Bridging the Gap between Research and the Community: Implementing Physical and Cognitive Interventions to Improve Spontaneous Walking Speed in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
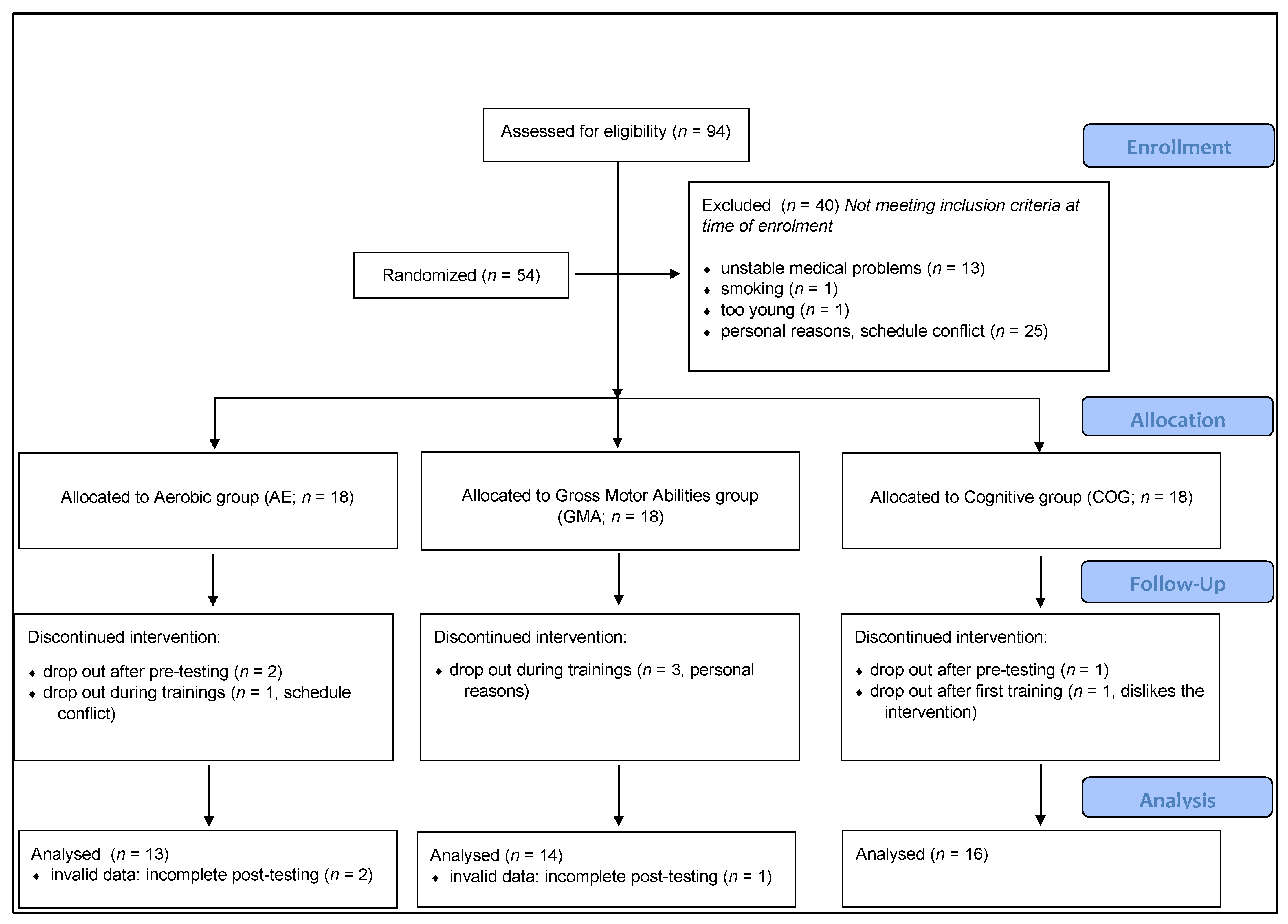
2.2. Participants
2.3. Intervention
2.3.1. Aerobic Intervention
2.3.2. Gross Motor Abilities Intervention
2.3.3. Cognitive Intervention
- -
- Dual-Task to train divided attention: The DT paradigm involved performing two discrimination tasks alone or concurrently. Participants had to answer as fast as possible, while making as few errors as possible, to one or two stimuli (fruits vs. modes of transport, letters vs. numbers or sounds vs. beeps) appearing in the center of the tablet by pressing on the appropriate button of a digit keyboard with their left and/or right thumbs. Stimuli were presented visually or orally, since each participant had headphones. After two training sessions, participants were asked to prioritize one hand over the other, depending on the trials, in order to increase the level of difficulty.
- -
- Stroop task to train inhibition and switching: The digit modified Stroop task consisted of four different conditions. First, in the Reading condition, digits from one to six were presented in small identical groups corresponding to their numerical values (e.g., four copies of the digit “4”) and participants had to press the corresponding digit (“1” to “3” with their left thumb on the keyboard; “4” to “6” with their right thumb on the keyboard) as fast as possible while making as few errors as possible. In the Counting condition, groups of one to six asterisks were presented and the participants had to report how many asterisks were present. In the Inhibition condition, digits were presented in small identical groups and the digits presented were incompatible with the number of digits presented (e.g., five copies of the digit “4”). Participants were asked to count how many digits were presented, and avoid reporting the identity of the digit. Finally, stimuli in the Switching condition were identical to those of the Inhibition condition, except that, for one random trial out of each sequence of five trials, the group of digits was surrounded by a white frame, indicating that, for those trials only, participants had to report the identity instead of the quantity of digit(s). To manipulate the level of difficulty in this task, stimuli and their position on the screen were often changed along the training weeks.
- -
- N-back task to train updating: The n-back task is a continuous cognitive task. Stimuli (digits from “1” to “9”) were presented sequentially and participants had to indicate if the current digit matched the one from n steps earlier in the sequence. Digits were presented visually, on the screen, and were also heard in each participant’s headphone as a voice spoke each digit as they were displayed. For the present study, the load factor n could vary from one to three. Two response buttons were displayed on the right side of the keyboard. The one above was for the response “is the same” and the one bellow was for “is different”. Only the right thumb was used for this task. During the first month, only 1- and 2-back were presented. At the beginning of the second month, 3-back was incorporated and for the third month of training, only 2- and 3-back were administered.
2.4. Feasibility Measures
2.4.1. Adherence
2.4.2. Participants’ Feedback
2.4.3. Long-Term Participation
2.5. Mobility Measures
2.6. Statistics
3. Results
3.1. Baseline Data
3.2. Feasibility
3.2.1. Adherence
3.2.2. Participants’ Feedback
3.2.3. Long-Term Participation
3.3. Mobility Measures
3.3.1. SWS
3.3.2. TUG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergland, A.; Jørgensen, L.; Emaus, N.; Strand, B.H. Mobility as a predictor of all-cause mortality in older men and women: 11.8 year follow-up in the Tromsø study. BMC Health Serv. Res. 2017, 17, 22. [Google Scholar] [CrossRef]
- Fritz, S.; Lusardi, M. White paper: ‘Walking speed: The sixth vital sign’. J. Geriatr. Phys. Ther. 2009, 32, 2–5. [Google Scholar] [CrossRef]
- Abellan Van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette, G.S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.E.; Perera, S.; Roumani, Y.F.; Chandler, J.M.; Studenski, S.A. Improvement in usual gait speed predicts better survival in older adults. J. Am. Geriatr. Soc. 2007, 55, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
- Levin, O.; Netz, Y.; Ziv, G. The beneficial effects of different types of exercise interventions on motor and cognitive functions in older age: A systematic review. Eur. Rev. Aging Phys. Act. 2017, 14, 20. [Google Scholar] [CrossRef]
- Henderson, R.M.; Leng, X.; Chmelo, E.A.; Brinkley, T.E.; Lyles, M.F.; Marsh, A.P.; Nicklas, B.J. Gait speed response to aerobic versus resistance exercise training in older adults. Aging Clin. Exp. Res. 2017, 29, 969–976. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Lesinski, M.; Gäbler, M.; VanSwearingen, J.M.; Malatesta, D.; Granacher, U. Effects of three types of exercise interventions on healthy old adults’ gait speed: A systematic review and meta-analysis. Sport. Med. 2015, 45, 1627–1643. [Google Scholar] [CrossRef]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 329–342. [Google Scholar] [CrossRef]
- Demnitz, N.; Esser, P.; Dawes, H.; Valkanova, V.; Johansen-Berg, H.; Ebmeier, K.P.; Sexton, C. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture 2016, 50, 164–174. [Google Scholar] [CrossRef]
- Bherer, L. Cognitive plasticity in older adults: Effects of cognitive training and physical exercise. Ann. N. Y. Acad. Sci. 2015, 1337, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marusic, U.; Verghese, J.; Mahoney, J.R. Cognitive-based interventions to improve mobility: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2018, 19, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Li, K.Z.; Roudaia, E.; Lussier, M.; Bherer, L.; Leroux, A.; McKinley, P.A. Benefits of cognitive dual-task training on balance performance in healthy older adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2010, 65, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Pothier, K.; Gagnon, C.; Fraser, S.A.; Lussier, M.; Desjardins-Crépeau, L.; Berryman, N.; Kergoat, M.J.; Vu, T.T.M.; Li, K.Z.H.; Bosquet, L.; et al. A comparison of the impact of physical exercise, cognitive training and combined intervention on spontaneous walking speed in older adults. Aging Clin. Exp. Res. 2018, 30, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Pothier, K.; Vrinceanu, T.; Intzandt, B.; Bosquet, L.; Karelis, A.D.; Lussier, M.; Vu, T.T.M.; Nigam, A.; Li, K.Z.H.; Berryman, N.; et al. A comparison of physical exercise and cognitive training interventions to improve determinants of functional mobility in healthy older adults. Exp. Gerontol. 2021, 149, 111331. [Google Scholar] [CrossRef]
- Manas, A.; Gómez-Redondo, P.; Valenzuela, P.L.; Morales, J.S.; Lucía, A.; Ara, I. Unsupervised home-based resistance training for community-dwelling older adults: A systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021, 69, 101368. [Google Scholar] [CrossRef]
- Waters, L.A.; Galichet, B.; Owen, N.; Eakin, E. Who participates in physical activity intervention trials? J. Phys. Act. Health 2011, 8, 85–103. [Google Scholar] [CrossRef]
- Trost, S.G.; Owen, N.; Bauman, A.E.; Sallis, J.F.; Brown, W. Correlates of adults’ participation in physical activity: Review and update. Med. Sci. Sport. Exerc. 2002, 34, 1996–2001. [Google Scholar] [CrossRef]
- Marusic, U.; Verghese, J.; Mahoney, J.R. Does cognitive training improve mobility, enhance cognition, and promote neural activation? Front. Aging Neurosci. 2022, 14, 845825. [Google Scholar] [CrossRef]
- Brahms, C.M.; Hortobágyi, T.; Kressig, R.W.; Granacher, U. The interaction between mobility status and exercise specificity in older adults. Exerc. Sport Sci. Rev. 2021, 49, 15–22. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A. Geriatric depression scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar] [PubMed]
- Bredin, S.S.; Gledhill, N.; Jamnik, V.K.; Warburton, D.E. PAR-Q+ and ePARmed-X+. Can. Fam. Physician 2013, 59, 273–277. [Google Scholar] [PubMed]
- Weschler, D. Administration and Scoring Manual. In Wechsler Adult Intelligence Scale, 3rd ed.; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Berryman, N.; Bherer, L.; Nadeau, S.; Lauzière, S.; Lehr, L.; Bobeuf, F.; Lussier, M.; Kergoat, M.J.; Vu, T.T.M.; Bosquet, L. Multiple roads lead to Rome: Combined high-intensity aerobic and strength training vs. gross motor activities leads to equivalent improvement in executive functions in a cohort of healthy older adults. Age 2014, 36, 9710. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Lussier, M.; Saillant, K.; Vrinceanu, T.; Hudon, C.; Bherer, L. Normative data for a tablet-based dual-task assessment in healthy older adults. Arch. Clin. Neuropsychol. 2021, 36, 1316–1325. [Google Scholar] [CrossRef]
- Pothier, K.; Soriano, G.; Lussier, M.; Naudin, A.; Costa, N.; Guyonnet, S.; Piau, A.; Ousset, P.J.; Nourhashemi, F.; Vellas, B.; et al. A web-based multidomain lifestyle intervention with connected devices for older adults: Research protocol of the eMIND pilot randomized controlled trial. Aging Clin. Exp. Res. 2018, 30, 1127–1135. [Google Scholar] [CrossRef]
- de Souto Barreto, P.; Pothier, K.; Soriano, G.; Lussier, M.; Bherer, L.; Guyonnet, S.; Piau, A.; Ousset, P.J.; Vellas, B. A web-based multidomain lifestyle intervention for older adults: The eMIND randomized controlled trial. J. Prev. Alzheimer’s Dis. 2021, 8, 142–150. [Google Scholar] [CrossRef]
- Graham, J.E.; Ostir, G.V.; Fisher, S.R.; Ottenbacher, K.J. Assessing walking speed in clinical research: A systematic review. J. Eval. Clin. Pract. 2008, 14, 552–562. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Dupuy, O.; Lussier, M.; Fraser, S.; Bherer, L.; Audiffren, M.; Bosquet, L. Effect of overreaching on cognitive performance and related cardiac autonomic control. Scand. J. Med. Sci. Sport. 2014, 24, 234–242. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, MI, USA, 1988. [Google Scholar]
- Himann, J.E.; Cunningham, D.A.; Rechnitzer, P.A.; Paterson, D.H. Age-related changes in speed of walking. Med. Sci. Sport. Exerc. 1988, 20, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Van Abbema, R.; De Greef, M.; Crajé, C.; Krijnen, W.; Hobbelen, H.; Van Der Schans, C. What type, or combination of exercise can improve preferred gait speed in older adults? A meta-analysis. BMC Geriatr. 2015, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Schrack, J.A.; Simonsick, E.M.; Ferrucci, L. The relationship of the energetic cost of slow walking and peak energy expenditure to gait speed in mid-to-late life. Am. J. Phys. Med. Rehabil./Assoc. Acad. Physiatr. 2013, 92, 28. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Andrews, A.W. Normal walking speed: A descriptive meta-analysis. Physiotherapy 2011, 97, 182–189. [Google Scholar] [CrossRef]
- Middleton, A.; Fritz, S.L.; Lusardi, M. Walking speed: The functional vital sign. J. Aging Phys. Act. 2015, 23, 314–322. [Google Scholar] [CrossRef]
- Stillman, C.M.; Cohen, J.; Lehman, M.E.; Erickson, K.I. Mediators of physical activity on neurocognitive function: A review at multiple levels of analysis. Front. Hum. Neurosci. 2016, 10, 626. [Google Scholar] [CrossRef]
- Dunstan, D.W.; Vulikh, E.; Owen, N.; Jolley, D.; Shaw, J.; Zimmet, P. Community center–based resistance training for the maintenance of glycemic control in adults with type 2 diabetes. Diabetes Care 2006, 29, 2586–2591. [Google Scholar] [CrossRef][Green Version]
- Grysztar, M.; Duplaga, M.; Wojcik, S.; Rodzinka, M. Effectiveness of peer-led health promotion interventions addressed to the elderly: Systematic review. Eur. J. Public Health 2017, 27, ckx186.036. [Google Scholar] [CrossRef]
| AE | GMA | COG | F or X2 | p | |
|---|---|---|---|---|---|
| n | 13 | 14 | 16 | - | - |
| Age | 67 (4.69) | 68.93 (5.90) | 68.19 (4.52) | 0.497 | 0.61 |
| Sex (women/men) | 8/5 | 10/4 | 10/6 | 0.367 | 0.83 |
| Education (number of years) | 13.85 (3.36) | 12.57 (2.73) | 14.81 (2.89) | 2.101 | 0.14 |
| BMI (kg/m−2) | 27.16 (4.02) | 26.79 (3.83) | 29.04 (5.64) | 1.020 | 0.37 |
| GDS | 2.46 (3.12) | 2.00 (2.11) | 3.69 (4.06) | 1.089 | 0.35 |
| MMSE (/30) | 28.54 (1.56) | 28.50 (1.65) | 28.75 (1.18) | 0.128 | 0.88 |
| Digit span (forward + backward) | 16.92 (4.07) | 17.00 (3.84) | 16.69 (4.39) | 0.024 | 0.98 |
| Similarities | 20.08 (4.90) | 20.50 (5.98) | 22.00 (3.52) | 0.646 | 0.53 |
| DSST | 64.15 (9.60) | 56.86 (16.93) | 62.94 (1.75) | 1.295 | 0.28 |
| Baseline Mean (SD) | 12 Weeks Mean (SD) | Mean Change (SD) [95% CI] | Hedges’ g | |
|---|---|---|---|---|
| Spontaneous Walking Speed (m.s−1) | ||||
| AE | 1.39 (0.09) | 1.45 (0.14) | 0.06 * (0.13) [−0.01, 0.14] | 0.500 |
| GMA | 1.48 (0.26) # | 1.48 (0.21) | 0.00 (0.13) [−0.07, 0.08] | 0.009 |
| COG | 1.28 (0.20) | 1.38 (0.16) | 0.10 * (0.11) [0.04, 0.16] | 0.505 |
| Timed-Up and Go Test (s) | ||||
| AE | 7.88 (1.10) | 7.72 (0.88) | −0.26 (0.51) [−0.57, 0.05] | −0.226 |
| GMA | 7.70 (2.16) | 8.02 (1.50) | 0.27 (1.46) [−0.61, 1.16] | 0.121 |
| COG | 7.99 (1.32) | 8.24 (1.38) | 0.25 (0.92) [−0.24, 0.73] | 0.174 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pothier, K.; Kaushal, N.; Vrinceanu, T.; Lussier, M.; Bailly, N.; Comte, F.; Vu, T.T.M.; Berryman, N.; Bherer, L. Bridging the Gap between Research and the Community: Implementing Physical and Cognitive Interventions to Improve Spontaneous Walking Speed in Older Adults. Int. J. Environ. Res. Public Health 2023, 20, 762. https://doi.org/10.3390/ijerph20010762
Pothier K, Kaushal N, Vrinceanu T, Lussier M, Bailly N, Comte F, Vu TTM, Berryman N, Bherer L. Bridging the Gap between Research and the Community: Implementing Physical and Cognitive Interventions to Improve Spontaneous Walking Speed in Older Adults. International Journal of Environmental Research and Public Health. 2023; 20(1):762. https://doi.org/10.3390/ijerph20010762
Chicago/Turabian StylePothier, Kristell, Navin Kaushal, Tudor Vrinceanu, Maxime Lussier, Nathalie Bailly, Francis Comte, Thien Tuong Minh Vu, Nicolas Berryman, and Louis Bherer. 2023. "Bridging the Gap between Research and the Community: Implementing Physical and Cognitive Interventions to Improve Spontaneous Walking Speed in Older Adults" International Journal of Environmental Research and Public Health 20, no. 1: 762. https://doi.org/10.3390/ijerph20010762
APA StylePothier, K., Kaushal, N., Vrinceanu, T., Lussier, M., Bailly, N., Comte, F., Vu, T. T. M., Berryman, N., & Bherer, L. (2023). Bridging the Gap between Research and the Community: Implementing Physical and Cognitive Interventions to Improve Spontaneous Walking Speed in Older Adults. International Journal of Environmental Research and Public Health, 20(1), 762. https://doi.org/10.3390/ijerph20010762






