The Role of Nutrition and Forest-Bathing in the Physical Rehabilitation of Physically Inactive Patients: From the Molecular Aspects to New Nature-Inspired Techniques
Abstract
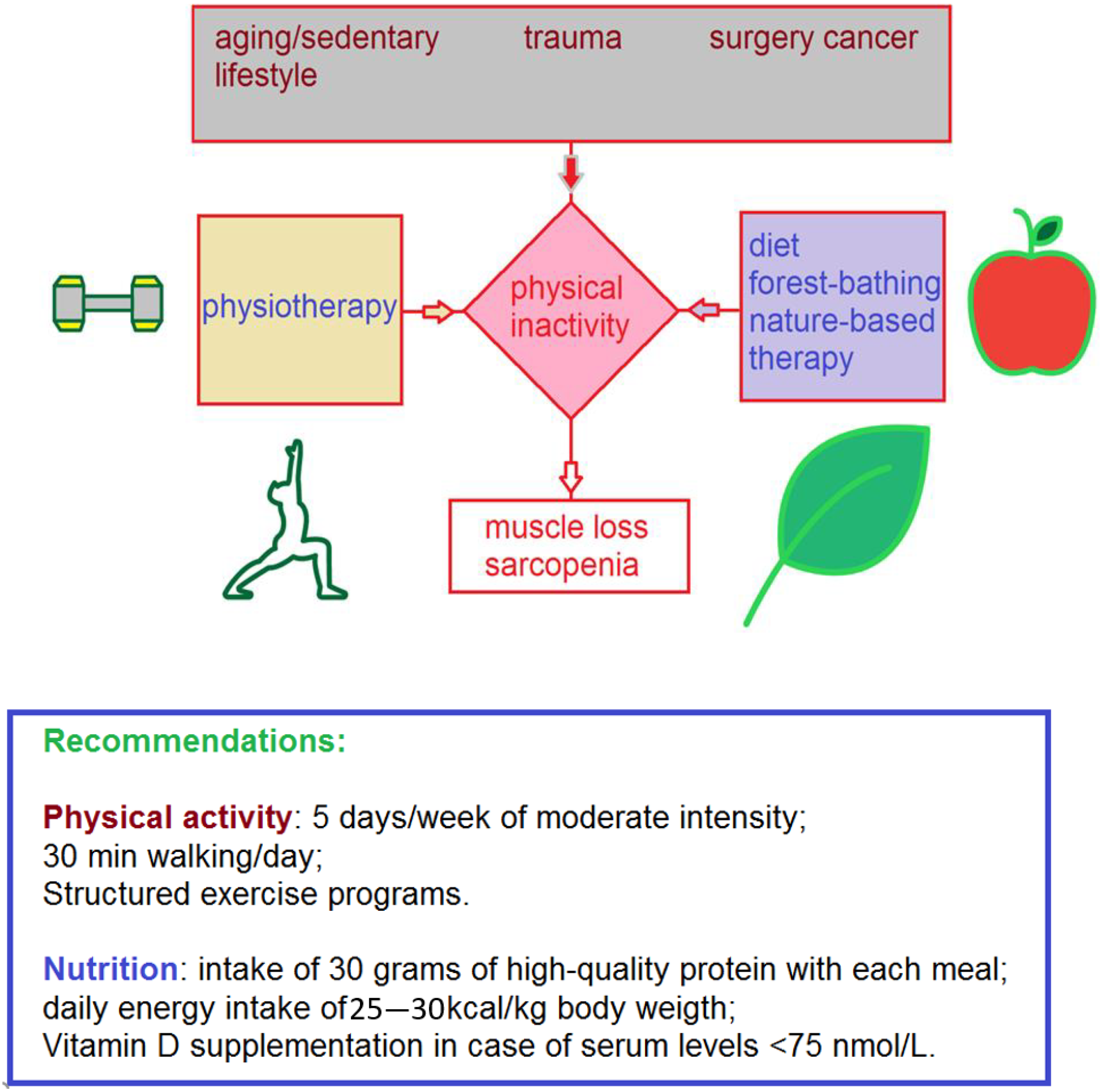
1. Introduction
2. Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bull, F.C.; Armstrong, T.P.; Dixon, T.; Ham, S.; Neiman, A.; Pratt, M. Physical inactivity. In Comparative Quantification of Health Risks Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; World Health Organization: Geneva, Switzerland, 2004; pp. 729–881. [Google Scholar]
- Kohl, H.W., 3rd; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S.; Group, L.P.A.S.W. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef]
- Blair, S.N. Physical inactivity: The biggest public health problem of the 21st century. Br. J. Sport. Med. 2009, 43, 1–2. [Google Scholar]
- Pratt, M.; Norris, J.; Lobelo, F.; Roux, L.; Wang, G. The cost of physical inactivity: Moving into the 21st century. Br. J. Sport. Med. 2014, 48, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Weyerer, S. Physical inactivity and depression in the community. Int. J. Sport. Med. 1992, 13, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yuan, Y.; Neale, A.; Jackson, L.; Mehaffey, M. Association between Natural Resources for Outdoor Activities and Physical Inactivity: Results from the Contiguous United States. Int. J. Environ. Res. Public Health 2016, 13, 830. [Google Scholar] [CrossRef]
- Figueiredo, N.; Rodrigues, F.; Morouço, P.; Monteiro, D. Active Commuting: An Opportunity to Fight Both Climate Change and Physical Inactivity. Sustainability 2021, 13, 4290. [Google Scholar] [CrossRef]
- Ono, S.; Yuktadatta, P.; Taniguchi, T.; Iitsuka, T.; Noguchi, M.; Tanaka, S.; Ito, H.; Nakamura, K.; Yasuhara, N.; Miyawaki, C.; et al. Financial Literacy and Exercise Behavior: Evidence from Japan. Sustainability 2021, 13, 4189. [Google Scholar] [CrossRef]
- Kiens, B.; Beyer, N.; Brage, S.; Hyldstrup, L.; Ottesen, L.S.; Overgaard, K.; Pedersen, B.K.; Puggaard, L.; Aagaard, P.G. Physical inactivity--consequences and correlations. Ugeskr. Laeger 2007, 169, 2442–2445. [Google Scholar]
- Freiberger, E.; Sieber, C.; Pfeifer, K. Physical activity, exercise, and sarcopenia–future challenges. Wien. Med. Wochenschr. 2011, 161, 416–425. [Google Scholar] [CrossRef]
- Zen, A.L.; Whooley, M.A.; Zhao, S.; Cohen, B.E. Post-traumatic stress disorder is associated with poor health behaviors: Findings from the heart and soul study. Health Psychol. 2012, 31, 194. [Google Scholar] [CrossRef]
- Knight, J.A. Physical inactivity: Associated diseases and disorders. Ann. Clin. Lab. Sci. 2012, 42, 320–337. [Google Scholar] [PubMed]
- Florin, T.A.; Fryer, G.E.; Miyoshi, T.; Weitzman, M.; Mertens, A.C.; Hudson, M.M.; Sklar, C.A.; Emmons, K.; Hinkle, A.; Whitton, J. Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. Cancer Epidemiol. Prev. Biomark. 2007, 16, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. Ca-Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Chu, E.; Sartorelli, A. Cancer chemotherapy. In Lange’s Basic and Clinical Pharmacology; Katzung, B.G., Ed.; University of California: San Francisco, CA, USA, 2018; pp. 948–976. [Google Scholar]
- Ruddon, R.W. Cancer Biology; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Onciu, M. Acute lymphoblastic leukemia. Hematol. /Oncol. Clin. N. Am. 2009, 23, 655–674. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe COVID-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef]
- Roviello, V.; Roviello, G.N. Less COVID-19 deaths in southern and insular Italy explained by forest bathing, Mediterranean environment, and antiviral plant volatile organic compounds. Environ. Chem. Lett. 2022, 20, 7–17. [Google Scholar] [CrossRef]
- Roviello, V.; Musumeci, D.; Mokhir, A.; Roviello, G.N. Evidence of protein binding by a nucleopeptide based on a thyminedecorated L-diaminopropanoic acid through CD and in silico studies. Curr. Med. Chem. 2021, 28, 5004–5015. [Google Scholar] [CrossRef]
- Costanzo, M.; De Giglio, M.A.; Roviello, G.N. Anti-Coronavirus Vaccines: Past Investigations on SARS-CoV-1 and MERS-CoV, the Approved Vaccines from BioNTech/Pfizer, Moderna, Oxford/AstraZeneca and others under Development against SARS-CoV-2 Infection. Curr. Med. Chem. 2022, 29, 4–18. [Google Scholar] [CrossRef]
- Van Bakel, B.; Van Den Heuvel, F.; Vos, J.; Rotbi, H.; Bakker, E.; Nijveldt, R.; Thijssen, D.; Eijsvogels, T. COVID-19 survivors are physically inactive with high levels of sedentary time, regardless of patient characteristics, disease severity or cardiac dysfunction. Eur. J. Prev. Cardiol. 2022, 29 (Suppl. S1), zwac056-313. [Google Scholar] [CrossRef]
- Damiot, A.; Pinto, A.J.; Turner, J.E.; Gualano, B. Immunological implications of physical inactivity among older adults during the COVID-19 pandemic. Gerontology 2020, 66, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, T.; Austad, C.; Kvien, T. OP0197 Many patients with rheumatoid arthritis remain physically inactive. Ann. Rheum. Dis. 2013, 71 (Suppl. S3), 121. [Google Scholar] [CrossRef]
- Fujita, S.; Volpi, E. Amino acids and muscle loss with aging. J. Nutr. 2006, 136, 277S–280S. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Camprubi-Robles, M.; Bear, D.; Cederholm, T.; Malafarina, V.; Welch, A.; Cruz-Jentoft, A. Muscle loss: The new malnutrition challenge in clinical practice. Clin. Nutr. 2019, 38, 2113–2120. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Chen, L.-K.; Lee, W.-J.; Peng, L.-N.; Liu, L.-K.; Arai, H.; Akishita, M.; Asian Working Group for Sarcopenia. Recent advances in sarcopenia research in Asia: 2016 update from the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2016, 17, 767.e1–767.e7. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; Bernabei, R. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef]
- English, K.L.; Paddon-Jones, D. Protecting muscle mass and function in older adults during bed rest. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 34. [Google Scholar] [CrossRef]
- Bassett, S.F. The assessment of patient adherence to physiotherapy rehabilitation. N. Z. J. Physiother. 2003, 31, 60–66. [Google Scholar]
- Tzani, I.; Tsichlaki, M.; Zerva, E.; Papathanasiou, G.; Dimakakos, E. Physiotherapeutic rehabilitation of lymphedema: State-of-the-art. Lymphology 2018, 51, 1–12. [Google Scholar]
- Harvey, L.A. Physiotherapy rehabilitation for people with spinal cord injuries. J. Physiother. 2016, 62, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.; Coulter, E.H.; Mattison, P.G.; Miller, L.; McFadyen, A.; Paul, L. Physiotherapy rehabilitation for people with progressive multiple sclerosis: A systematic review. Arch. Phys. Med. Rehabil. 2016, 97, 141–151.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. Effect of forest bathing trips on human immune function. Environ. Health Prev. Med. 2010, 15, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, B.-J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef]
- Hansen, M.M.; Jones, R.; Tocchini, K. Shinrin-yoku (forest bathing) and nature therapy: A state-of-the-art review. Int. J. Environ. Res. Public Health 2017, 14, 851. [Google Scholar] [CrossRef]
- Bielinis, E.; Takayama, N.; Boiko, S.; Omelan, A.; Bielinis, L. The effect of winter forest bathing on psychological relaxation of young Polish adults. Urban For. Urban Green. 2018, 29, 276–283. [Google Scholar] [CrossRef]
- Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Hirata, K.; Suzuki, H.; Miyazaki, Y. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2007, 20 (Suppl. S2), 3–8. [Google Scholar] [CrossRef]
- Paddon-Jones, D. Interplay of stress and physical inactivity on muscle loss: Nutritional countermeasures. J. Nutr. 2006, 136, 2123–2126. [Google Scholar] [CrossRef]
- Tanner, R.E.; Brunker, L.B.; Agergaard, J.; Barrows, K.M.; Briggs, R.A.; Kwon, O.S.; Young, L.M.; Hopkins, P.N.; Volpi, E.; Marcus, R.L.; et al. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J. Physiol. 2015, 593, 4259–4273. [Google Scholar] [CrossRef]
- Yoo, S.Z.; No, M.H.; Heo, J.W.; Park, D.H.; Kang, J.H.; Kim, S.H.; Kwak, H.B. Role of exercise in age-related sarcopenia. J. Exerc. Rehabil. 2018, 24, 551–558. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Fundamentals of resistance training: Progression and exercise prescription. Med. Sci. Sport. Exerc. 2004, 36, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Paul, C. Strength and conditioning: Scientific aspects including principles of rehabilitation. In A Comprehensive Guide to Sports Physiology and Injury Management: An Interdisciplinary Approach; Elsevier: London, UK, 2020; Volume 25. [Google Scholar]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Steele, J.; Bruce-Low, S.; Smith, D. Evidence based resistance training recommendations. Med. Sport. 2011, 15, 147–162. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement from the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.K.; Magnusson, H.; von Schewelov, T.; Rosengren, B.E. Prevention of falls in the elderly—A review. Osteoporos Int. 2013, 24, 747–762. [Google Scholar] [CrossRef]
- American College of Sports Medicine. In ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013.
- Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010.
- Gluchowski, A.; Bilsborough, H.; Mcdermott, J.; Hawley-Hague, H.; Todd, C. ‘A Lot of People Just Go for Walks, and Don’t Do Anything Else’: Older Adults in the UK Are Not Aware of the Strength Component Embedded in the Chief Medical Officers’ Physical Activity Guidelines—A Qualitative Study. Int. J. Environ. Res. Public Health 2022, 19, 10002. [Google Scholar] [CrossRef]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef]
- Zasadzka, E.; Kropińska, S.; Pawlaczyk, M.; Krzymińska-Siemaszko, R.; Lisiński, P.; Wieczorowska-Tobis, K. Effects of inpatient physical therapy on the functional status of elderly individuals. J. Phys. Ther. Sci. 2016, 28, 426–431. [Google Scholar] [CrossRef]
- O’Sullivan Susan, B.; Thomas, J.; Schmitz George, F. Physical Rehabilitation; FA Davis: Duxbury, VT, USA, 2019. [Google Scholar]
- Porter, S. Tidy’s Physiotherapy E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Langhammer, B.; Astrid, B.; Elisabeth, R. The importance of physical activity exercise among older people. BioMed. Res. Int. 2018, 2018, 7856823. [Google Scholar] [CrossRef]
- Edemekong, P.F.; Bomgaars, D.L.; Sukumaran SSchoo, C. Activities of Daily Living. In Treasure Island; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Billot, M.; Calvani, R.; Urtamo, A.; Sánchez-Sánchez, J.L.; Ciccolari-Micaldi, C.; Chang, M.; Roller-Wirnsberger, R.; Wirnsberger, G.; Sinclair, A.; Vaquero-Pinto, N.; et al. Preserving Mobility in Older Adults with Physical Frailty and Sarcopenia: Opportunities, Challenges, and Recommendations for Physical Activity Interventions. Clin. Interv. Aging. 2020, 15, 1675–1690. [Google Scholar] [CrossRef]
- Colibazzi, V.; Coladonato, A.; Zanazzo MRomanini, E. Evidence based rehabilitation after hip arthroplasty. HIP Int. 2020, 30 (Suppl. S2), 20–29. [Google Scholar] [CrossRef] [PubMed]
- Cattagni, T.; Scaglioni, G.; Laroche, D.; Van Hoecke, J.; Gremeaux VMartin, A. Ankle muscle strength discriminates fallers from non-fallers. Front. Aging Neurosci. 2014, 6, 336. [Google Scholar] [CrossRef] [PubMed]
- Arnetz, J.E.; Almin, I.; Bergström, K.; Franzén, Y.; Nilsson, H. Active patient involvement in the establishment of physical therapy goals: Effects on treatment outcome and quality of care. Adv. Physiother. 2004, 6, 50–69. [Google Scholar] [CrossRef]
- Hurst, C.; Robinson, S.M.; Witham, M.D.; Dodds, R.M.; Granic, A.; Buckland, C.; Sayer, A.A. Resistance exercise as a treatment for sarcopenia: Prescription and delivery. Age Ageing 2022, 51, afac003. [Google Scholar] [CrossRef] [PubMed]
- Veronika, S.; Liliana, S.; Ruth, P.; Alison, P. “What do you expect from physiotherapy?”: A detailed analysis of goal setting in physiotherapy. Disabil. Rehabil. 2014, 36, 1679–1686. [Google Scholar] [CrossRef]
- Burgess, E.; Hassmén, P.; Welvaert, M.; Pumpa, K. Behavioural treatment strategies improve adherence to lifestyle intervention programmes in adults with obesity: A systematic review and meta-analysis. Clin. Obes. 2017, 7, 105–114. [Google Scholar] [CrossRef]
- Topp, R.; Frost, K.L. Exercise for the inactive hypertensive patient. Ethn. Dis. 2006, 16 (Suppl. S4), S4–S27. [Google Scholar]
- McGrane, N.; Cusack, T.; O’Donoghue, G.; Stokes, E. Motivational strategies for physiotherapists. Phys. Ther. Rev. 2014, 19, 136–142. [Google Scholar] [CrossRef]
- Biro, E.; Veres-Balajti, I.; Kosa, K. Social support contributes to resilience among physiotherapy students: A cross sectional survey and focus group study. Physiotherapy 2016, 102, 189–195. [Google Scholar] [CrossRef]
- Kunstler, B.E.; Cook, J.L.; Freene, N.; Finch, C.F.; Kemp, J.L.; O’Halloran, P.D.; Gaida, J.E. Physiotherapists use a small number of behaviour change techniques when promoting physical activity: A systematic review comparing experimental and observational studies. J. Sci. Med. Sport 2018, 21, 609–615. [Google Scholar] [CrossRef]
- Young, H.E.; Black Jr, A.C. Adult stem cells. In The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology: An Official Publication of the American Association of Anatomists; John Wiley and Sons: New York, NY, USA, 2004; Volume 276, pp. 75–102. [Google Scholar]
- Alison, M.; Islam, S. Attributes of adult stem cells. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2009, 217, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Wagers, A.J.; Weissman, I.L. Plasticity of adult stem cells. Cell 2004, 116, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. What is an adult stem cell? Science 2015, 350, 1319–1320. [Google Scholar] [CrossRef] [PubMed]
- Poulsom, R.; Alison, M.R.; Forbes, S.J.; Wright, N.A. Adult stem cell plasticity. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2002, 197, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, S.; Garroni, G.; Ventura, C.; Danani, A.; Nečas, A.; Maioli, M. Stem cells and physical energies: Can we really drive stem cell fate? Physiol. Res. 2019, 68, S375–S384. [Google Scholar] [CrossRef] [PubMed]
- Charbord, P. Bone marrow mesenchymal stem cells: Historical overview and concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, M.; Schipani, E. Bone marrow mesenchymal stem cells. J. Cell Biochem. 2010, 109, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.E.; Caplan, A.I. Bone marrow mesenchymal stem cells. Stem Cells Handb. 2004, 107–117. [Google Scholar] [CrossRef]
- Miao, Z.; Jin, J.; Chen, L.; Zhu, J.; Huang, W.; Zhao, J.; Qian, H.; Zhang, X. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 2006, 30, 681–687. [Google Scholar] [CrossRef]
- Mills, C. M1 and M2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 6. [Google Scholar] [CrossRef]
- Bai, H.; Kyu-Cheol, N.; Wang, Z.; Cui, Y.; Liu, H.; Liu, H.; Feng, Y.; Zhao, Y.; Lin, Q.; Li, Z. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J. Tissue Eng. 2020, 11, 2041731420947242. [Google Scholar] [CrossRef] [PubMed]
- Nolan, P.; Forest, M. Healthy trees: Do woodlands really make us healthier. Countrys. Recreat. 2005, 13, 1. [Google Scholar]
- Song, C.; Ikei, H.; Miyazaki, Y. Physiological Effects of Nature Therapy: A Review of the Research in Japan. Int. J. Environ. Res. Public Health 2016, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Annerstedt, M.; Währborg, P. Nature-assisted therapy: Systematic review of controlled and observational studies. Scand. J. Public Health 2011, 39, 371–388. [Google Scholar] [CrossRef]
- Roviello, V.; Gilhen-Baker, M.; Vicidomini, C.; Roviello, G.N. Forest-bathing and physical activity as weapons against COVID-19: A review. Environ. Chem. Lett. 2021, 1–10. [Google Scholar] [CrossRef]
- Roviello, V.; Scognamiglio, P.L.; Caruso, U.; Vicidomini, C.; Roviello, G.N. Evaluating In Silico the Potential Health and Environmental Benefits of Houseplant Volatile Organic Compounds for an Emerging ‘Indoor Forest Bathing’Approach. Int. J. Environ. Res. Public Health 2021, 19, 273. [Google Scholar] [CrossRef]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. In silico investigation on the interaction of chiral phytochemicals from opuntia ficus-indica with SARS-CoV-2 Mpro. Symmetry 2021, 13, 1041. [Google Scholar] [CrossRef]
- Kegge, W.; Pierik, R. Biogenic volatile organic compounds and plant competition. Trends Plant Sci. 2010, 15, 126–132. [Google Scholar] [CrossRef]
- Loreto, F.; Dicke, M.; SCHNITZLER, J.P.; Turlings, T.C. Plant volatiles and the environment. Plant Cell Environ. 2014, 37, 1905–1908. [Google Scholar] [CrossRef]
- Iijima, Y. Recent advances in the application of metabolomics to studies of biogenic volatile organic compounds (BVOC) produced by plant. Metabolites 2014, 4, 699–721. [Google Scholar] [CrossRef]
- Šimpraga, M.; Takabayashi, J.; Holopainen, J.K. Language of plants: Where is the word? J. Integr. Plant Biol. 2016, 58, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Ormeño, E.; Goldstein, A.; Niinemets, Ü. Extracting and trapping biogenic volatile organic compounds stored in plant species. TrAC Trends Anal. Chem. 2011, 30, 978–989. [Google Scholar] [CrossRef]
- de Christo Scherer, M.M.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J. Tissue Viability 2019, 28, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Beresford-Kroeger, D. Bioplans for Forest Therapy. In The International Handbook of Forest Therapy; Cambridge Scholars Publishing: Newcastle Upon Tyne, UK, 2019. [Google Scholar]
- Li, Q.; Kawada, T. Effect of forest therapy on the human psycho-neuro-endocrino-immune network. Nihon Eiseigaku Zasshi. Jpn. J. Hyg. 2011, 66, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Borbone, N.; De Marino, S.; Iorizzi, M.; Zollo, F.; Debitus, C.; Esposito, G.; Iuvone, T. Minor steroidal alkaloids from the marine sponge Corticium sp. J. Nat. Prod. 2002, 65, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Marzano, M.; Falanga, A.P.; Marasco, D.; Borbone, N.; D’Errico, S.; Piccialli, G.; Roviello, G.N.; Oliviero, G. Evaluation of an analogue of the marine ε-PLL peptide as a ligand of G-quadruplex DNA structures. Mar. Drugs 2020, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Marasco, D.; Vicidomini, C.; Krupa, P.; Cioffi, F.; Huy, P.D.Q.; Li, M.S.; Florio, D.; Broersen, K.; De Pandis, M.F.; Roviello, G.N. Plant isoquinoline alkaloids as potential neurodrugs: A comparative study of the effects of benzo [c] phenanthridine and berberine-based compounds on β-amyloid aggregation. Chem. Biol. Interact. 2021, 334, 109300. [Google Scholar] [CrossRef]
- Costanzo, V.; Gilhen-Baker, M.; Beresford-Kroeger, D.; Roviello, G.N. Tree-inhabiting polypore fungi as sources of a cornucopia of bioactive compounds. Future Microbiol. 2022, 17, 899–902. [Google Scholar] [CrossRef]
- Zjawiony, J.K. Biologically active compounds from Aphyllophorales (polypore) fungi. J. Nat. Prod. 2004, 67, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Roviello, V.; Gilhen-Baker, M.; Roviello, G.N.; Lichtfouse, E. River therapy. Environ. Chem. Lett. 2022, 20, 2729–2734. [Google Scholar] [CrossRef]
- Roviello, V.; Gilhen-Baker, M.; Vicidomini, C.; Roviello, G.N. The Healing Power of Clean Rivers: In Silico Evaluation of the Antipsoriatic Potential of Apiin and Hyperoside Plant Metabolites Contained in River Waters. Int. J. Environ. Res. Public Health 2022, 19, 5. [Google Scholar] [CrossRef] [PubMed]

| Compound | Molecular Weight [g/mol] | Solubility in Water [mg/L] | Vapor Pressure [mmHg] |
|---|---|---|---|
| terpinolene | 136.23 | 9.5 | 0.74 |
| α-phellandrene | 136.23 | insoluble | 1.4 |
| Compound | Antioxidant Activity (IC50 μM) 1 ± SD 2 | Stimulatory Effect at 200 μM on L929 Fibroblasts (%) ± SD 2 |
|---|---|---|
| terpinolene | 409.4 ± 1.6 | 36.3 ± 4.8 |
| α-phellandrene | 216.9 ± 5.7 | 39.1 ± 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, S.; Gilhen-Baker, M.; Roviello, G.N. The Role of Nutrition and Forest-Bathing in the Physical Rehabilitation of Physically Inactive Patients: From the Molecular Aspects to New Nature-Inspired Techniques. Int. J. Environ. Res. Public Health 2023, 20, 793. https://doi.org/10.3390/ijerph20010793
Baker S, Gilhen-Baker M, Roviello GN. The Role of Nutrition and Forest-Bathing in the Physical Rehabilitation of Physically Inactive Patients: From the Molecular Aspects to New Nature-Inspired Techniques. International Journal of Environmental Research and Public Health. 2023; 20(1):793. https://doi.org/10.3390/ijerph20010793
Chicago/Turabian StyleBaker, Steven, Melinda Gilhen-Baker, and Giovanni N. Roviello. 2023. "The Role of Nutrition and Forest-Bathing in the Physical Rehabilitation of Physically Inactive Patients: From the Molecular Aspects to New Nature-Inspired Techniques" International Journal of Environmental Research and Public Health 20, no. 1: 793. https://doi.org/10.3390/ijerph20010793
APA StyleBaker, S., Gilhen-Baker, M., & Roviello, G. N. (2023). The Role of Nutrition and Forest-Bathing in the Physical Rehabilitation of Physically Inactive Patients: From the Molecular Aspects to New Nature-Inspired Techniques. International Journal of Environmental Research and Public Health, 20(1), 793. https://doi.org/10.3390/ijerph20010793








