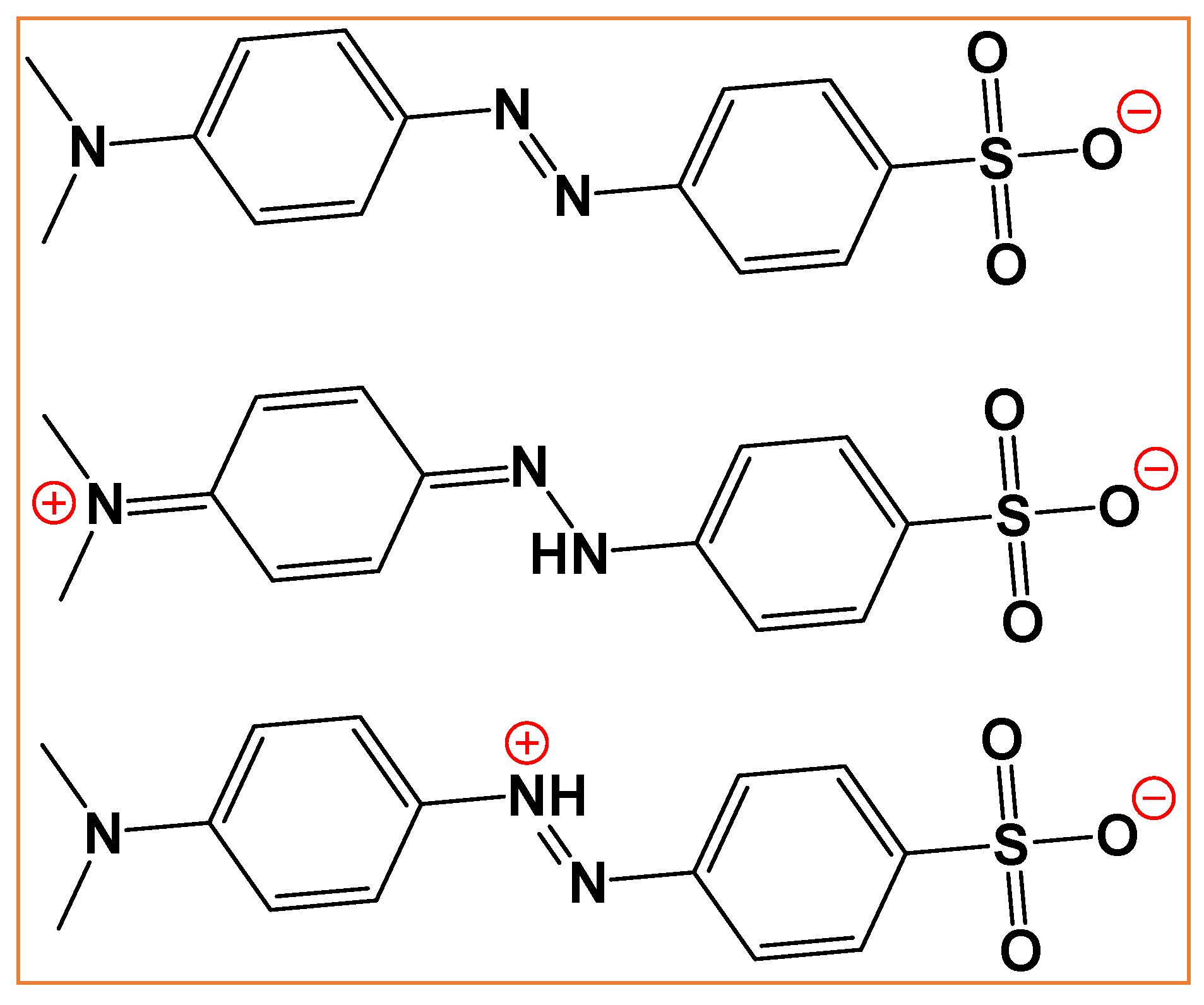
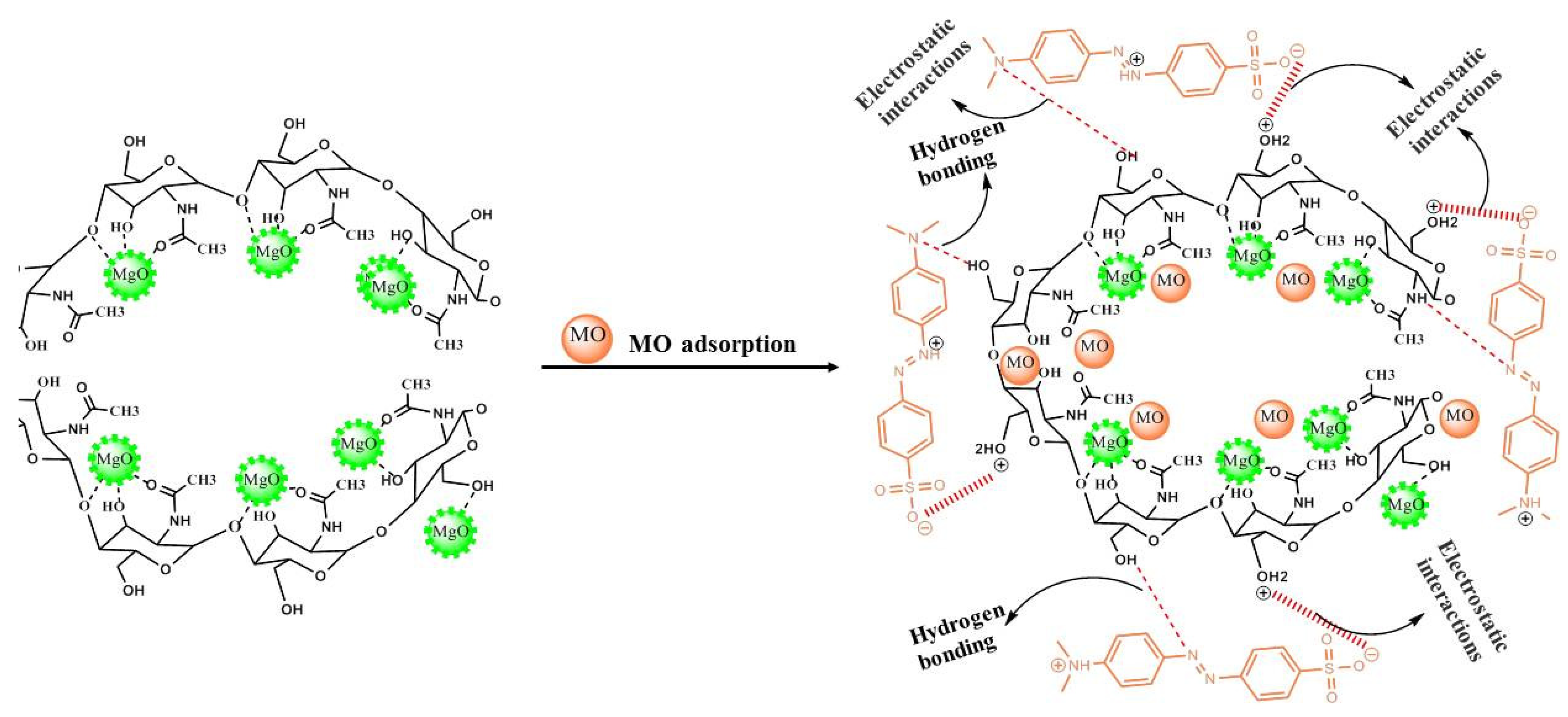
3.6. MO Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altalhi, T.; Jethave, G.; Fegade, U.; Mersal, G.A.M.; Ibrahim, M.M.; Mahmoud, M.H.H.; Kumeria, T.; Isai, K.A.; Sonawane, M. Adsorption of Magenta Dye on PbO Doped MgZnO: Interpretation of Statistical Physics Parameters Using Double-Layer Models. Int. J. Environ. Res. Public Health 2022, 19, 12199. [Google Scholar] [CrossRef] [PubMed]
- Sabarudin, A.; Madjid, A.D.R. Preparation and Kinetic Studies of Cross-Linked Chitosan Beads Using Dual Crosslinkers of Tripolyphosphate and Epichlorohydrin for Adsorption of Methyl Orange. Sci. World J. 2021, 2021, 6648457. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Xing, J.; Li, Z.; Li, Q. Cr-doped ZnO nanoparticles: Synthesis, characterization, adsorption property, and recyclability. ACS Appl. Mater. Interfaces 2015, 7, 27449–27457. [Google Scholar] [CrossRef]
- Chen, P.; Liang, Y.; Xu, Y.; Zhao, Y.; Song, S. Synchronous photosensitized degradation of methyl orange and methylene blue in water by visible-light irradiation. J. Mol. Liq. 2021, 334, 116159. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Ye, J.; Lin, Z.; Yang, X. Electrochemical oxidation of methyl orange by a Magnéli phase Ti4O7 anode. Chemosphere 2020, 241, 125084. [Google Scholar] [CrossRef]
- Yadav, A.; Patel, R.V.; Singh, C.P.; Labhasetwar, P.K.; Shahi, V.K. Experimental study and numerical optimization for removal of methyl orange using polytetrafluoroethylene membranes in vacuum membrane distillation process. Colloids Surf. Physicochem. Eng. Asp. 2022, 635, 128070. [Google Scholar] [CrossRef]
- Al-Bastaki, N. Removal of methyl orange dye and Na2SO4 salt from synthetic waste water using reverse osmosis. Chem. Eng. Process. Process Intensif. 2004, 43, 1561–1567. [Google Scholar] [CrossRef]
- Pandey, S. A comprehensive review on recent developments in bentonite-based materials used as adsorbents for wastewater treatment. J. Mol. Liq. 2017, 241, 1091–1113. [Google Scholar] [CrossRef]
- Vidya, J.; Bosco, A.J.; Haribaaskar, K.; Balamurugan, P. Polyaniline-BiVO4 nanocomposite as an efficient adsorbent for the removal of methyl orange from aqueous solution. Mater. Sci. Semicond. Process. 2019, 103, 04645. [Google Scholar] [CrossRef]
- Haque, E.; Jun, J.W.; Jhung, S.H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. [Google Scholar] [CrossRef]
- Hosseini, S.; Khan, M.A.; Malekbala, M.R.; Cheah, W.; Choong, T.S.Y. Carbon coated monolith, a mesoporous material for the removal of methyl orange from aqueous phase: Adsorption and desorption studies. Chem. Eng. J. 2011, 171, 1124–1131. [Google Scholar] [CrossRef]
- Cheah, W.; Hosseini, S.; Khan, M.A.; Chuah, T.G.; Choong, T.S.Y. Acid modified carbon coated monolith for methyl orange adsorption. Chem. Eng. J. 2013, 215–216, 747–754. [Google Scholar] [CrossRef]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Handayani, A.D.; Sutrisno; Indraswati, N.; Ismadji, S. Extraction of astaxanthin from giant tiger (Panaeus monodon) shrimp waste using palm oil: Studies of extraction kinetics and thermodynamic. Bioresour. Technol. 2008, 99, 4414–4419. [Google Scholar] [CrossRef] [PubMed]
- Taser, B.; Ozkan, H.; Adiguzel, A.; Orak, T.; Baltaci, M.O.; Taskin, M. Preparation of chitosan from waste shrimp shells fermented with Paenibacillus jamilae BAT1. Int. J. Biol. Macromol. 2021, 183, 1191–1199. [Google Scholar] [CrossRef]
- Liang, X.; Fan, X.; Li, R.; Li, S.; Shen, S.; Hu, D. Efficient removal of Cr (VI) from water by quaternized chitin/branched polyethylenimine biosorbent with hierarchical pore structure. Bioresour. Technol. 2018, 250, 178–184. [Google Scholar] [CrossRef]
- Daneshvar, E.; Sohrabi, M.S.; Kousha, M.; Bhatnagar, A.; Aliakbarian, B.; Converti, A.; Norrström, A.C. Shrimp shell as an efficient bioadsorbent for Acid Blue 25 dye removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2014, 45, 2926–2934. [Google Scholar] [CrossRef]
- Fabbricino, M.; Pontoni, L. Use of non-treated shrimp-shells for textile dye removal from wastewater. J. Environ. Chem. Eng. 2016, 4, 4100–4106. [Google Scholar] [CrossRef]
- He, C.; Lin, H.; Dai, L.; Qiu, R.; Tang, Y.; Wang, Y.; Duan, P.G.; Ok, Y.S. Waste shrimp shell-derived hydrochar as an emergent material for methyl orange removal in aqueous solutions. Environ. Int. 2020, 134, 105340. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, W.; Zhang, L. Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J. Hazard. Mater. 2012, 209–210, 218–225. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Ladha, D.G.; Wadhwani, P.M.; Shah, N.K. Comparative study of chitin and chitosan beads for the adsorption of hazardous anionic azo dye Congo Red from wastewater. Desalination Water Treat. 2016, 57, 9247–9262. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Bartczak, P.; Jesionowski, T. Enhanced removal of hazardous dye form aqueous solutions and real textile wastewater using bifunctional chitin/lignin biosorbent. Int. J. Biol. Macromol. 2017, 99, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Gautam, D.; Hooda, S. Magnetic Graphene Oxide/Chitin Nanocomposites for Efficient Adsorption of Methylene Blue and Crystal Violet from Aqueous Solutions. J. Chem. Eng. Data 2020, 65, 4052–4062. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; Thakur, B.; Vo, D.V.N.; Gao, B.; Al-Kahtani, A.A.; Stadler, F.J. Adsorptional-photocatalytic removal of fast sulphon black dye by using chitin-cl-poly(itaconic acid-co-acrylamide)/zirconium tungstate nanocomposite hydrogel. J. Hazard. Mater. 2021, 416, 125714. [Google Scholar] [CrossRef]
- Ding, B.; Huang, S.; Pang, K.; Duan, Y.; Zhang, J. Nitrogen-Enriched Carbon Nanofiber Aerogels Derived from Marine Chitin for Energy Storage and Environmental Remediation. ACS Sustain. Chem. Eng. 2018, 6, 177–185. [Google Scholar] [CrossRef]
- Labidi, A.; Salaberria, A.M.; Fernandes, S.C.M.; Labidi, J.; Abderrabba, M. Functional Chitosan Derivative and Chitin as Decolorization Materials for Methylene Blue and Methyl Orange from Aqueous Solution. Materials 2019, 12, 361. [Google Scholar] [CrossRef]
- Makhluf, S.; Dror, R.; Nitzan, Y.; Abramovich, Y.; Jelinek, R.; Gedanken, A. Microwave-Assisted Synthesis of Nanocrystalline MgO and Its Use as a Bacteriocide. Adv. Funct. Mater. 2005, 15, 1708–1715. [Google Scholar] [CrossRef]
- Haldorai, Y.; Shim, J.J. An efficient removal of methyl orange dye from aqueous solution by adsorption onto chitosan/MgO composite: A novel reusable adsorbent. Appl. Surf. Sci. 2014, 292, 447–453. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, S.; Yu, J. Facile fabrication of mesoporous MgO microspheres and their enhanced adsorption performance for phosphate from aqueous solutions. Colloids Surf. Physicochem. Eng. Asp. 2011, 379, 102–108. [Google Scholar] [CrossRef]
- Hu, J.; Song, Z.; Chen, L.; Yang, H.; Li, J.; Richards, R. Adsorption Properties of MgO(111) Nanoplates for the Dye Pollutants from Wastewater. J. Chem. Eng. Data 2010, 55, 3742–3748. [Google Scholar] [CrossRef]
- Nga, N.K.; Hong, P.T.T.; Lam, T.D.; Huy, T.Q. A facile synthesis of nanostructured magnesium oxide particles for enhanced adsorption performance in reactive blue 19 removal. J. Colloid Interface Sci. 2013, 398, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Mahmoudi, M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J. Hazard. Mater. 2009, 168, 806–812. [Google Scholar] [CrossRef]
- Atangana, E.; Oberholster, P.J. Modified Biopolymer (Chitin–Chitosan Derivatives) for the Removal of Heavy Metals in Poultry Wastewater. J. Polym. Environ. 2020, 28, 388–398. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, S.B.; Yun, H.J.; Won, S.W. Polyethylenimine-crosslinked chitin biosorbent for efficient recovery of Pd(II) from acidic solution: Characterization and adsorption mechanism. Carbohydr. Polym. Technol. Appl. 2021, 2, 100091. [Google Scholar] [CrossRef]
- Zarghani, M.; Akhlaghinia, B. Magnetically separable Fe3O4@chitin as an eco-friendly nanocatalyst with high efficiency for green synthesis of 5-substituted-1H-tetrazoles under solvent-free conditions. RSC Adv. 2016, 6, 31850–31860. [Google Scholar] [CrossRef]
- Zahir, M.H.; Rahman, M.M.; Irshad, K.; Rahman, M.M. Shape-Stabilized Phase Change Materials for Solar Energy Storage: MgO and Mg(OH)(2) Mixed with Polyethylene Glycol. Nanomaterials 2019, 9, 1773. [Google Scholar] [CrossRef] [PubMed]
- Nga, N.K.; Chau, N.T.T.; Viet, P.H. Preparation and characterization of a chitosan/MgO composite for the effective removal of reactive blue 19 dye from aqueous solution. J. Sci. Adv. Mater. Devices 2020, 5, 65–72. [Google Scholar] [CrossRef]
- Qin, Q.; Li, M.; Lan, P.; Liao, Y.; Sun, S.; Liu, H. Novel CaCO3/chitin aerogel: Synthesis and adsorption performance toward Congo red in aqueous solutions. Int. J. Biol. Macromol. 2021, 181, 786–792. [Google Scholar] [CrossRef]
- de Silva, R.T.; Mantilaka, M.M.M.G.P.G.; Ratnayake, S.P.; Amaratunga, G.A.J.; de Silva, K.M.N. Nano-MgO reinforced chitosan nanocomposites for high performance packaging applications with improved mechanical, thermal and barrier properties. Carbohydr. Polym. 2016, 157, 739–747. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Adeosun, S.O.; Lawal, G.I.; Jun, S.; Olaleye, S.A. Acetylation, crystalline and morphological properties of structural polysaccharide from shrimp exoskeleton. Eng. Sci. Technol. Int. J. 2017, 20, 1155–1165. [Google Scholar] [CrossRef]
- Esvandi, Z.; Foroutan, R.; Mirjalili, M.; Sorial, G.A.; Ramavandi, B. Physicochemical Behavior of Penaeuse semisulcatuse Chitin for Pb and Cd Removal from Aqueous Environment. J. Polym. Environ. 2019, 27, 263–274. [Google Scholar] [CrossRef]
- Billah, R.E.K.; Khan, M.A.; Park, Y.K.; Am, A.; Majdoubi, H.; Haddaji, Y.; Jeon, B.H. A comparative study on hexavalent chromium adsorption onto chitosan and chitosan-based composites. Polymers 2021, 13, 3427. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A.; Bashir, M.A.; Najam, T.; Javed, M.S.; Suleman, S.; Hussain, S.; Kumar, O.P.; Shah, S.S.A.; ur Rehman, A. Combining structurally ordered intermetallic nodes: Kinetic and isothermal studies for removal of malachite green and methyl orange with mechanistic aspects. Microchem. J. 2021, 164, 105973. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Ye, H.; Chen, J.; Xiao, W.; Zhou, W.; Garba, Z.N.; Lawan, I.; Wang, L.; Yuan, Z. Modification of sugar-based carbon using lanthanum and cobalt bimetal species for effective adsorption of methyl orange. Environ. Technol. Innov. 2021, 23, 101769. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, J.; Zhou, J.; Lei, J. Study on adsorption of methylene blue by a novel composite material of TiO2 and alum sludge. RSC Adv. 2018, 8, 32799–32807. [Google Scholar] [CrossRef]
- Sun, B.; Yuan, Y.; Li, H.; Li, X.; Zhang, C.; Guo, F.; Liu, X.; Wang, K.; Zhao, X.S. Waste-cellulose-derived porous carbon adsorbents for methyl orange removal. Chem. Eng. J. 2019, 371, 55–63. [Google Scholar] [CrossRef]
- Alshareef, S.A.; Alqadami, A.A.; Khan, M.A.; Alanazi, H.S.; Siddiqui, M.R.; Jeon, B.-H. Simultaneous co-hydrothermal carbonization and chemical activation of food wastes to develop hydrochar for aquatic environmental remediation. Biores. Technol. 2022, 347, 126363. [Google Scholar] [CrossRef]
- Khan, M.A.; Alqadami, A.A.; Wabaidur, S.M.; Siddiqui, M.R.; Jeon, B.-H.; Alshareef, S.A.; Alothman, Z.A.; Hamedelniel, A.E. Oil industry waste based non-magnetic and magnetic hydrochar to sequester potentially toxic post-transition metal ions from water. J. Hazard. Mater. 2020, 400, 123247. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Yarramuthi, V.; Kim, D.-S. Methyl orange removal from aqueous solution using goethite, chitosan beads and goethite impregnated with chitosan beads. J. Mol. Liq. 2017, 240, 329–339. [Google Scholar] [CrossRef]
- Wang, M.; Day, S.; Wu, Z.; Wan, X.; Ye, X.; Cheng, B. A new type of porous Zn (II) metal-organic gel designed for effective adsorption to methyl orange dye. Colloids Surf. Physicochem. Eng. Asp. 2021, 628, 127335. [Google Scholar] [CrossRef]
- El Maguana, Y.; Elhadiri, N.; Benchanaa, M.; Chikri, R. Adsorption Thermodynamic and Kinetic Studies of Methyl Orange onto Sugar Scum Powder as a Low-Cost Inorganic Adsorbent. J. Chem. 2020, 2020, 9165874. [Google Scholar] [CrossRef]
- Buhani; Suharso; Miftahza, N.; Permatasari, D.; Sumadi. Improved Adsorption Capacity of Nannochloropsis sp. through Modification with Cetyltrimethylammonium Bromide on the Removal of Methyl Orange in Solution. Adsorpt. Sci. Technol. 2021, 2021, 1641074. [Google Scholar] [CrossRef]
- Tran, T.T.A.; Duong, H.T.L.; Pham, T.T.P.; Nguyen, T.; Nguyen, T.D.; Tran, B.A. Preparation of Magnetic Composite Polyaniline/Fe3O4−Hydrotalcite and Performance in Removal of Methyl Orange. Adsorpt. Sci. Technol. 2021, 2021, 4150073. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mavhungu, A.; Moropeng, M.L.; Mbaya, R. Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater. Heliyon 2022, 8, e09930. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Handlingar 1898, 24, 1–39. [Google Scholar]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solutions. Journal of the Sanitary Engineering Division, American Society of Civil Engineers. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Jasper, E.E.; Ajibola, V.O.; Onwuka, J.C. Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 2020, 10, 132. [Google Scholar] [CrossRef]
- Wan, X.; Zhan, Y.; Long, Z.; Zeng, G.; He, Y. Core@double-shell structured magnetic halloysite nanotube nano-hybrid as efficient recyclable adsorbent for methylene blue removal. Chem. Eng. J. 2017, 330, 491–504. [Google Scholar] [CrossRef]
- Cao, G.; Gao, M.; Shen, T.; Guo, S.; Zhao, B.; Zhao, Q. Asymmetric gemini surfactants modified vermiculite- and silica nanosheets-based adsorbents for removing methyl orange and crystal violet. Colloids Surf. Physicochem. Eng. Asp. 2020, 596, 124735. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Saha, P.D. Adsorption of Crystal Violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Abdul Mubarak, N.S.; Chuan, T.W.; Khor, H.P.; Jawad, A.H.; Wilson, L.D.; Sabar, S. Immobilized Fe-Loaded Chitosan Film for Methyl Orange Dye Removal: Competitive Ions, Reusability, and Mechanism. J. Polym. Environ. 2021, 29, 1050–1062. [Google Scholar] [CrossRef]
- Fradj, A.B.; Boubakri, A.; Hafiane, A.; Hamouda, S.B. Removal of azoic dyes from aqueous solutions by chitosan enhanced ultrafiltration. Results Chem. 2020, 2, 100017. [Google Scholar] [CrossRef]
- Ramakrishnan, R.K.; Padil, V.V.T.; Wacławek, S.; Černík, M.; Varma, R.S. Eco-Friendly and Economic, Adsorptive Removal of Cationic and Anionic Dyes by Bio-Based Karaya Gum—Chitosan Sponge. Polymers 2021, 13, 251. [Google Scholar] [CrossRef]

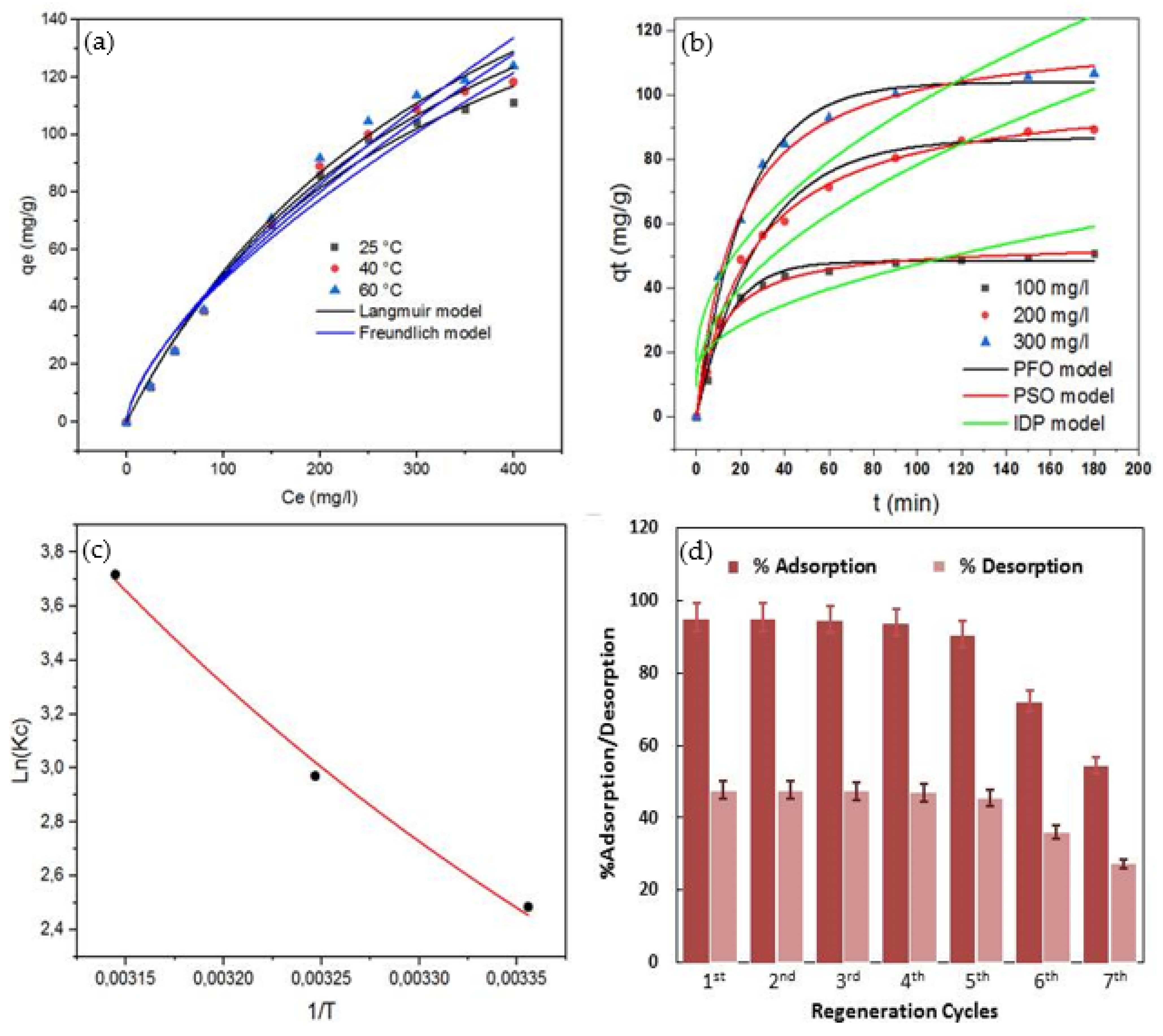
| Temperature (°C) | Isotherm Models | |||||
|---|---|---|---|---|---|---|
| Langmuir | Freundlich | |||||
| qm (mg/g) | KL (L/mg) | R2 | KF (mg/g) (L/mg)1/n | n | R2 | |
| 25 | 206.88 | 0.003 | 0.991 | 2.45 | 0.65 | 0.960 |
| 40 | 231.95 | 0.022 | 0.993 | 2.21 | 0.67 | 0.968 |
| 60 | 251.62 | 0.002 | 0.992 | 2.11 | 0.69 | 0.969 |
| Adsorbent | Experimental Conditions | qm (mg/g) | Reference |
|---|---|---|---|
| ZrMOX particles | Co: 20–60 mg/L; T: 50 °C; m: 0.5 g; pH: 4–6; t: 5 h | 143.68 | [1] |
| Crosslinked chitosan beads | Co: 20 mg/L; T: 25 °C; m: 0.02 g; pH: 3; t: 12 h | 79.55 | [2] |
| Goethite/chitosan beads | Co:10–100 mg/L; T: 62 °C K; pH: 3; m:0.1 g; t: 3 h | 84.00 | [49] |
| Zn-MOG | Co: 50 mg/L; T: 25 °C; m: 0.005 g; t: 1 h | 130.04 | [50] |
| Sugar scum powder | Co: 100 mg/L; T: 22 °C; m: 16 g/L; pH: 7.2.; t: 1 h | 15.24 | [51] |
| AlgN-CTAB | Co: 0–300 mg/L; T: 27 °C; m: 0.1 g; pH: 5; t: 1 h | 76.48 | [52] |
| Pan/MHT | Co: 20 mg/L; T: 25 °C; pH: 3–4; m: 0.02 g: t: 2 h | 156.25 | [53] |
| CHt@MgO | Co: 25–400 mg/L; T: 60 °C; pH: 6; m: 0.02 g: t: 2 h | 251.62 | This study |
| Co (mg/L) | qe,exp (mg/g) | Kinetic Models | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PFO | PSO | IPD | ||||||||
| qe,cal (mg/g) | k1 (1/min) | R2 | qe,cal (mg/g) | k2 (mg/g-min) | R2 | kip (mg/g-min1/2) | C | R2 | ||
| 100 | 49.6 | 48.37 | 0.07 | 0.982 | 53.50 | 0.002 | 0.973 | 3.42 | 13.23 | 0.743 |
| 200 | 88.6 | 86.61 | 0.03 | 0.987 | 102.35 | 0.0004 | 0.996 | 6.88 | 9.56 | 0.915 |
| 300 | 105.7 | 104.07 | 0.04 | 0.993 | 120.01 | 0.0005 | 0.989 | 8.01 | 17.29 | 0.848 |
| ∆H° (kJ/mol) | ∆S° (J/mol-K) | ∆G° (kJ/mol) | ||
|---|---|---|---|---|
| 298 K | 313 K | 333 K | ||
| 48.42 | 182.78 | −6.156 | −7.606 | −9.829 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majdoubi, H.; Alqadami, A.A.; Billah, R.E.K.; Otero, M.; Jeon, B.-H.; Hannache, H.; Tamraoui, Y.; Khan, M.A. Chitin-Based Magnesium Oxide Biocomposite for the Removal of Methyl Orange from Water. Int. J. Environ. Res. Public Health 2023, 20, 831. https://doi.org/10.3390/ijerph20010831
Majdoubi H, Alqadami AA, Billah REK, Otero M, Jeon B-H, Hannache H, Tamraoui Y, Khan MA. Chitin-Based Magnesium Oxide Biocomposite for the Removal of Methyl Orange from Water. International Journal of Environmental Research and Public Health. 2023; 20(1):831. https://doi.org/10.3390/ijerph20010831
Chicago/Turabian StyleMajdoubi, Hicham, Ayoub Abdullah Alqadami, Rachid EL Kaim Billah, Marta Otero, Byong-Hun Jeon, Hassan Hannache, Youssef Tamraoui, and Moonis Ali Khan. 2023. "Chitin-Based Magnesium Oxide Biocomposite for the Removal of Methyl Orange from Water" International Journal of Environmental Research and Public Health 20, no. 1: 831. https://doi.org/10.3390/ijerph20010831
APA StyleMajdoubi, H., Alqadami, A. A., Billah, R. E. K., Otero, M., Jeon, B.-H., Hannache, H., Tamraoui, Y., & Khan, M. A. (2023). Chitin-Based Magnesium Oxide Biocomposite for the Removal of Methyl Orange from Water. International Journal of Environmental Research and Public Health, 20(1), 831. https://doi.org/10.3390/ijerph20010831









