Underdiagnosis in Background of Emerging Public Health Challenges Related to Peri-Implant Diseases: An Interventional Split-Mouth Study
Abstract
1. Introduction
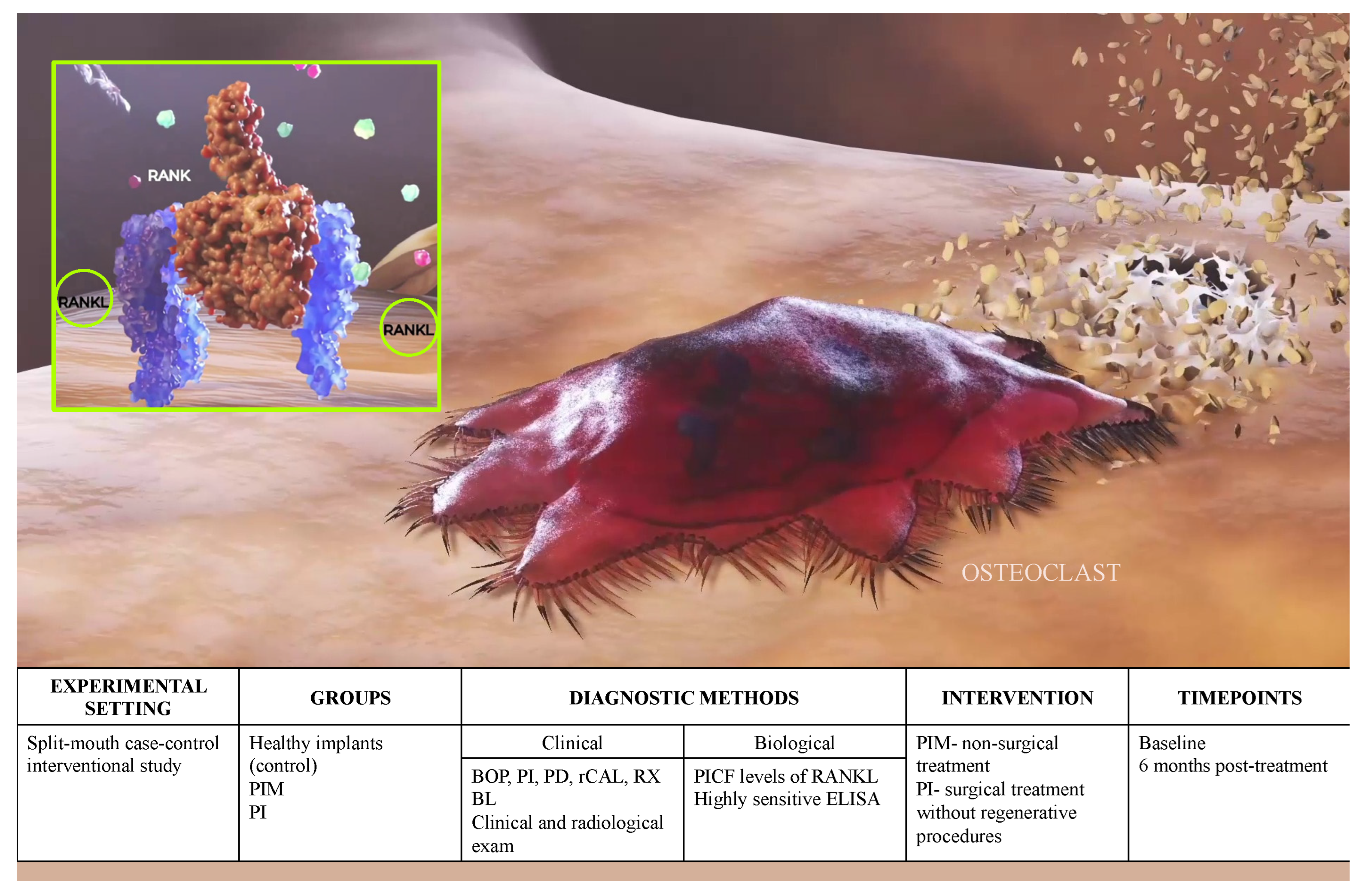
2. Materials and Methods
2.1. Study Population
2.2. Definitions and Study Criteria
- Healthy controls (HC)- bleeding on probing (BOP) ≤ 1/6 points, probing depth < 3 mm and radiological bone loss (RXBL) < 2 mm;
- PIM-BOP > 1/6 sites, PD > 4 mm and RXBL < 2 mm compared to moment of prosthetic loading;
- Peri-implantitis-BOP > 25%, PD > 4 mm and RXBL > 2 mm from the moment of prosthetic loading;
- Treatment success: The absence of deep PPD with BoP/suppuration and no additional bone loss.
- Peri-implant defects requiring regenerative procedures;
- Intake of antibiotics and/or anti-inflammatory agents in the preceding 3 months;
- Previous periodontal treatment in preceding year;
- Aggressive and severe forms of periodontitis;
- Pregnancy and/or lactation in female patients;
- Implant supported restoration with signs of biomechanical overload.
2.3. Clinical Outcome Variables
2.4. Anti-Infective Mechanical Treatment (AIMT)
- PIM: supra + subgingival debridement of the implant surface, implant neck and the abutment for elimination of dental plaque and/or calculus using graphite and/or titanium curettes (Deppeler SA, Rolle, Switzerland). Implant surface was additionally decontaminated using 0.2% chlorhexidine gel (Curasept ADS®, Curaprox, Curaden International AG, Kriens, Switzerland);
- PI: open-flap debridement + implant surface decontamination. Briefly, following infiltrative local anesthesia (2% lidocaine with 1:100,000 adrenaline), intrasulcular incisions were created for elevation of buccal and lingual full-thickness flaps. Scaling was performed using graphite and/or titanium curettes, while the chemical implant surface decontamination was performed using gauze soaked infiltrated povidone iodine and 0.2% chlorhexidine gel for 2–3 min each (Curasept ADS®, Curaprox, Curaden International AG, Kriens, Switzerland) [29]. The flaps were repositioned and stabilized with interrupted sutures. Postoperative care consisted of rinsing with a 0.12% chlorhexidine gluconate mouthwash (Curasept ADS®, Curaprox, Curaden International AG, Kriens, Switzerland) twice a day for 2 weeks, while the sutures were removed 10 days following surgery.
2.5. Biomarker Measurement
2.6. Data Analysis
2.7. RANKL Validation for Diagnostic Use
3. Results
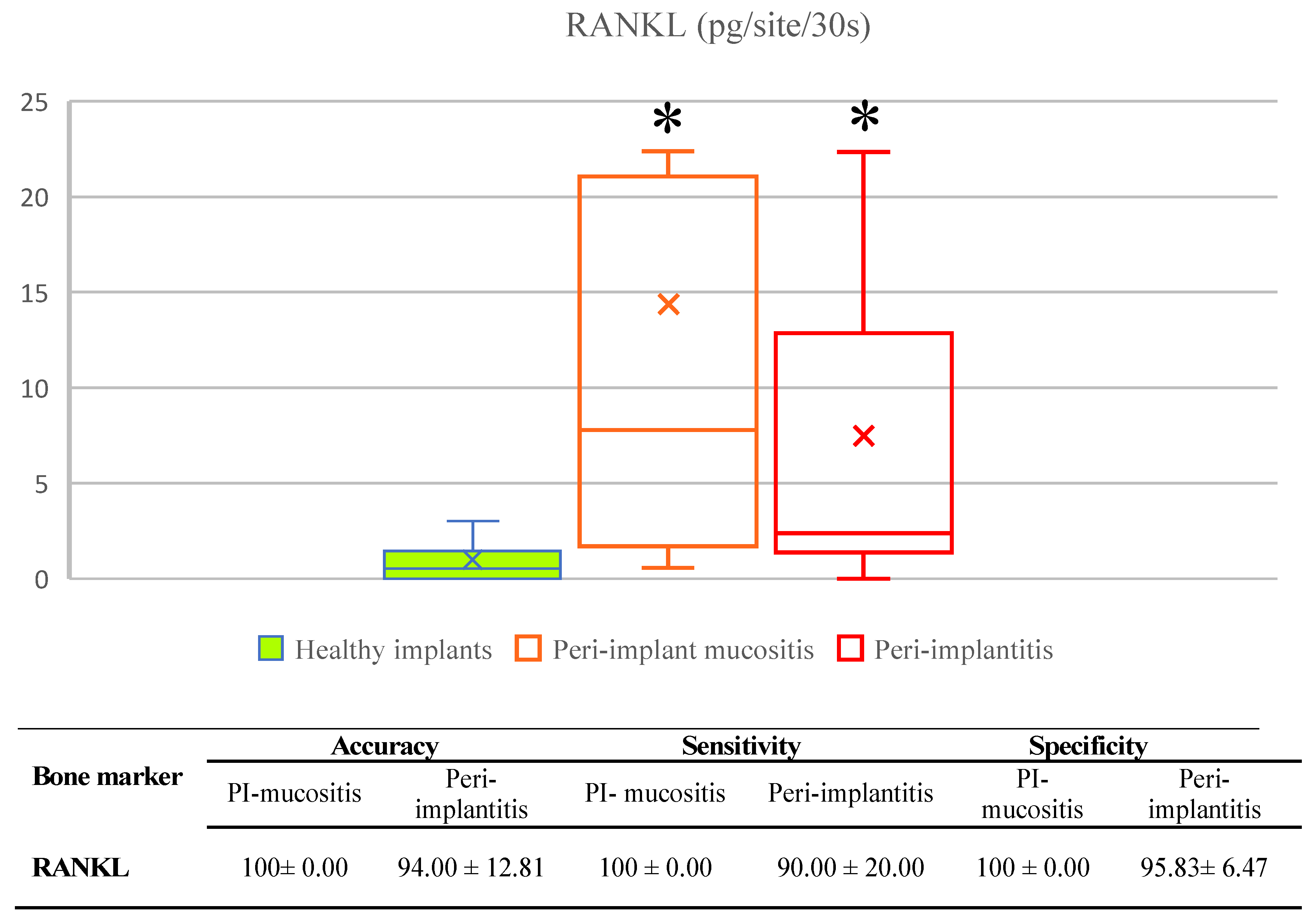
3.1. Validation of Biomarkers
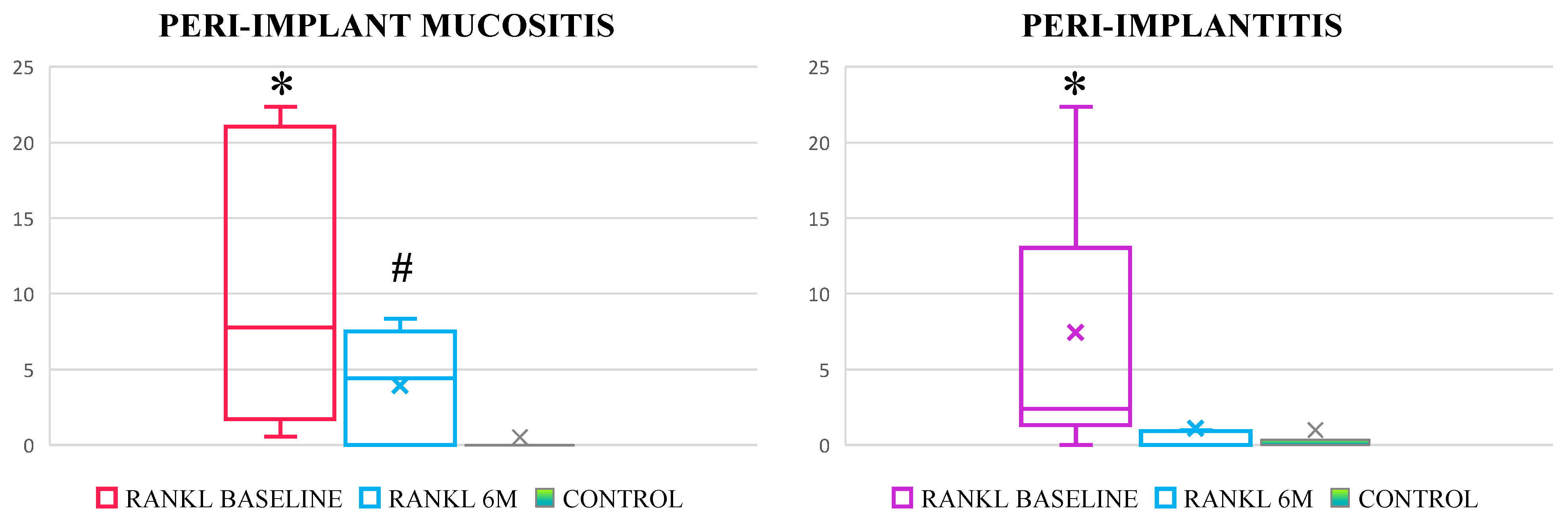
3.2. Effectiveness of Standard Treatment of Peri-Implant Mucositis and Peri-Implantitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tonetti, M.S.; Chapple, I.L.C.; Jepsen, S.; Sanz, M. Primary and Secondary Prevention of Periodontal and Peri-Implant Diseases: Introduction to, and Objectives of the 11th European Workshop on Periodontology Consensus Conference. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Rakic, M.; Galindo-Moreno, P.; Monje, A.; Radovanovic, S.; Wang, H.-L.; Cochran, D.; Sculean, A.; Canullo, L. How Frequent Does Peri-Implantitis Occur? A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2018, 22, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Lima, C.; Pedrinaci, I.; Araoz, A.; Soldini, M.C.; Sanz, M. Prevalence and Risk/Protective Indicators of Peri-Implant Diseases: A University-Representative Cross-Sectional Study. Clin. Oral Implants Res. 2021, 32, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Figuero, E.; Graziani, F.; Sanz, I.; Herrera, D.; Sanz, M. Management of Peri-Implant Mucositis and Peri-Implantitis. Periodontology 2014, 66, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Becker, K.; Sager, M. Efficacy of Professionally Administered Plaque Removal with or without Adjunctive Measures for the Treatment of Peri-Implant Mucositis. A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2015, 42, S202–S213. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Berglundh, T.; Genco, R.; Aass, A.M.; Demirel, K.; Derks, J.; Figuero, E.; Giovannoli, J.L.; Goldstein, M.; Lambert, F.; et al. Primary Prevention of Peri-Implantitis: Managing Peri-Implant Mucositis. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S152–S157. [Google Scholar] [CrossRef]
- Koldsland, O.C.; Wohlfahrt, J.C.; Aass, A.M. Surgical Treatment of Peri-implantitis: Prognostic Indicators of Short-term Results. J. Clin. Periodontol. 2018, 45, 100–113. [Google Scholar] [CrossRef]
- Brägger, U. Use of Radiographs in Evaluating Success, Stability and Failure in Implant Dentistry. Periodontology 1998, 17, 77–88. [Google Scholar] [CrossRef]
- Lang, N.P.; Wetzel, A.C.; Stich, H.; Caffesse, R.G. Histologic Probe Penetration in Healthy and Inflamed Peri-implant Tissues. Clin. Oral Implants Res. 1994, 5, 191–201. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S286–S291. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-Implantitis. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S246–S266. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Berglundh, J.; Derks, J.; Sanz, M.; Berglundh, T. Diagnosis of Peri-Implantitis in the Absence of Baseline Data: A Diagnostic Accuracy Study. Clin. Oral Implants Res. 2021, 32, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Rakic, M.; Monje, A.; Radovanovic, S.; Petkovic-Curcin, A.; Vojvodic, D.; Tatic, Z. Is the Personalized Approach the Key to Improve Clinical Diagnosis of Peri-Implant Conditions? The Role of Bone Markers. J. Periodontol. 2020, 91, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef]
- Carinci, F.; Romanos, G.E.; Scapoli, L. Molecular Tools for Preventing and Improving Diagnosis of Peri-Implant Diseases. Periodontology 2019, 81, 41–47. [Google Scholar] [CrossRef]
- Watts, N.B. Clinical Utility of Biochemical Markers of Bone Remodeling. Clin. Chem. 1999, 45, 1359–1368. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N. The RANKL-OPG System in Clinical Periodontology. J. Clin. Periodontol. 2012, 39, 239–248. [Google Scholar] [CrossRef]
- Rakic, M.; Lekovic, V.; Nikolic-Jakoba, N.; Vojvodic, D.; Petkovic-Curcin, A.; Sanz, M. Bone Loss Biomarkers Associated with Peri-Implantitis. A Cross-Sectional Study. Clin. Oral Implants Res. 2013, 24, 1110–1116. [Google Scholar] [CrossRef]
- Theoleyre, S.; Wittrant, Y.; Tat, S.K.; Fortun, Y.; Redini, F.; Heymann, D. The Molecular Triad OPG/RANK/RANKL: Involvement in the Orchestration of Pathophysiological Bone Remodeling. Cytokine Growth Factor Rev. 2004, 15, 457–475. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.-L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin Ligand Is a Cytokine That Regulates Osteoclast Differentiation and Activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Yoshinaga, Y.; Ukai, T.; Abe, Y.; Hara, Y. Expression of Receptor Activator of Nuclear Factor Kappa B Ligand Relates to Inflammatory Bone Resorption, with or without Occlusal Trauma, in Rats. J. Periodontal Res. 2007, 42, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Rakic, M.; Struillou, X.; Petkovic-Curcin, A.; Matic, S.; Canullo, L.; Sanz, M.; Vojvodic, D. Estimation of Bone Loss Biomarkers as a Diagnostic Tool for Peri-Implantitis. J. Periodontol. 2014, 85, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.R.; Powers, J.H. Biomarkers and Surrogate Endpoints in Clinical Trials. Stat. Med. 2012, 31, 2973–2984. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- WMA—The World Medical Association-WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/ (accessed on 9 November 2022).
- Sanz, M.; Chapple, I.L. Working Group 4 of the VIII European Workshop on Periodontology* Clinical Research on Peri-Implant Diseases: Consensus Report of Working Group 4. J. Clin. Periodontol. 2012, 39, 202–206. [Google Scholar] [CrossRef]
- Fleming, T.R.; DeMets, D.L. Surrogate End Points in Clinical Trials: Are We Being Misled? Ann. Intern. Med. 1996, 125, 605–613. [Google Scholar] [CrossRef]
- Van Aken, J. Optimum Conditions for Intraoral Roentgenograms. Oral Surg. Oral Med. Oral Pathol. 1969, 27, 475–491. [Google Scholar] [CrossRef]
- de Waal, Y.C.M.; Raghoebar, G.M.; Meijer, H.J.A.; Winkel, E.G.; van Winkelhoff, A.J. Implant Decontamination with 2% Chlorhexidine during Surgical Peri-Implantitis Treatment: A Randomized, Double-Blind, Controlled Trial. Clin. Oral Implants Res. 2015, 26, 1015–1023. [Google Scholar] [CrossRef]
- Nakashima, K.; Demeurisse, C.; Cimasoni, G. The Recovery Efficiency of Various Materials for Sampling Enzymes and Polymorphonuclear Leukocytes from Gingival Crevices. J. Clin. Periodontol. 1994, 21, 479–483. [Google Scholar] [CrossRef]
- Quinlan, J.R. C4.5: Programs for Machine Learning; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-08-050058-4. [Google Scholar]
- Dental Implants Market Size & Growth, Industry Report, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/dental-implants-market (accessed on 20 July 2020).
- Mancini, L. Peri-Implant Health and Diagnostic Considerations. Int. J. Environ. Res. Public. Health 2022, 19, 12008. [Google Scholar] [CrossRef]
- Monje, A.; Caballé-Serrano, J.; Nart, J.; Peñarrocha, D.; Wang, H.-L.; Rakic, M. Diagnostic Accuracy of Clinical Parameters to Monitor Peri-Implant Conditions: A Matched Case-Control Study. J. Periodontol. 2018, 89, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, A.; Beltrán, V.; Betancur, D.; Sam, Y.-H.; Moaven, H.; Tarjomani, A.; Donos, N.; Sousa, V. Molecular Biomarkers in Peri-Implant Health and Disease: A Cross-Sectional Pilot Study. Int. J. Mol. Sci. 2022, 23, 9802. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.M.; de Mendonça, A.C.; Máximo, M.B.B.; Santos, V.R.; Bastos, M.F.; Nociti, F.H. Effect of Anti-Infective Mechanical Therapy on Clinical Parameters and Cytokine Levels in Human Peri-Implant Diseases. J. Periodontol. 2009, 80, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Nelson, K.; Tarnow, D.P.; Wang, H.-L.; Giannobile, W.V. Is Metal Particle Release Associated with Peri-Implant Bone Destruction? An Emerging Concept. J. Dent. Res. 2018, 97, 259–265. [Google Scholar] [CrossRef]
- Knight, E.T.; Thomson, W.M. A Public Health Perspective on Personalized Periodontics. Periodontology 2018, 78, 195–200. [Google Scholar] [CrossRef]
- Duarte, P.M.; Serrão, C.R.; Miranda, T.S.; Zanatta, L.C.S.; Bastos, M.F.; Faveri, M.; Figueiredo, L.C.; Feres, M. Could Cytokine Levels in the Peri-Implant Crevicular Fluid Be Used to Distinguish between Healthy Implants and Implants with Peri-Implantitis? A Systematic Review. J. Periodontal Res. 2016, 51, 689–698. [Google Scholar] [CrossRef]
- Alassy, H.; Parachuru, P.; Wolff, L. Peri-Implantitis Diagnosis and Prognosis Using Biomarkers in Peri-Implant Crevicular Fluid: A Narrative Review. Diagnostics 2019, 9, 214. [Google Scholar] [CrossRef]
- Rakic, M.; Petkovic-Curcin, A.; Struillou, X.; Matic, S.; Stamatovic, N.; Vojvodic, D. CD14 and TNFα Single Nucleotide Polymorphisms Are Candidates for Genetic Biomarkers of Peri-Implantitis. Clin. Oral Investig. 2015, 19, 791–801. [Google Scholar] [CrossRef]
- Rakić, M.; Nikolić-Jakoba, N.; Struillou, X.; Petković-Ćurčin, A.; Stamatović, N.; Matić, S.; Janković, S.; Aleksić, Z.; Vasilić, Đ.; Leković, V.; et al. Receptor Activator of Nuclear Factor Kappa B (RANK) as a Determinant of Peri-Implantitis. Vojnosanit. Pregl. 2013, 70, 346–351. [Google Scholar] [CrossRef]
| Periodontal Status | Peri-Implant Mucositis | Peri-Implantitis | |||
|---|---|---|---|---|---|
| Number of teeth (n; mean and range) | 17.8 (0–25) | 16.4 (5–25) | |||
| Full-mouth PI (% mean ± SD) | 25.3 ± 3.9 | 24.6 ± 4.1 | |||
| Full-mouth BOP (% means ± SD) | 19.5 ± 4.2 | 18.39 ± 5.7 | |||
| Full-mouth PD (mm; mean ± SD) | 4.1 ± 0.7 | 3.9 ± 1.9 | |||
| Full-mouth dental CAL (mm; mean ± SD) | 3.2 ± 0.7 | 3.1 ± 1.2 | |||
| Implant-site parameters | Controls | Baseline | 6 months | Baseline | 6 months |
| BOP | 0 | 95.25 ± 9.63 | 29.17 ± 22.13 *# | 100.0 | 19.92 ± 3.8 *# |
| PI | 21.66 ± 10.51 | 87.25 ± 28.5 | 25.7 ± 14.21 * | 84.88 ± 25.52 | 19.87 ± 14.41 * |
| PD (mm) | 1.2 ± 1.57 | 4.10 ± 1.50 | 3.25 ± 1.2 *# | 6.15 ± 1.75 | 4.28 ± 1.75 *# |
| rCAL (mm) | 0 | 0 | 0 | 3.78 ± 1.77 | 2.67 ± 0.88 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djuran, B.; Tatic, Z.; Perunovic, N.; Pejcic, N.; Vukovic, J.; Petkovic-Curcin, A.; Vojvodic, D.; Rakic, M. Underdiagnosis in Background of Emerging Public Health Challenges Related to Peri-Implant Diseases: An Interventional Split-Mouth Study. Int. J. Environ. Res. Public Health 2023, 20, 477. https://doi.org/10.3390/ijerph20010477
Djuran B, Tatic Z, Perunovic N, Pejcic N, Vukovic J, Petkovic-Curcin A, Vojvodic D, Rakic M. Underdiagnosis in Background of Emerging Public Health Challenges Related to Peri-Implant Diseases: An Interventional Split-Mouth Study. International Journal of Environmental Research and Public Health. 2023; 20(1):477. https://doi.org/10.3390/ijerph20010477
Chicago/Turabian StyleDjuran, Boris, Zoran Tatic, Neda Perunovic, Natasa Pejcic, Jovana Vukovic, Aleksandra Petkovic-Curcin, Danilo Vojvodic, and Mia Rakic. 2023. "Underdiagnosis in Background of Emerging Public Health Challenges Related to Peri-Implant Diseases: An Interventional Split-Mouth Study" International Journal of Environmental Research and Public Health 20, no. 1: 477. https://doi.org/10.3390/ijerph20010477
APA StyleDjuran, B., Tatic, Z., Perunovic, N., Pejcic, N., Vukovic, J., Petkovic-Curcin, A., Vojvodic, D., & Rakic, M. (2023). Underdiagnosis in Background of Emerging Public Health Challenges Related to Peri-Implant Diseases: An Interventional Split-Mouth Study. International Journal of Environmental Research and Public Health, 20(1), 477. https://doi.org/10.3390/ijerph20010477









