Adsorption Characteristics of Phosphate Based on Al-Doped Waste Ceramsite: Batch and Column Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ceramsite
2.3. Experimental Setup
2.4. Experimental Procedure
2.4.1. Static Adsorption of PO43−-P by Al-Ceramsite
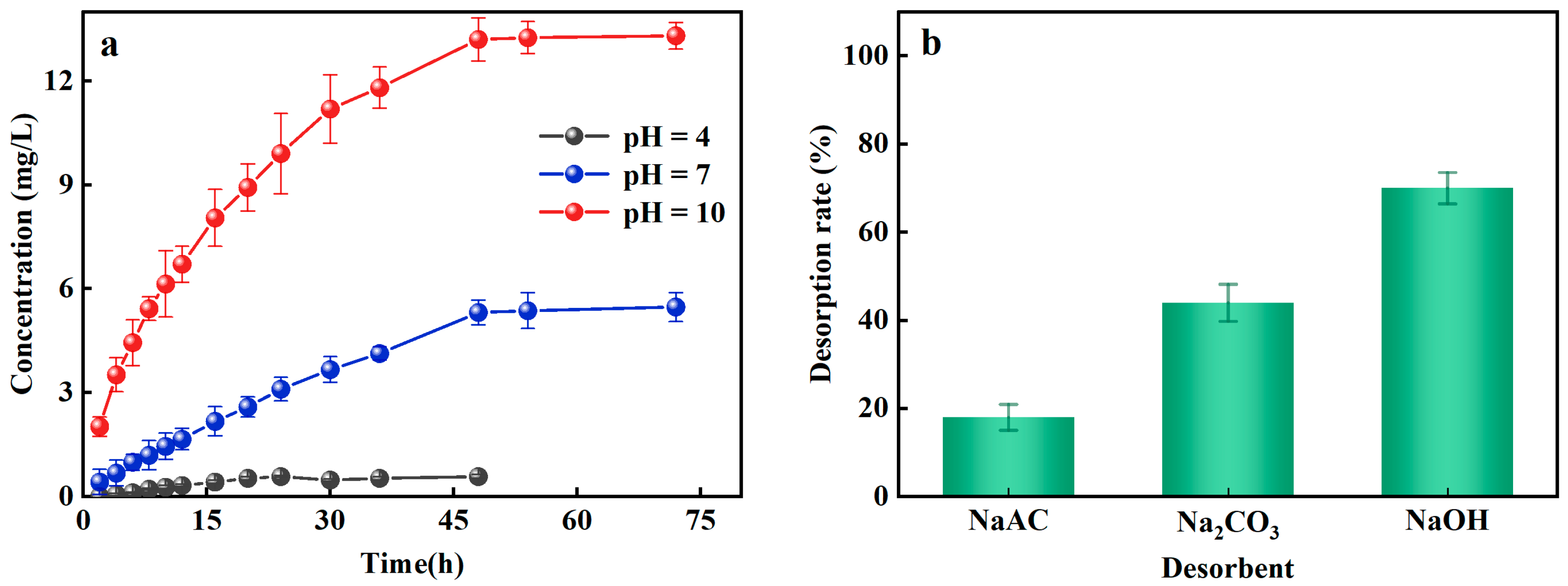
2.4.2. Effect of Phosphorus Desorption
2.4.3. PO43−-P Removal by Dynamic Adsorption Column
2.5. Analytical Methods
3. Results and Discussion
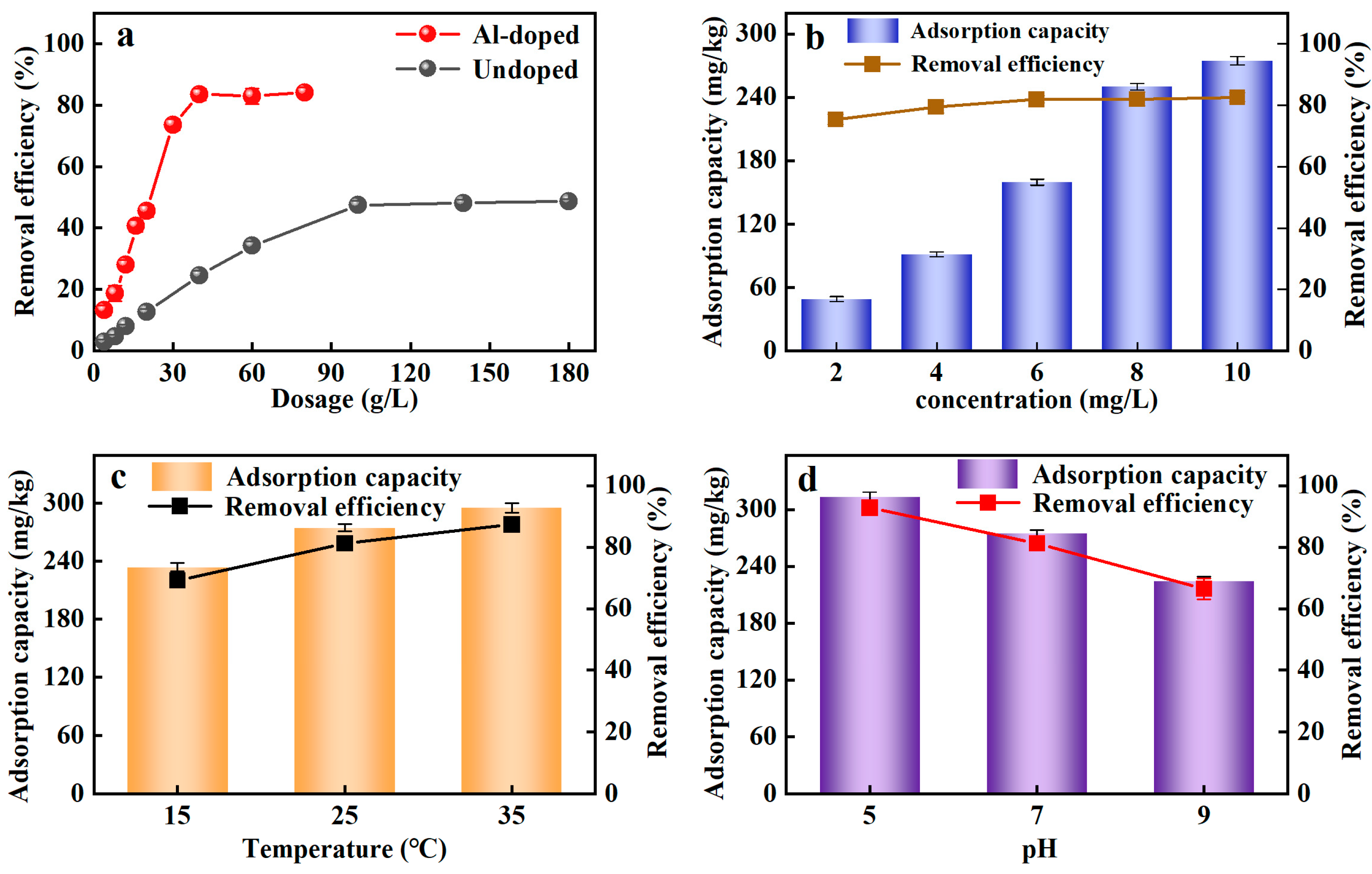
3.1. Optimization of Adsorption Conditions
3.1.1. Advantages of Al-Ceramsite
3.1.2. Effect of PO43−-P Concentration
3.1.3. Effect of Temperature
3.1.4. Effect of pH
3.2. Effect of Coexisting Ions on PO43−-P Removal
3.3. Desorption Characteristics of Al-Ceramsite
3.4. PO43−-P Removal by Dynamic Adsorption of Al-Ceramsite
3.4.1. Effect of Submerged Zone Depth on the Column
3.4.2. Effect of Filter Media Height on the Column
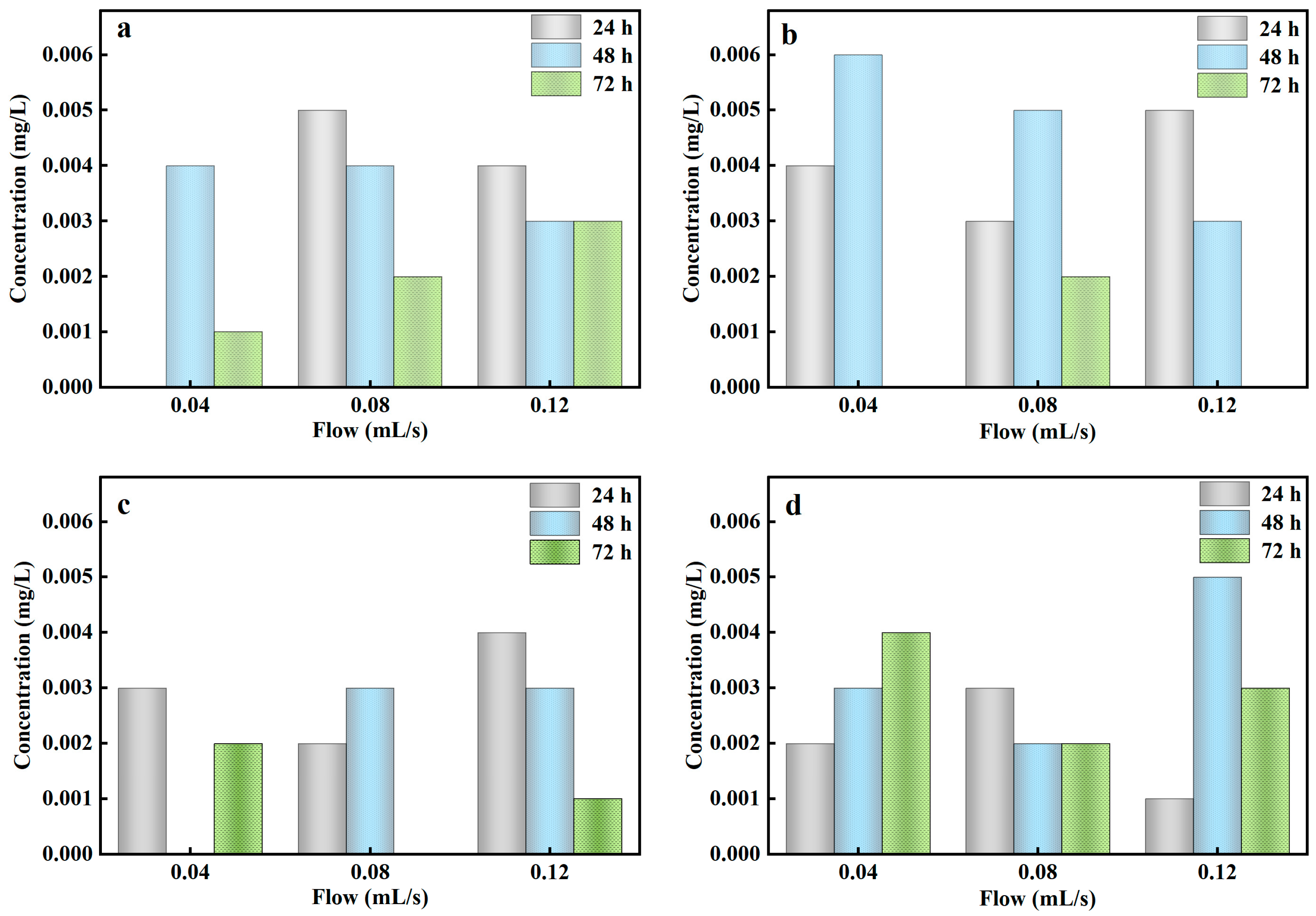
3.4.3. Effect of Influent Flow in the Biofilter
3.4.4. Effect of Antecedent Dry Day on the Column
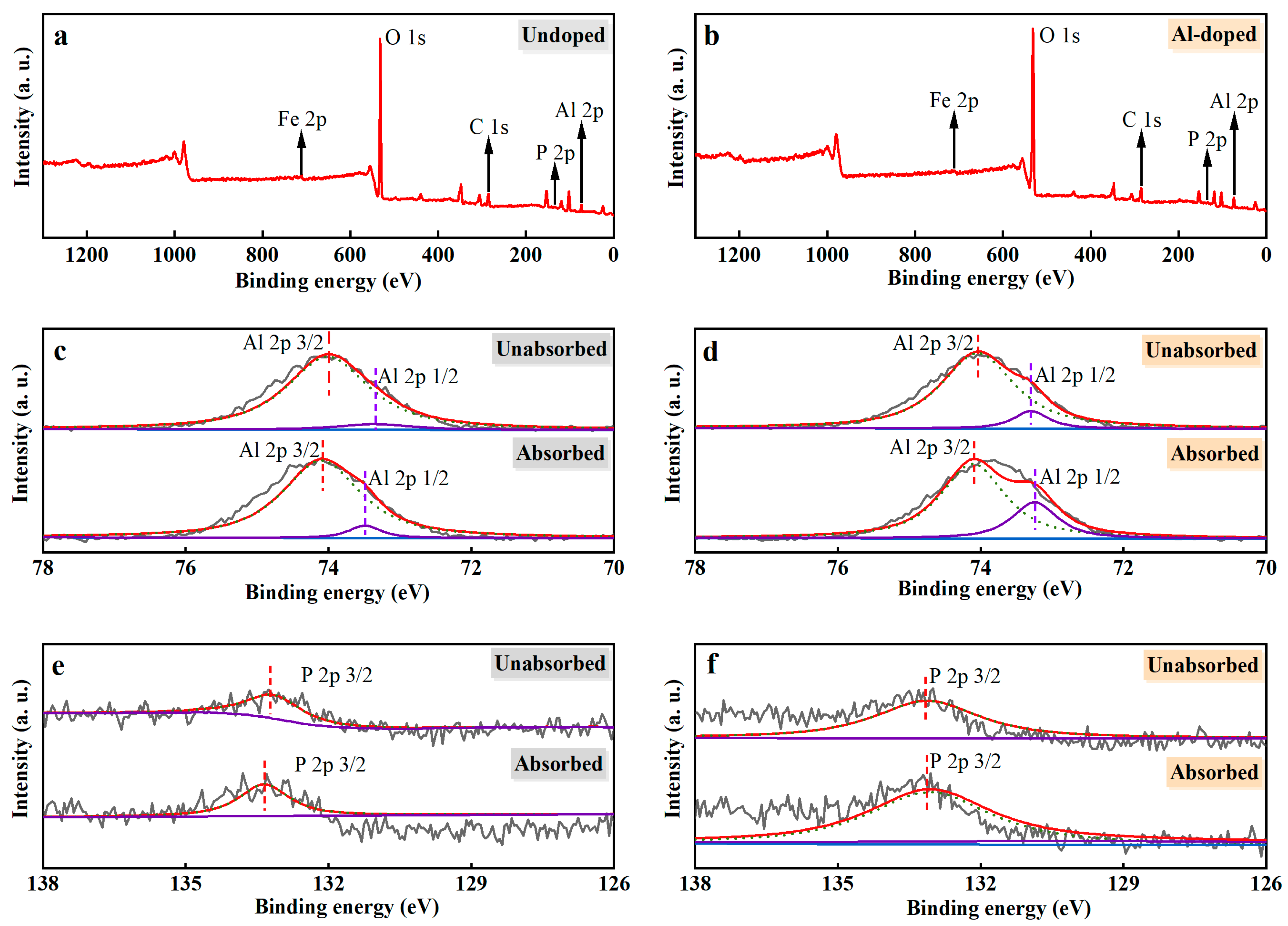
3.5. XPS of Ceramsite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, D.; Hale, L.; Kar, G.; Soolanayakanahally, R.; Adl, S. Phosphorus recovery and reuse by pyrolysis: Applications for agriculture and environment. Chemosphere 2018, 194, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ouyang, X.; Shen, J.; Li, Y.; Sun, W.; Jiang, W.; Wu, J. Nitrogen and phosphorus runoff losses were influenced by chemical fertilization but not by pesticide application in a double rice-cropping system in the subtropical hilly region of China. Sci. Total Environ. 2020, 715, 136852. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Tong, J.; Yang, Z.; Zeng, G.; Zhou, Y.; Wang, D.; Song, P.; Xu, R.; Zhang, C.; Cheng, M. Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: Performance and mechanism. J. Colloid Interface Sci. 2017, 493, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Tian, Y.; Wang, Y.; Li, N.; Liu, Y.; Pang, Z.; Huang, X.; Li, M.; Zhang, J.; Zuo, W. Periclase-induced generation of flowerlike clay-based layered double hydroxides: A highly efficient phosphate scavenger and solid-phase fertilizer. Chem. Eng. J. 2019, 359, 902–913. [Google Scholar] [CrossRef]
- Braun, J.C.; Borba, C.E.; Godinho, M.; Perondi, D.; Schontag, J.M.; Wenzel, B.M. Phosphorus adsorption in Fe-loaded activated carbon: Two-site monolayer equilibrium model and phenomenological kinetic description. Chem. Eng. J. 2019, 361, 751–763. [Google Scholar] [CrossRef]
- Zhan, Y.; Yu, Y.; Lin, J.; Wu, X.; Wang, Y.; Zhao, Y. Simultaneous control of nitrogen and phosphorus release from sediments using iron-modified zeolite as capping and amendment materials. J. Environ. Manage. 2019, 249, 109369. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Sun, Y.; Chen, Y.; Qian, J. La(OH)3 loaded magnetic mesoporous nanospheres with highly efficient phosphate removal properties and superior pH stability. Chem. Eng. J. 2019, 360, 342–348. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Qiao, S.; Zhou, J. Magnetic Fe0/iron oxide-coated diatomite as a highly efficient adsorbent for recovering phosphorus from water. Chem. Eng. J. 2021, 412, 128696. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, Y.; Sheng, L. Preparation of ceramsite from municipal sludge and its application in water treatment: A review. J. Environ. Manage. 2021, 287, 112374. [Google Scholar] [CrossRef]
- Zheng, X.; Jin, M.; Xu, H.; Chen, W.; Zhang, Y.; Yang, M.; Shao, X.; Xu, Z.; Wang, W. Enhanced simultaneous nitrogen and phosphorus removal in a denitrifying biological filter using waterworks sludge ceramsite coupled with iron-carbon. Int. J. Env. Res. Public Health 2019, 16, 2646. [Google Scholar] [CrossRef]
- Li, T.; Sun, T.; Li, D. Preparation, sintering behavior, and expansion performance of ceramsite filter media from dewatered sewage sludge, coal fly ash, and river sediment. J. Mater. Cycles. Waste Manage. 2018, 20, 71–79. [Google Scholar] [CrossRef]
- Lian, F.; Gao, S.; Fu, Q.; Wu, Y.; Wang, J.; Huang, Q.; Hu, S. A comprehensive study of phosphorus removal and recovery with a Fe-loaded sulfoaluminate cement (FSC) adsorbent. J. Water Process Eng. 2021, 39, 101744. [Google Scholar] [CrossRef]
- Li, X.; Ji, M.; Nghiem, L.D.; Zhao, Y.; Liu, D.; Yang, Y.; Wang, Q.; Trinh, Q.T.; Vo, D.V.N.; Pham, V.Q. A novel red mud adsorbent for phosphorus and diclofenac removal from wastewater. J. Mol. Liq. 2020, 303, 112286. [Google Scholar] [CrossRef]
- Liu, X.; Yang, S.; Liu, S.; Yang, Y. Performance and mechanism of phosphorus removal by slag ceramsite filler. Process Saf. Environ. Prot. 2021, 148, 858–866. [Google Scholar] [CrossRef]
- Lin, J.Y.; Li, D.; Kim, M.; Lee, I.; Kim, H.; Huang, C.P. Process optimization for the synthesis of ceramsites in terms of mechanical strength and phosphate adsorption capacity. Chemosphere 2021, 278, 130239. [Google Scholar] [CrossRef]
- Gu, S.; Fu, B.; Ahn, J.W.; Fang, B. Mechanism for phosphorus removal from wastewater with fly ash of municipal solid waste incineration, Seoul, Korea. J. Clean. Prod. 2021, 280, 124430. [Google Scholar] [CrossRef]
- Liu, D.; Quan, X.; Zhu, H.; Huang, Q.; Zhou, L. Evaluation of modified waste concrete powder used as a novel phosphorus remover. J. Clean. Prod. 2020, 257, 120646. [Google Scholar] [CrossRef]
- Tam, V.W.; Soomro, M.; Evangelista, A.C.J. A review of recycled aggregate in concrete applications (2000–2017). Constr. Build. Mater. 2018, 172, 272–292. [Google Scholar] [CrossRef]
- Liu, S.; Yang, C.; Liu, W.; Yi, L.; Qin, W. A novel approach to preparing ultra-lightweight ceramsite with a large amount of fly ash. Front. Env. Sci. Eng. 2020, 14, 62. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Meng, Y.; Aihemaiti, A.; Xu, Y.; Xiang, H.; Gao, Y.; Chen, X. Preparation, environmental application and prospect of biochar-supported metal nanoparticles: A review. J. Hazard. Mater. 2020, 388, 122026. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhang, D.; Zheng, X.; Ye, X.; Niu, X.; Lin, Z.; Fu, M.; Zhou, S. Adsorption recovery of phosphate from waste streams by Ca/Mg-biochar synthesis from marble waste, calcium-rich sepiolite and bagasse. J. Clean. Prod. 2021, 288, 125638. [Google Scholar] [CrossRef]
- Xing, Y.; Huang, X.; Yu, J.; Gong, C.; Zhang, C. Removal of phosphorus from wastewater by metal salt doping waste-based ceramsite. Desalin. Water Treat. 2022, 272, 126–137. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Water Works Association and Water Environmental Federation: Washington, DC, USA, 2005. [Google Scholar]
- Shao, Q.; Zhang, Y.; Liu, Z.; Long, L.; Liu, Z.; Chen, Y.; Hu, X.M.; Lu, M.; Huang, L.Z. Phosphorus and nitrogen recovery from wastewater by ceramsite: Adsorption mechanism, plant cultivation and sustainability analysis. Sci. Total Environ. 2022, 805, 150288. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, J.; Rong, H.; Zhou, X.; Chen, F.; Li, X.; Wang, T.; Hou, H. Adsorption mechanism of lead ions on porous ceramsite prepared by co-combustion ash of sewage sludge and biomass. Sci. Total Environ. 2020, 702, 135017. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Li, Q.; Su, Z.; Sheng, S.; Fu, J. Preparation, optimization, and application of sustainable ceramsite substrate from coal fly ash/waterworks sludge/oyster shell for phosphorus immobilization in constructed wetlands. J. Clean. Prod. 2018, 175, 572–581. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Q.; Gao, S.; Poon, C.S.; Zhou, Y.; Li, J.S. Novel recycling of incinerated sewage sludge ash (ISSA) and waste bentonite as ceramsite for Pb-containing wastewater treatment: Performance and mechanism. J. Environ. Manage. 2021, 288, 112382. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, X.; Xu, Z.; Hu, W.; Zhou, Y.; Wan, Z.; Yang, Y.; Wei, Y.; Yang, J.; Tsang, D.C. Fabrication of sustainable manganese ferrite modified biochar from vinasse for enhanced adsorption of fluoroquinolone antibiotics: Effects and mechanisms. Sci. Total Environ. 2020, 709, 136079. [Google Scholar] [CrossRef]
- Wang, D.; Hu, W.; Chen, N.; Yu, Y.; Tian, C.; Feng, C. Removal of phosphorus from aqueous solutions by granular mesoporous ceramic adsorbent based on Hangjin clay. Desalin. Water Treat. 2016, 57, 22400–22412. [Google Scholar] [CrossRef]
- Cusack, P.B.; Healy, M.G.; Ryan, P.C.; Burke, I.T.; O’donoghue, L.M.; Ujaczki, É.; Courtney, R. Enhancement of bauxite residue as a low-cost adsorbent for phosphorus in aqueous solution, using seawater and gypsum treatments. J. Clean. Prod. 2018, 179, 217–224. [Google Scholar] [CrossRef]
- Zhou, L.; Li, N.; Owens, G.; Chen, Z. Simultaneous removal of mixed contaminants, copper and norfloxacin, from aqueous solution by ZIF-8. Chem. Eng. J. 2019, 362, 628–637. [Google Scholar] [CrossRef]
- Zhang, H.; Khanal, S.K.; Jia, Y.; Song, S.; Lu, H. Fundamental insights into ciprofloxacin adsorption by sulfate-reducing bacteria sludge: Mechanisms and thermodynamics. Chem. Eng. J. 2019, 378, 122103. [Google Scholar] [CrossRef]
- Lundehøj, L.; Jensen, H.C.; Wybrandt, L.; Nielsen, U.G.; Christensen, M.L.; Quist-Jensen, C.A. Layered double hydroxides for phosphorus recovery from acidified and non-acidified dewatered sludge. Water Res. 2019, 153, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Hu, F.; Zhang, T.; Qiu, F.; Dai, H. Amine-functionalized magnetic bamboo-based activated carbon adsorptive removal of ciprofloxacin and norfloxacin: A batch and fixed-bed column study. Bioresource Technol. 2018, 249, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Saroha, A.K. Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total Environ. 2017, 578, 16–33. [Google Scholar] [CrossRef]
- Niu, J.; Ding, P.; Jia, X.; Hu, G.; Li, Z. Study of the properties and mechanism of deep reduction and efficient adsorption of Cr (VI) by low-cost Fe3O4-modified ceramsite. Sci. Total Environ. 2019, 688, 994–1004. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Kochkodan, V.; Subeh, M.; Mckay, G.; Atieh, M.; Al-Ansari, T. Phosphate removal from synthetic and treated sewage effluent by carbide derive carbon. J. Water Process Eng. 2020, 36, 101323. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, X.; Pan, B.; Zhang, W.; Hua, M.; Lv, L.; Zhang, W. Preferable removal of phosphate from water using hydrous zirconium oxide-based nanocomposite of high stability. J. Hazard. Mater. 2015, 284, 35–42. [Google Scholar] [CrossRef]
- Liu, J.; Cao, J.; Hu, Y.; Han, Y.; Zhou, J. Adsorption of phosphate ions from aqueous solutions by a CeO2 functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial. Water. Sci. Technol. 2017, 76, 2867–2875. [Google Scholar] [CrossRef]
- Shin, E.W.; Han, J.S.; Jang, M.; Min, S.H.; Park, J.K.; Rowell, R.M. Phosphate adsorption on aluminum-impregnated mesoporous silicates: Surface structure and behavior of adsorbents. Environ. Sci. Technol. 2004, 38, 912–917. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Deletic, A.; Mccarthy, D.T.; Hatt, B.E.; Payne, E.G.; Chandrasena, G.; Li, Y.; Pham, T.; Jamali, B. The impact of stormwater biofilter design and operational variables on nutrient removal-a statistical modelling approach. Water Res. 2021, 188, 116486. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wei, D.; Hu, J.; Zhang, J.; Liu, Z.; Li, A.; Li, R. Glyphosate and nutrients removal from simulated agricultural runoff in a pilot pyrrhotite constructed wetland. Water Res. 2020, 168, 115154. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Zou, L.; Zhao, X.; Xu, Y.; Xu, F.; Li, Y.; Wang, Y. Characterization and properties of iron oxide-coated zeolite as adsorbent for removal of copper(II) from solution in fixed bed column. Chem. Eng. J. 2009, 149, 123–131. [Google Scholar] [CrossRef]
- Chandrasena, G.I.; Pham, T.; Payne, E.G.; Deletic, A.; Mccarthy, D.T.E. E. coli removal in laboratory scale stormwater biofilters: Influence of vegetation and submerged zone. J. Hydrol. 2014, 519, 814–822. [Google Scholar] [CrossRef]
- Chandrasena, G.; Deletic, A.; Ellerton, J.; Mccarthy, D.T. Evaluating Escherichia coli removal performance in stormwater biofilters: A laboratory-scale study. Water Sci. Technol. 2012, 66, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Afrooz, A.N.; Boehm, A.B. Effects of submerged zone, media aging, and antecedent dry period on the performance of biochar-amended biofilters in removing fecal indicators and nutrients from natural stormwater. Ecol. Eng. 2017, 102, 320–330. [Google Scholar] [CrossRef]
- Shi, W.; Fu, Y.; Jiang, W.; Ye, Y.; Kang, J.; Liu, D.; Ren, Y.; Li, D.; Luo, C.; Xu, Z. Enhanced phosphate removal by zeolite loaded with Mg-Al-La ternary (hydr) oxides from aqueous solutions: Performance and mechanism. Chem. Eng. J. 2019, 357, 33–44. [Google Scholar] [CrossRef]
- Fang, L.; Liu, R.; Li, J.; Xu, C.; Huang, L.Z.; Wang, D. Magnetite/Lanthanum hydroxide for phosphate sequestration and recovery from lake and the attenuation effects of sediment particles. Water Res. 2018, 130, 243–254. [Google Scholar] [CrossRef]


| Characterization | Un-Ceramsite | Al-Ceramsite |
|---|---|---|
| Particle size (mm) | 4–6 | 4–6 |
| BET surface area (m2/g) | 1.71 | 5.31 |
| Pore volume (cm2/g) | 9.45 × 10−3 | 2.87 × 10−2 |
| Average pore size (nm) | 22.16 | 21.19 |
| Al (wt%) | 7.92 | 15.82 |
| No. Adsorbent | Composition | Dosage (g/L) | Qe (mg/g) | Reference |
|---|---|---|---|---|
| Hangjin clay granular ceramic | Hangjin clay, montmorillonite, corn straw powders | 10 | 5.96 | [30] |
| Waste ceramsite | Coal fly ash, waterworks sludge, oyster shell | 20 | 4.51 | [27] |
| Modified bauxite residue | Gypsum, seawater, bauxite residue | 40 | 0.35 | [31] |
| La-ceramsite | Sewage sludge, coal fly ash, clay | 20 | 0.104 | [25] |
| Al-ceramsite | Sludge, cement block, coal fly ash | 40 | 0.313 | This study |
| NaOH Concentration (mol/L) | Desorption Rate (%) |
|---|---|
| 0.25 | 40 |
| 0.5 | 58 |
| 1 | 70 |
| 2 | 73 |
| Height (cm) | PO43−-P Concentration (mg/L) | ||||
|---|---|---|---|---|---|
| 24 h | 36 h | 48 h | 60 h | 72 h | |
| 2 | 0.051 | 0.049 | 0.042 | 0.043 | 0.040 |
| 5 | 0.032 | 0.029 | 0.031 | 0.022 | 0.025 |
| 8 | 0.029 | 0.030 | 0.021 | 0.020 | 0.019 |
| Height (cm) | PO43−-P Concentration (mg/L) | ||||
|---|---|---|---|---|---|
| 24 h | 36 h | 48 h | 60 h | 72 h | |
| 5 | 0.032 | 0.029 | 0.031 | 0.022 | 0.025 |
| 10 | 0.017 | 0.013 | 0.018 | 0.011 | 0.010 |
| 15 | 0.005 | 0.009 | 0.001 | 0 | 0 |
| 20 | 0.004 | 0.006 | 0.003 | 0 | 0 |
| Flow (mL/s) | PO43−-P Concentration (mg/L) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 0.04 | 0.003 | 0.001 | 0.002 |
| 0.08 | 0.004 | 0 | 0.001 |
| 0.12 | 0.002 | 0.004 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhu, J.; Yu, J.; Fu, Y.; Gong, C.; Huang, X. Adsorption Characteristics of Phosphate Based on Al-Doped Waste Ceramsite: Batch and Column Experiments. Int. J. Environ. Res. Public Health 2023, 20, 671. https://doi.org/10.3390/ijerph20010671
Ma Y, Zhu J, Yu J, Fu Y, Gong C, Huang X. Adsorption Characteristics of Phosphate Based on Al-Doped Waste Ceramsite: Batch and Column Experiments. International Journal of Environmental Research and Public Health. 2023; 20(1):671. https://doi.org/10.3390/ijerph20010671
Chicago/Turabian StyleMa, Yameng, Jia Zhu, Jianghua Yu, Yicheng Fu, Chao Gong, and Xiao Huang. 2023. "Adsorption Characteristics of Phosphate Based on Al-Doped Waste Ceramsite: Batch and Column Experiments" International Journal of Environmental Research and Public Health 20, no. 1: 671. https://doi.org/10.3390/ijerph20010671
APA StyleMa, Y., Zhu, J., Yu, J., Fu, Y., Gong, C., & Huang, X. (2023). Adsorption Characteristics of Phosphate Based on Al-Doped Waste Ceramsite: Batch and Column Experiments. International Journal of Environmental Research and Public Health, 20(1), 671. https://doi.org/10.3390/ijerph20010671







