Phytoplankton-Zooplankton Community Structure in Coal Mining Subsidence Lake
Abstract
1. Introduction
2. Materials and Methods
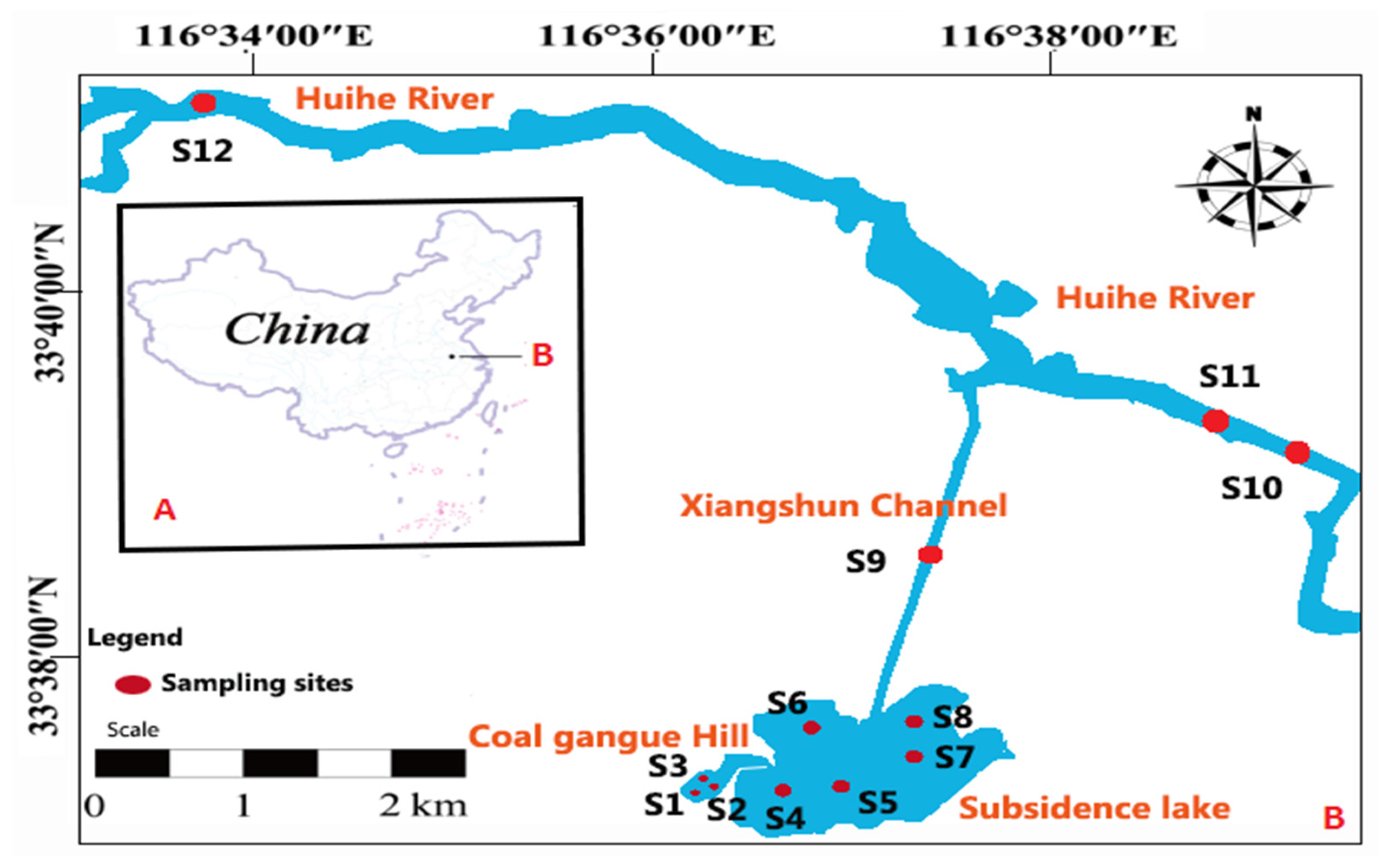
2.1. Study Area and Locations of Samples
2.2. Samples Collection
2.3. Physicochemical Analysis
2.4. Biological Data Preparation and Analysis
2.5. Statistical Analysis
3. Results
3.1. Water Characteristics and Physicochemical Parameters
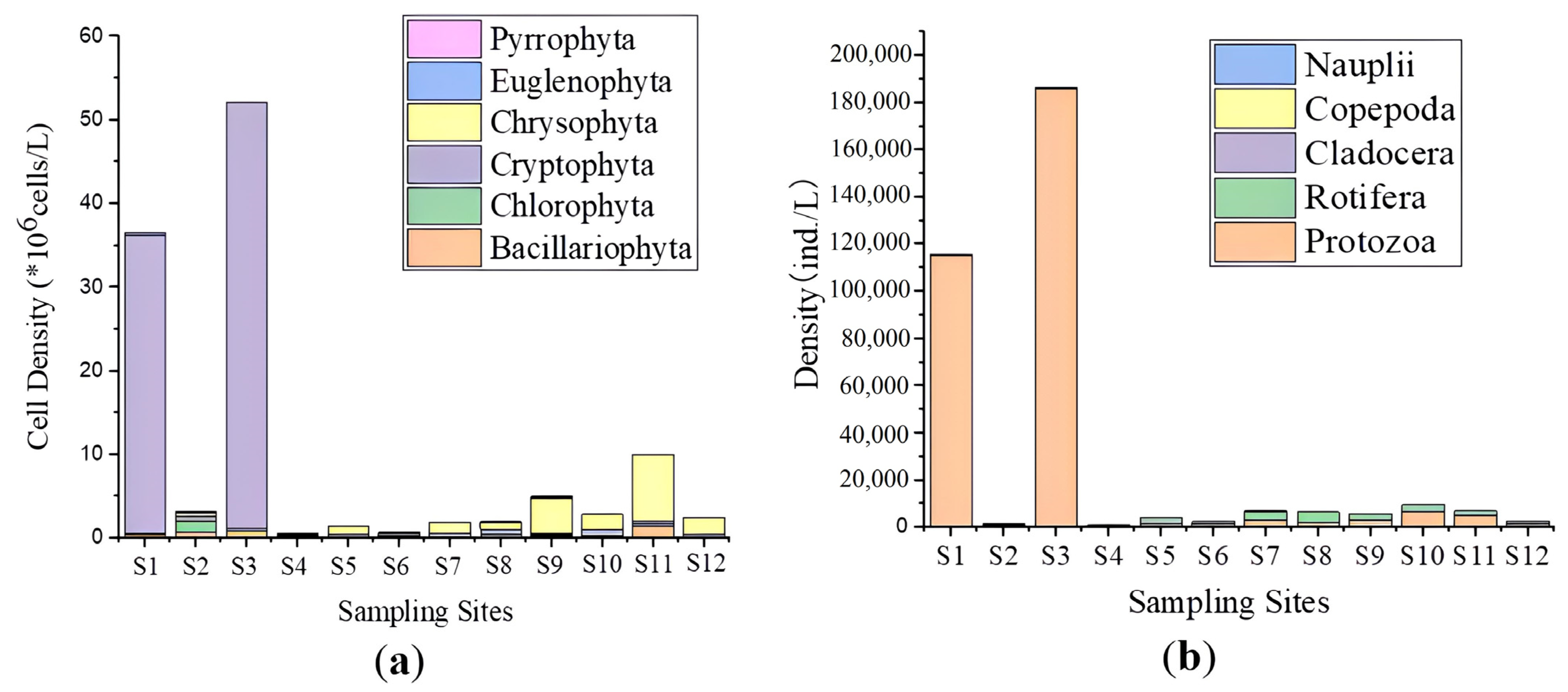
3.2. Phytoplankton Community Characteristics
3.3. Zooplankton Community Characteristics
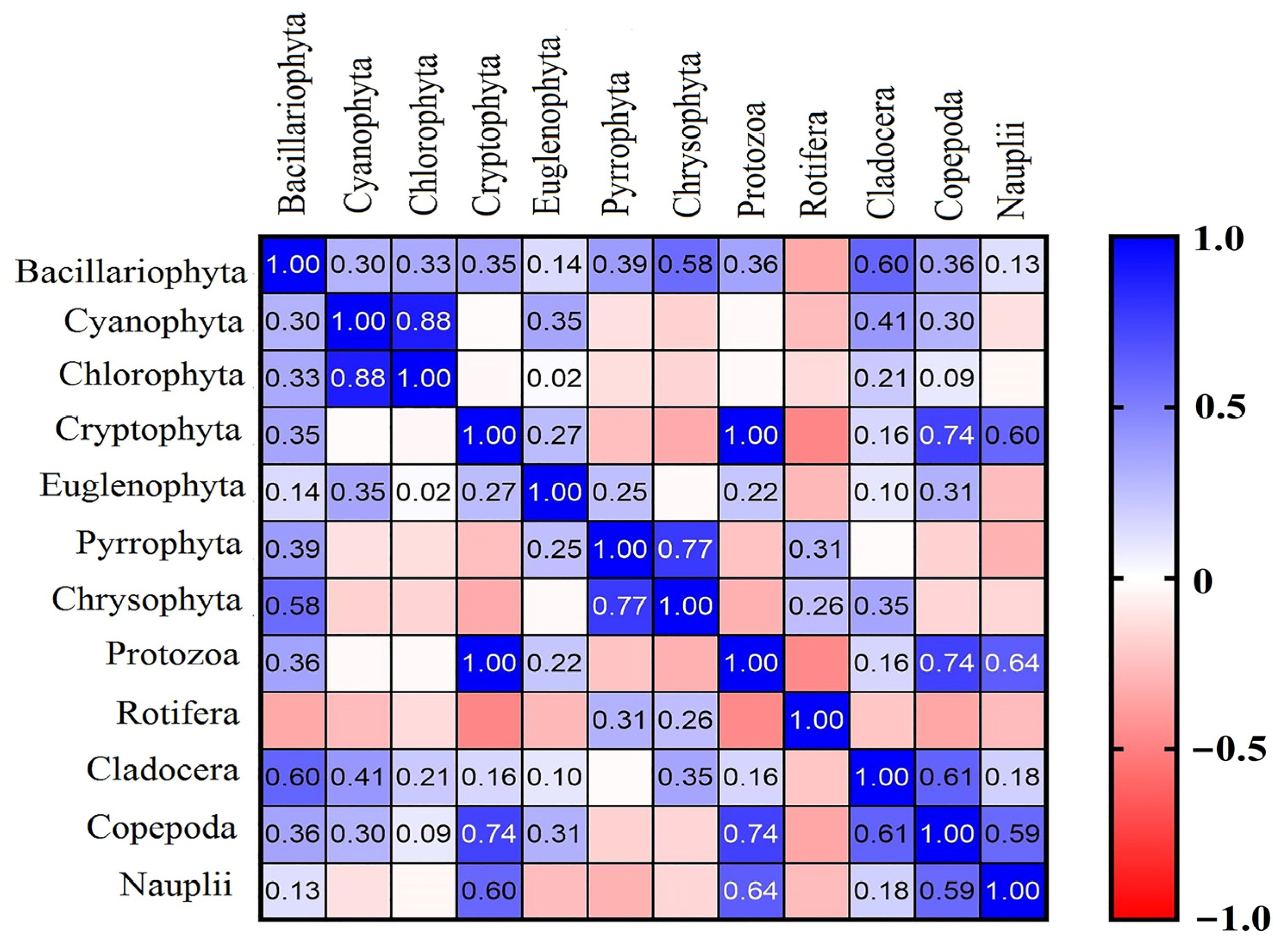
3.4. Correlation between Water Quality Parameters and Phytoplankton and Zooplankton
4. Discussion
4.1. Phytoplankton Characteristics
4.2. Zooplankton Characteristics
4.3. Interactions between Phytoplankton and Zooplankton
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirchi, A.; Hadian, S.; Madani, K.; Rouhani, O.M.; Rouhani, A.M. World Energy Balance Outlook and OPEC Production Capacity: Implications for Global Oil Security. Energies 2012, 5, 2626–2651. [Google Scholar] [CrossRef]
- Price, J.G. The world is changing. GSA Today 2016, 26, 4–10. [Google Scholar] [CrossRef]
- Yang, Z.; Li, W.; Pei, Y.; Qiao, W.; Wu, Y. Classification of the type of eco-geological environment of a coal mine district: A case study of an ecologically fragile region in Western China. J. Clean. Prod. 2018, 174, 1513–1526. [Google Scholar] [CrossRef]
- Masood, N.; Hudson-Edwards, K.; Farooqi, A. True cost of coal: Coal mining industry and its associated environmental impacts on water resource development. J. Sustain. Min. 2020, 19, 135–149. [Google Scholar] [CrossRef]
- Ouyang, Z.; Gao, L.; Yang, C. Distribution, sources and influence factors of polycyclic aromatic hydrocarbon at different depths of the soil and sediments of two typical coal mining subsidence areas in Huainan, China. Ecotoxicol. Environ. Saf. 2018, 163, 255–265. [Google Scholar] [CrossRef]
- Kai, X.; Yanqiu, Z.; Qitao, Y.; Jiaping, Y. Optimal resource utilization and ecological restoration of aquatic zones in the coal mining subsidence areas of the Huaibei Plain in Anhui Province, China. Desalination Water Treat. 2013, 51, 19–21. [Google Scholar]
- Stojiljkovic, E.; Grozdanovic, M.; Marjanovic, D. Impact of the underground coal mining on the environment. Acta Montan. Slovaca 2014, 19, 6–14. [Google Scholar]
- Fikri, H.M.; Erna, R.; Abdul, R.Z.; Yosep, R.; Wawan, K.; Marlon, I.A. A study of River quality and pollution index in the water around Coal Mining area. J. Biodivers. Environ. Sci. 2019, 15, 66–76. [Google Scholar]
- Hu, J.; Chen, X.; Chen, Y.; Li, C.; Ren, M.; Jiang, C.; Chen, Y.; An, S.; Xu, Y.; Zheng, L. Nitrate sources and transformations in surface water of a mining area due to intensive mining activities: Emphasis on effects on distinct subsidence waters. J. Environ. Manag. 2021, 298, 113451. [Google Scholar] [CrossRef]
- Xue, X.; Wang, L.; Xing, H.; Zhao, Y.; Li, X.; Wang, G.; Wang, Z. Characteristics of phytoplankton-zooplankton communities and the roles in the transmission of antibiotic resistance genes under the pressure of river contamination. Sci. Total Environ. 2021, 780, 146452. [Google Scholar] [CrossRef]
- Pourafrasyabi, M.; Ramezanpour, Z. Phytoplankton as bio-indicator of water quality in Sefid Rud River—Iran (South Caspian Sea). Caspian J. Environ. Sci. 2014, 12, 31–40. [Google Scholar]
- Ligez, S.; Wilk-Woźniak, E. The occurrence of a Euglena pascheri and Lepocinclis ovum bloom in an oxbow lake in southern Poland under extreme environmental conditions. J. Ecol. Indic. 2011, 11, 925–929. [Google Scholar] [CrossRef]
- UNESCO. Coastal lagoon research, present and future. UNESCO Technol. Pap. Mar. Sci. 1981, 32, 51–79. [Google Scholar]
- Cloern, J.E.; Powell, T.M.; Huzzley, L.M. Spatial and temporal variability in South Francisco Bay (USA). II. Temporal changes in salinity, suspended sediments, phytoplankton biomass and productivity over tidal time scales. Estuary Coast. J. Shelf Sci. 1989, 28, 599–613. [Google Scholar] [CrossRef]
- Garmendia, M.; Borja, Á.; Franco, J.; Revilla, M. Phytoplankton composition indicators for the assessment of eutrophication in marine waters: Present state and challenges within the European directives. Mar. Pollut. Bull. 2013, 66, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Gharib, S.M.; El-Sherif, A.Z.M.; Abdelhalim, M.; Radwan, A.A. Phytoplankton and environmental variables as a water quality indicator for the beaches at Matrouh, southeastern Mediterranean Sea, Egypt: An assessment. Oceanologia 2011, 53, 819–836. [Google Scholar] [CrossRef]
- Yusuf, Z.H. Phytoplankton as bioindicators of water quality in Nasarawa reservoir, Katsina State Nigeria. Acta Limnol. Bras. 2020, 32, e4. [Google Scholar] [CrossRef]
- Mukhortova, O.V.; Bolotov, S.E.; Tarasova, N.G. Zooplankton of an urbanized water body and factors determining its development (a case study of Bol’shoe Vasil’evskoe Lake, Tolyatti, Samara oblast). Povolzh. Ekol. Zh. 2015, 4, 409–421. [Google Scholar]
- Imant, E.N.; Novoselov, A.P. Dynamics of Zooplankton Composition in the Lower Northern Dvina River and Some Factors Determining Zooplankton Abundance. Russ. J. Ecol. 2021, 52, 59–69. [Google Scholar] [CrossRef]
- Goździejewska, A.M.; Skrzypczak, A.R.; Paturej, E.; Koszałka, J. Zooplankton diversity of drainage system reservoirs at an opencast mine. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 33. [Google Scholar] [CrossRef]
- Rissik, D.; Senden, D.V.; Doherty, M.; Ingleton, T.; Ajani, P.; Bowling, L.; Gibbs, M.; Gladstone, M.; Kobayashi, T.; Suthers, I.; et al. Plankton-related environmental and water-quality issues. In Plankton—A Guide to Their Ecology and Monitoring for Water Quality, 1st ed.; Suthers, I.M., Rissik, D., Eds.; CSIRO Publishing: Melbourne, Australia, 2009; pp. 39–72. [Google Scholar]
- Florescu, L.I.; Moldoveanu, M.M.; Catană, R.D.; Păceșilă, I.; Dumitrache, A.; Gavrilidis, A.A.; Iojă, C.I. Assessing the Effects of Phytoplankton Structure on Zooplankton Communities in Different Types of Urban Lakes. Diversity 2022, 14, 231. [Google Scholar] [CrossRef]
- Pant, B.; Lohani, V.; Trakroo, M.D.; Tewari, H. Study of water quality by physicochemical analysis of a Himalayan lake of Uttarakhand, India. Econ. Environ. Conserv. 2017, 23, 1128–1134. [Google Scholar]
- Ghidini, A.R.; Serafim-Ghidini, A.; Serafim, M., Jr.; Perbiche-Neves, G.; Brito, L. Distribution of planktonic cladocerans (Crustacea: Branchiopoda) of a shallow eutrophic reservoir (Paraná State, Brazil). Pan-Am. J. Aquat. Sci. 2009, 4, 294–305. [Google Scholar]
- El Ghizi, S.; El Aadel, N.; Sadik, M.; El Bouch, M.; Hasnaoui, M. The physicochemical characteristics and the pollution level of Dayet Er-Roumi Lake in Morocco. E3S Web Conf. 2021, 234, 00036. [Google Scholar] [CrossRef]
- Brogueira, M.J.; Oliveira, M.D.R.; Cabeçadas, G. Phytoplankton community structure defined by key environmental variables in Tagus estuary, Portugal. Mar. Environ. Res. 2007, 64, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, N.E.; Gharib, S. The interaction between phytoplankton and zooplankton in a Lake-Sea connection, Alexandria, Egypt. Int. J. Ocean. Oceanogr. 2006, 1, 151–165. [Google Scholar]
- Wang, L.; Wang, C.; Deng, D.; Zhao, X.; Zhou, Z. Temporal and spatial variations in phytoplankton: Correlations with environmental factors in Shengjin Lake, China. Environ. Sci. Pollut. Res. 2015, 22, 14144–14156. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzi, S.; Valavi, H. Effects of Environmental Factors on Phytoplankton Abundance and Diversity in Kaftar Lake. J. Fish. Aquat. Sci. 2011, 6, 130–140. [Google Scholar] [CrossRef]
- Abdulwahab, A.; Rabee, A.M. Ecological factors affecting the distribution of the zooplankton community in the Tigris River at Baghdad region, Iraq. Egypt. J. Aquat. Res. 2015, 41, 187–196. [Google Scholar] [CrossRef]
- Kong, L.; Jiang, C.; Zheng, L.; Cheng, H.; Ren, M.; Min, F.; Fang, L. Characters of Hydrochemistry and Their Influenced Factors of Different Waters in the Linhuan Coal Mining Subsidence Area of Huaibei City. J. Lake Sci. 2017, 29, 10. [Google Scholar]
- Chen, X.; Zheng, L.; Jiang, C.; Huang, W.; Dong, X. Characteristics of Surface Water Chemistry and Sulfur Hydrogen Oxygen Isotope Composition in Linhuan Mining Area of Huaibei City, Anhui Province. Earth Environ. 2019, 47, 9. [Google Scholar]
- Reynolds, C. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Wickstead, J.H. An Introduction to Study of Tropical Plankton; Hutchinson and Co: London, UK, 1965. [Google Scholar]
- Shannon, C.E.; Wiener, W. The Mathematical Theory of Communication; University Illinois Press: Urbana, IL, USA, 1963; 360p. [Google Scholar]
- Garibotti, I.A.; Vernet, M.; Ferrario, M.E.; Smith, R.C.; Ross, R.M.; Quetin, L.B. Phytoplankton spatial distribution patterns along the western Antarctic Peninsula (Southern Ocean). Mar. Ecol. Prog. Ser. 2003, 261, 21–39. [Google Scholar] [CrossRef]
- Fukai, Y.; Abe, Y.; Matsuno, K.; Yamaguchi, A. Spatial changes in the summer diatom community of the northern Bering Sea in 2017 and 2018. Deep Sea Res. II Top. Stud. Oceanogr. 2020, 181–182, 104903. [Google Scholar] [CrossRef]
- Becker, V.; Caputo, L.; Ordóñez, J.; Marcé, R.; Armengol, J.; Crossetti, L.O.; Huszar, V.L. Driving factors of the phytoplankton functional groups in a deep Mediterranean reservoir. Water Res. 2010, 44, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Hou, Z.; Li, Z.; Chu, Z.; Yang, P.; Zheng, B. Succession of phytoplankton functional groups and their driving factors in a subtropical plateau lake. Sci. Total Environ. 2018, 631–632, 1127–1137. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Internal phosphorus loading in shallow Danish lakes. Hydrobiologia 1999, 191, 139–148. [Google Scholar] [CrossRef]
- Ding, Y.; Pan, B.; Zhao, X.; Zhao, G.; Han, X.; Li, M. Will a heavy sediment load affect responses of phytoplankton functional groups to aquatic environmental changes in different water body types? Sci. Total. Environ. 2022, 837, 155863. [Google Scholar] [CrossRef]
- Wan, Y.; Zhou, Z.; Wang, C.; Lin, Z.; Wu, Q. Community structure characteristics of phytoplankton in Digou coal mining subsidence lake. J. Biol. 2018, 35, 75. [Google Scholar]
- Xu, X.; Yi, Q.T.; Wang, X.M.; Zhang, L.; Xie, K. Phytoplankton community in the small subsided lakes around Huainan coal mine subsidence areas. Acta Hydrobiol. Sin. 2015, 39, 740–750. [Google Scholar]
- He, R.; Luo, H.; He, N.; Chen, W.; Yang, F.; Huang, W.; Li, N.; Sun, L.; Peng, S. Phytoplankton Communities and Their Relationship with Environmental Factors in the Waters around Macau. Int. J. Environ. Res. Public Health 2022, 19, 7788. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Zhao, L.; Zhang, F.; Huang, H. Phytoplankton Diversity Effects on Community Biomass and Stability along Nutrient Gradients in a Eutrophic Lake. Int. J. Environ. Res. Public Health 2017, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Burpee, B.; Saros, J.E.; Northington, R.M.; Simon, K.S. Microbial nutrient limitation in Arctic lakes in a permafrost landscape of southwest Greenland. Biogeosciences 2016, 13, 365–374. [Google Scholar] [CrossRef]
- Kissman, C.E.H.; Williamson, C.E.; Rose, K.C.; Saros, J.E. Nutrients associated with terrestrial dissolved organic matter drive changes in zooplankton:phytoplankton biomass ratios in an alpine lake. Freshw. Biol. 2016, 62, 40–51. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kang, C.-K. Causes of COD increases in Gwangyang Bay, South Korea. J. Environ. Monit. 2010, 12, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Yongo, E.; Outa, N. Spatial distribution and abundance of zooplankton communities in Lake Victoria, Kenya. Int. J. Fish. Aquat. Res. 2017, 2, 33–35. [Google Scholar]
- Nowicki, C.J.; Bunnell, D.B.; Armenio, P.M.; Warner, D.M.; Vanderploeg, H.A.; Cavaletto, J.F.; Mayer, C.M.; Adams, J.V. Biotic and abiotic factors influencing zooplankton vertical distribution in Lake Huron. J. Great Lakes Res. 2017, 43, 1044–1054. [Google Scholar] [CrossRef]
- Sousa, W.; Attayde, J.L.; Rocha, E.D.S.; Eskinazi-Sant’Anna, E.M. The response of zooplankton assemblages to variations in the water quality of four man-made lakes in semi-arid northeastern Brazil. J. Plankton Res. 2008, 30, 699–708. [Google Scholar] [CrossRef]
- Lin, Z.; Wan, Y.; Xu, M.; Wang, C.; Wu, Q.; Zhou, Z. Metazoan zooplankton community structure and environmental drivers of Digou coal mining subsided lakes in Huainan. J. Lake Sci. 2018, 30, 171–182. [Google Scholar] [CrossRef]
- Yaeger, R.G. Protozoa: Structure, Classification, Growth, and Development. In Medical Microbiology, 4th ed.; Chapter, 77; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Segers, H. Global diversity of rotifers (Rotifera) in freshwater. Hydrobiologia 2008, 595, 49–59. [Google Scholar] [CrossRef]
- Sharma, B.K. Biological monitoring of freshwaters with reference to role of freshwater Rotifera as biomonitors. In Water Quality Assessment Biomonitoring and Zooplanktonic Diversity; Ministry of Environment and Forests, Government of India: New Delhi, India, 2001; pp. 83–97. [Google Scholar]
- Gilbert, J.J. Food niches of planktonic rotifers: Diversification and implications. Limnol. Oceanogr. 2022, 67, 2218–2251. [Google Scholar] [CrossRef]
- Bledzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) Key to Species Identification, with Notes on Ecology, Distribution, Methods and Introduction to Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Sługocki, Ł.; Czerniawski, R.; Kowalska-Góralska, M.; Senze, M.; Reis, A.; Carrola, J.S.; Teixeira, C.A. The Impact of Land Use Transformations on Zooplankton Communities in a Small Mountain River (The Corgo River, Northern Portugal). Int. J. Environ. Res. Public Health 2019, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, M.; Ejsmont-Karabin, J.; Karpowicz, M. Reservoir-river relationships in lowland, shallow, eutrophic systems: An impact of zooplankton from hypertrophic reservoir on river zooplankton. Pol. J. Ecol. 2013, 61, 759–768. [Google Scholar]
- Richardson, W.B. Microcrustacea in flowing warer—Experimantal analysis of washout times and a field-test. Freshwater Biol. 1992, 28, 217–230. [Google Scholar] [CrossRef]
- De Bie, T.; Declerck, S.; Martens, K.; De Meester, L.; Brendonck, L. A comparative analysis of cladoceran communities from different water body types: Patterns in community composition and diversity. Hydrobiologia 2008, 597, 19–27. [Google Scholar] [CrossRef]
- Czerniawski, R. Spatial pattern of potamozooplankton community of the slowly flowing fishless stream in relation to abiotic and biotic factors. Pol. J. Ecol. 2012, 60, 323–338. [Google Scholar]
- Czerniawski, R.; Sługocki, Ł. Analysis of zooplankton assemblages from man-made ditches in relation to current velocity. Oceanol. Hydrobiol. Stud. 2017, 46, 199–211. [Google Scholar] [CrossRef]
- Hillebrand, H.; Bennett, D.M.; Cadotte, M.W. Consequences of Dominance: A Review of Evenness Effects on Local and Regional Ecosystem Processes. Ecology 2008, 89, 1510–1520. [Google Scholar] [CrossRef]
- Ryszard, G.; Kowalczewska-Madura, K. Interactions between phytoplankton and zooplankton in the hypertrophic Swarzędzkie Lake in western Poland. J. Plankton Res. 2008, 30, 33–42. [Google Scholar]
- Mellina, E.; Rasmussen, J.B.; Mills, E.L. Impact of Zebra mussels (Dressena polymorpha) on phytoplankton cycling and chlorophyll in lakes. Can. J. Fish. Aquat. Sci. 1995, 52, 2553–2579. [Google Scholar] [CrossRef]
- Prins, T.C.; Escaravage, V.; Smaal, A.C.; Peters, J.C.H. Nutrient cycling and phytoplankton dynamics in relation to mussel grazing in a mesocosm experiment. Ophelia 1995, 41, 289–315. [Google Scholar] [CrossRef]
- Havens, K.E. The importance of rotiferan and crustacean zooplankton as grazers of algal productivity in a freshwater estuary. Arch. Hydrobiol. 1991, 122, 1–22. [Google Scholar] [CrossRef]
- Sanders, R.W.; Wickham, S.A. Planktonic protozoa and metazoa: Predation, food quality and population control. Mar. Microb. Food Webs 1993, 7, 197–223. [Google Scholar]
- Sterner, R.W. The role of grazers in phytoplankton succession. In Plankton Ecology: Seasonal Succession in Plankton Communities; Sommer, U., Ed.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 107–170. [Google Scholar]
- Bergquist, A.M.; Carpenter, S.R. Limnetic Herbivory: Effects on Phytoplankton Populations and Primary Production. Ecology 1986, 67, 1351–1360. [Google Scholar] [CrossRef]
- Kawecka, B.; Eloranta, P.V. The Outline of Algae Ecology in Freshwater and Terrestrial Environments; PWN Scientific Publishers: Warsaw, Poland, 1994; pp. 1–252. [Google Scholar]
- Alcaraz, M.; Almeda, R.; Calbet, A.; Saiz, E.; Duarte, C.M.; Lasternas, S.; Agusti, S.; Santiago, R.; Movilla, J.; Alonso, A. The role of arctic zooplankton in biogeochemical cycles: Respiration and excretion of ammonia and phosphate during summer. Polar Biol. 2010, 33, 1719–1731. [Google Scholar] [CrossRef]
- Coello-Camba, A.; Llabrés, M.; Duarte, C.M.; Agusti, S. Zooplankton excretion metabolites stimulate Southern Ocean phytoplankton growth. Polar Biol. 2017, 40, 2035–2045. [Google Scholar] [CrossRef]
- Sterner, R.W. Herbivores’ Direct and Indirect Effects on Algal Populations. Science 1986, 231, 605–607. [Google Scholar] [CrossRef]


| Water Quality Variables | Phytoplankton Density | Zooplankton Density |
|---|---|---|
| SD | −0.559 | −0.479 |
| CODcr | 0.795 ** | 0.726 ** |
| TP | 0.948 *** | 0.919 *** |
| PO43− | 0.763 ** | 0.745 ** |
| TN | 0.701 * | 0.613 * |
| NH4+ | 0.424 | 0.434 |
| SO42− | 0.179 | 0.162 |
| NO3− | −0.135 | −0.100 |
| F− | 0.078 | 0.083 |
| DO | −0.184 | −0.187 |
| T° | −0.336 | −0.355 |
| TDS | 0.377 | 0.280 |
| EC | 0.423 | 0.326 |
| pH | −0.375 | −0.391 |
| CL− | 0.264 | 0.213 |
| Phytoplankton density | 1.000 | 0.987 *** |
| Zooplankton density | 0.987 *** | 1.000 |
| Cyanophyta | Bacillariophyta | Chlorophyta | Cryptophyta | Euglenophyta | Pyrrophyta | Chrysophyta | |
|---|---|---|---|---|---|---|---|
| Protozoa | −0.031 | 0.364 | −0.031 | 0.998 *** | 0.221 | −0.238 | −0.309 |
| Rotifera | −0.268 | −0.338 | −0.144 | −0.477 | −0.282 | 0.305 | 0.255 |
| Cladocera | 0.405 | 0.604 * | 0.208 | 0.159 | 0.095 | −0.017 | 0.351 |
| Copepoda | 0.297 | 0.357 | 0.086 | 0.736 ** | 0.309 | −0.178 | −0.166 |
| Nauplii | −0.124 | 0.128 | −0.036 | 0.596 * | −0.264 | −0.305 | −0.164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, T.; Amzil, H.; Fang, W.; Xu, L.; Lu, A.; Wang, S.; Wang, X.; Chen, Y.; Pan, J.; Wei, X. Phytoplankton-Zooplankton Community Structure in Coal Mining Subsidence Lake. Int. J. Environ. Res. Public Health 2023, 20, 484. https://doi.org/10.3390/ijerph20010484
Fan T, Amzil H, Fang W, Xu L, Lu A, Wang S, Wang X, Chen Y, Pan J, Wei X. Phytoplankton-Zooplankton Community Structure in Coal Mining Subsidence Lake. International Journal of Environmental Research and Public Health. 2023; 20(1):484. https://doi.org/10.3390/ijerph20010484
Chicago/Turabian StyleFan, Tingyu, Hayat Amzil, Wangkai Fang, Liangji Xu, Akang Lu, Shun Wang, Xingming Wang, Yingxiang Chen, Jinhong Pan, and Xiangping Wei. 2023. "Phytoplankton-Zooplankton Community Structure in Coal Mining Subsidence Lake" International Journal of Environmental Research and Public Health 20, no. 1: 484. https://doi.org/10.3390/ijerph20010484
APA StyleFan, T., Amzil, H., Fang, W., Xu, L., Lu, A., Wang, S., Wang, X., Chen, Y., Pan, J., & Wei, X. (2023). Phytoplankton-Zooplankton Community Structure in Coal Mining Subsidence Lake. International Journal of Environmental Research and Public Health, 20(1), 484. https://doi.org/10.3390/ijerph20010484






