COVID-19 Vaccination and Late-Onset Myasthenia Gravis: A New Case Report and Review of the Literature
Abstract
1. Introduction
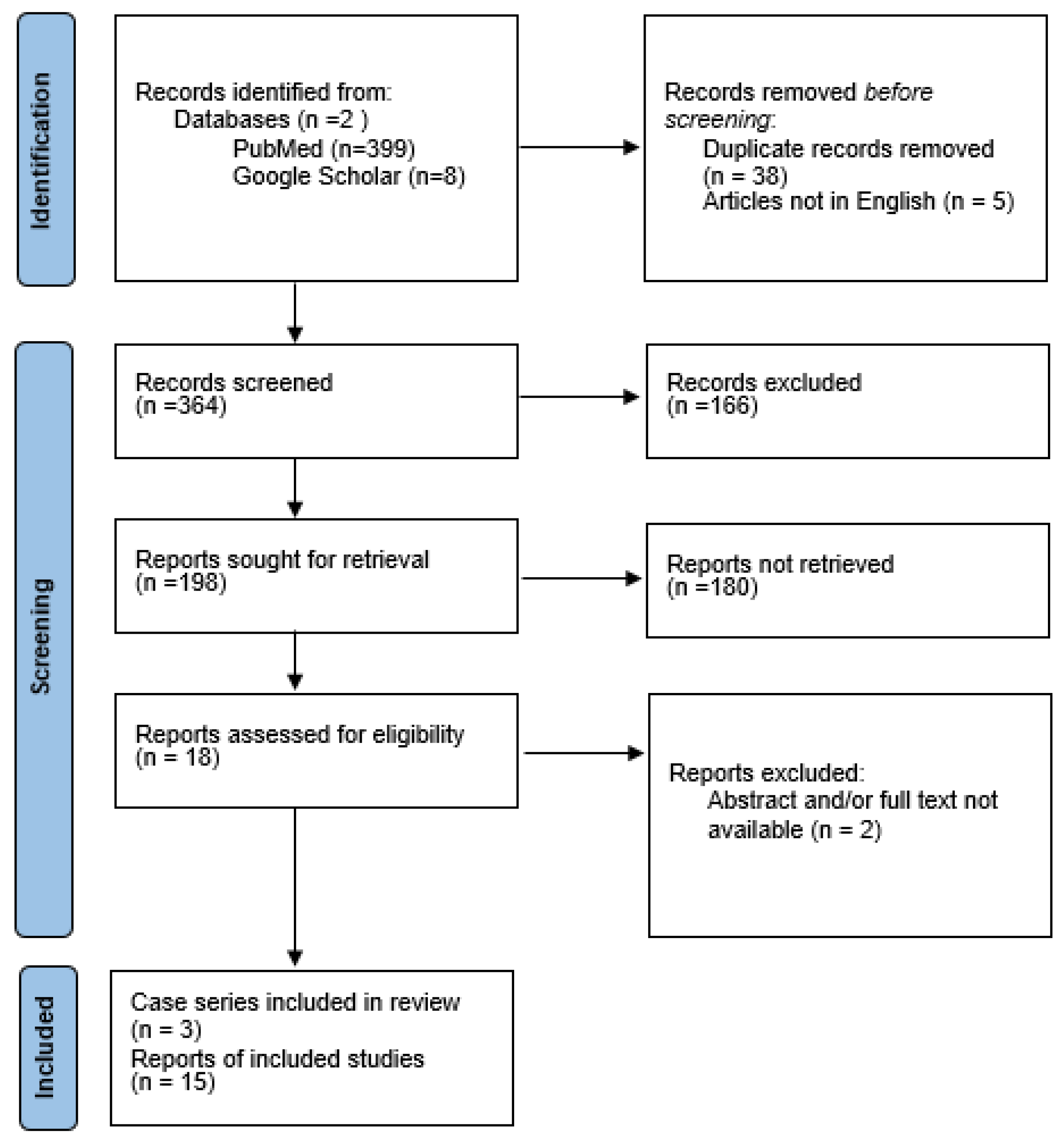
2. Materials and Methods
2.1. Literature Revision
2.2. Statistical Analysis
3. Results
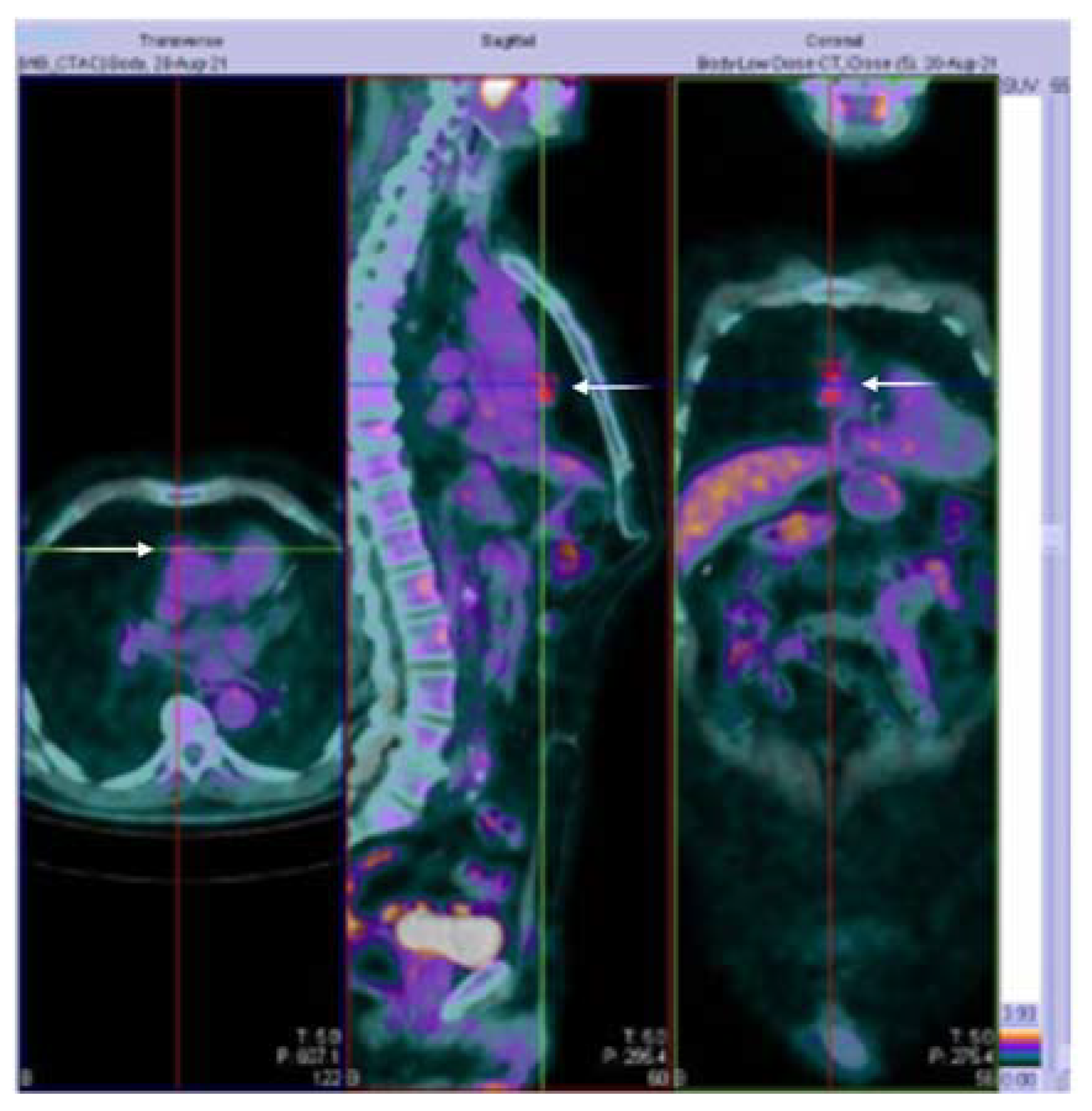
3.1. Case Presentation
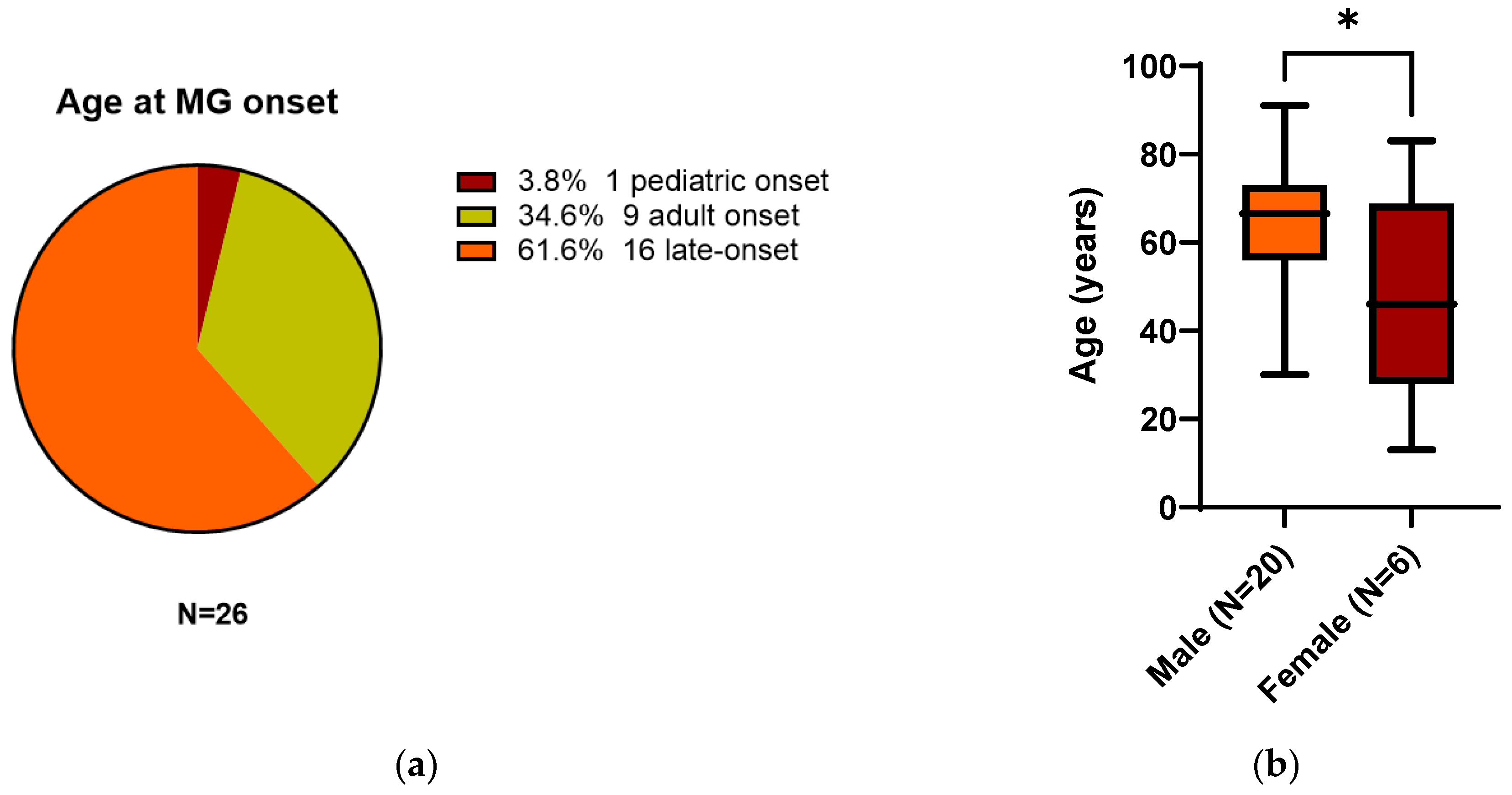
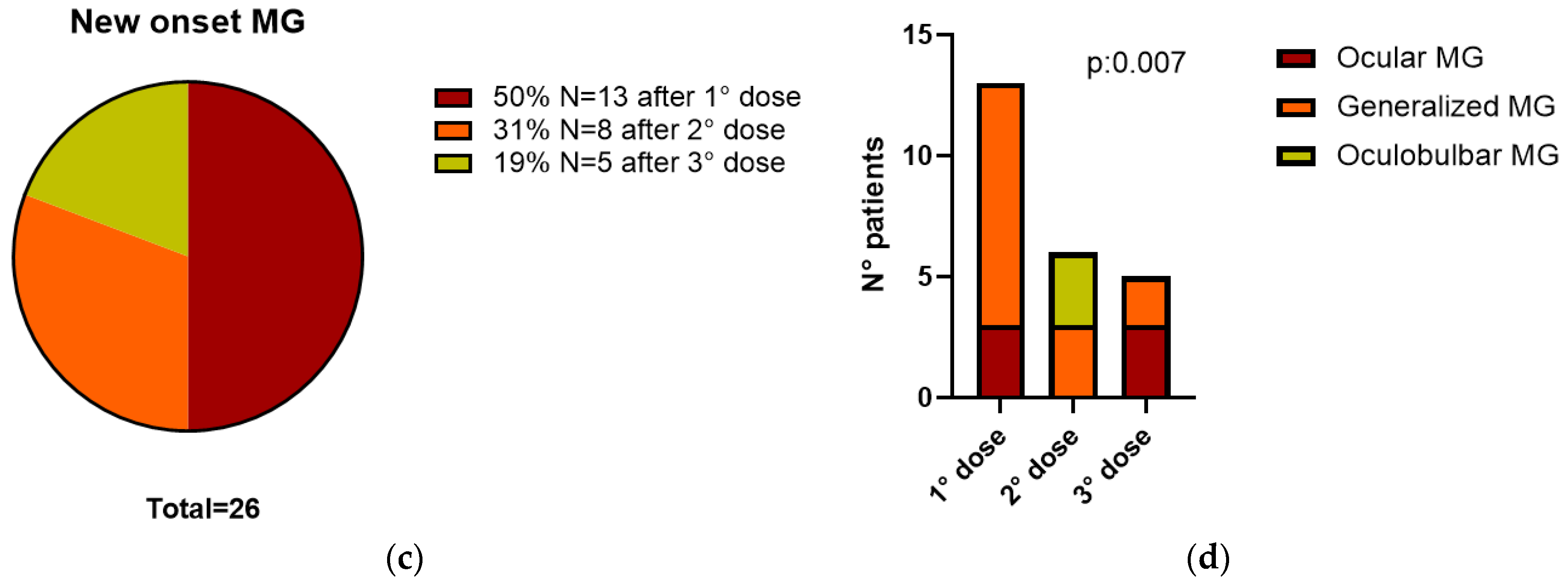
3.2. All 26 Reported Patients
4. Discussion
5. Conclusions
| No. | Age/Sex | Vaccine Name/Dose | Days Onset | Abs Status | First Symptom | MG Type | Treatment | Follow-Up | Chest-CT | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65/M | Pfizer-BioNTech/3° | 21 | AchR+ | Diplopia | Ocular | P300 mg/S10 mg | Good recovery at 2MTHs | no | [40] |
| 2 | 82/M | Pfizer-BioNTech/2° | 2 | AchR+ | Slurred speech | Generalized | IV-P/IV-IG/S | Improved | no | [14] |
| 3 | 91/M | Pfizer-BioNTech/2° | 10 | AchR+ | Oculobulbar | Oculobulbar | P90 mg | Unchanged | no | [41] |
| 4 | 80/M | Moderna mRNA1273/2° | 6 | AchR+ | Oculobulbar | Oculobulbar | P90 mg/PLAEX/AZA150 mg | Mild ptosis at 3MTHs | no | [41] |
| 5 | 55/M | Moderna mRNA1273/1° | 3 | AchR+ | ULs-neck-diplopia | Generalized | P240 mg/IV-IG/S50 mg | Mild UL at 3MTHs | no | [41] |
| 6 | 73/M | O.A ChAdOx1/1° | 8 | AchR and RF+ | Monolateral ptosis | Ocular | P240 mg | NR | no | [42] |
| 7 | 30/M | Moderna mRNA1273/1° | 2 | AchR+ | Diplopia | Generalized | P90 mg/S10 mg | NR | no | [26] |
| 8 | 35/M | O.A ChAdOx1/1° | 7 | AchR+ | Diplopia | Ocular | NR | NR | no | [43] |
| 9 | 33/F | Pfizer-BioNTech/2° | 0 | Double-seronegative | GW and diplopia | Generalized | P360 mg | Partial improvement | TH | [44] |
| 10 | 72/M | Pfizer-BioNTech/2° | 1 | NR | NR | NR | S60 mg/PLAEX | Recovered | NR | [45] |
| 11 | 73/M | Pfizer-BioNTech/2° | 7 | NR | Ocular signs | Generalized | PLAEX/P/S | NR | NR | [45] |
| 12 | 65/M | Pfizer-BioNTech/3° | 3 | AchR+ | Diplopia | Ocular | PLAEX/P180 mg | Improvement at 3MTHs | no | [46] |
| 13 | 60/M | Moderna mRNA-1273/3° | 6 | AchR and ANA+ | Dysarthria | Generalized | P/S | Improvement | no | [47] |
| 14 | 13/F | Pfizer-BioNTech/1° | 14 | AchR negative | NR | Generalized | P/S | NR | NR | [38] |
| 15 | 59/M | O.A ChAdOx1/1° | 2 | AchR+ | NR | Generalized | P/S | NR | NR | [38] |
| 16 | 63/M | Pfizer-BioNTech/3° | 3 | AchR+ | NR | Ocular | P | NR | NR | [38] |
| 17 | 73/M | Pfizer-BioNTech/3° | 12 | AchR+ | NR | Generalized | P/IV-IG/S | NR | NR | [38] |
| 18 | 50/M | Pfizer-BioNTech/1° | 7 | AchR+ | NR | Ocular | P | NR | NR | [38] |
| 19 | 83/F | Pfizer-BioNTech/1° | 6 | AchR+ | NR | Generalized | P/IV-IG/S | NR | NR | [38] |
| 20 | 77/M | O.A ChAdOx1/1° | 3 | AchR+ | NR | Generalized | P/PLEX/S | NR | NR | [38] |
| 21 | 53/M | O.A ChAdOx1/1° | 1 | AchR+ | diplopia | Generalized | P360 mg/S15 mg | Improvement at 1MTH | no | [48] |
| 22 | 68/M | Sinopharm/2° | 3 | AchR+ | Dysarthria/dysphagia | Oculobulbar | P180 mg/IVIG/S | Improvement | no | [49] |
| 23 | 46/F | Pfizer-BioNTech/1° | 2 | Triple-seronegative | Monolateral ptosis | Generalized | P/PLAEX/S/M | Stabilization | no | [50] |
| 24 | 46/F | Pfizer-BioNTech/1° | 5 | AchR+ | LL weakness | Generalized | P/PLAEX/S/M | Stabilization | TH | [51] |
| 25 | 64/F | Pfizer-BioNTech/2° | 12 | AchR+ | NR | NR | NR | NR | NR | [30] |
| 26 * | 73/M | O.A ChAdOx1/1° | 28 | AchR+ | Diplopia | Generalized | P360 mg/S12.5 mg/AZA | Improvement | T |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acter, T.; Uddin, N.; Das, J.; Akhter, A.; Choudhury, T.R.; Kim, S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: A global health emergency. Sci. Total Environ. 2020, 730, 138996. [Google Scholar] [CrossRef] [PubMed]
- Tondo, G.; Virgilio, E.; Naldi, A.; Bianchi, A.; Comi, C. Safety of COVID-19 Vaccines: Spotlight on Neurological Complications. Life 2022, 12, 1338. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.I.; Ghany, S.; Gilkes, T.; Umakanthan, S. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad. Med. J. 2022, 98, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Hasanzad, M.; Namazi, H.; Larijani, B. COVID-19 anti-vaccine attitude and hesitancy. J. Diabetes Metab. Disord. 2022, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Causality Assessment of an Adverse Event Following Immunization (AEFI): User Manual for the Revised WHO Classification. Available online: https://www.who.int/publications-detail-redirect/9789241516990 (accessed on 15 October 2022).
- The Use of the WHO-UMC System for Standardised Case Causality Assessment. Available online: https://www.who.int/publications/m/item/WHO-causality-assessment (accessed on 15 October 2022).
- Estephan, E.D.P.; Baima, J.P.S.; Zambon, A.A. Myasthenia gravis in clinical practice. Arq. Neuro-Psiquiatr. 2022, 80, 257–265. [Google Scholar] [CrossRef]
- Huda, S.; Woodhall, M.R.; Vincent, A.; Heckmann, J.M. Characteristics Of acetylcholine-receptor-antibody-negative myasthenia gravis in a South African cohort. Muscle Nerve 2016, 54, 1023–1029. [Google Scholar] [CrossRef]
- Vincent, A.; Newsom-Davis, J. Acetylcholine receptor antibody as a diagnostic test for myasthenia gravis: Results in 153 validated cases and 2967 diagnostic assays. J. Neurol. Neurosurg. Psychiatry 1985, 48, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Rossi, C.M.; Melazzini, F.; Gastaldi, M.; Bugatti, S.; Rotondi, M.; Bianchi, P.I.; Gentile, A.; Chiovato, L.; Montecucco, C.; et al. Seronegative autoimmune diseases: A challenging diagnosis. Autoimmun. Rev. 2022, 21, 103143. [Google Scholar] [CrossRef]
- Kwon, Y.N.; Woodhall, M.; Sung, J.-J.; Kim, K.-K.; Lim, Y.-M.; Kim, H.; Kim, J.-E.; Baek, S.-H.; Kim, B.-J.; Park, J.-S.; et al. Clinical pitfalls and serological diagnostics of MuSK myasthenia gravis. J. Neurol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Spagni, G.; Gastaldi, M.; Businaro, P.; Chemkhi, Z.; Carrozza, C.; Mascagna, G.; Falso, S.; Scaranzin, S.; Franciotta, D.; Evoli, A.; et al. Comparison of Fixed and Live Cell-Based Assay for the Detection of AChR and MuSK Antibodies in Myasthenia Gravis. Neurol. Neuroimmunol. Neuroinflammation 2023, 10, e200038. [Google Scholar] [CrossRef]
- Damato, V.; Spagni, G.; Monte, G.; Woodhall, M.; Jacobson, L.; Falso, S.; Smith, T.; Iorio, R.; Waters, P.; Irani, S.R.; et al. Clinical value of cell-based assays in the characterisation of seronegative myasthenia gravis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.; Pougnier, C. A Case of COVID-19 Vaccine Associated New Diagnosis Myasthenia Gravis. J. Prim. Care Community Health 2021, 12, 21501327211051933. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E. Myasthenia Gravis. N. Engl. J. Med. 2016, 375, 2570–2581. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Verschuuren, J.J. Myasthenia gravis: Subgroup classification and therapeutic strategies. Lancet Neurol. 2015, 14, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M. Dysphonia as first symptom of late-onset myasthenia gravis. J. Gen. Intern. Med. 2006, 21, C4–C6. [Google Scholar] [CrossRef] [PubMed]
- Sih, M.; Soliven, B.; Mathenia, N.; Jacobsen, J.; Rezania, K. Head-drop: A frequent feature of late-onset myasthenia gravis. Muscle Nerve 2017, 56, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Toba, H.; Kondo, K.; Sadohara, Y.; Otsuka, H.; Morimoto, M.; Kajiura, K.; Nakagawa, Y.; Yoshida, M.; Kawakami, Y.; Takizawa, H.; et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography and the relationship between fluorodeoxyglucose uptake and the expression of hypoxia-inducible factor-1α, glucose transporter-1 and vascular endothelial growth factor in thymic epithelial tumours. Eur. J. Cardiothorac. Surg. 2013, 44, e105–e112. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, N.; Johkoh, T.; Mihara, N.; Honda, O.; Kozuka, T.; Koyama, M.; Hamada, S.; Okumura, M.; Ohta, M.; Eimoto, T.; et al. Using the World Health Organization Classification of thymic epithelial neoplasms to describe CT findings. AJR Am. J. Roentgenol. 2002, 179, 881–886. [Google Scholar] [CrossRef]
- Carr, A.S.; Cardwell, C.R.; McCarron, P.O.; McConville, J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.H. The epidemiology of myasthenia gravis. Semin. Neurol. 2004, 24, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Bedlack, R.S.; Sanders, D.B. On the concept of myasthenic crisis. J. Clin. Neuromuscul. Dis. 2002, 4, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Tugasworo, D.; Kurnianto, A.; Retnaningsih; Andhitara, Y.; Ardhini, R.; Budiman, J. The relationship between myasthenia gravis and COVID-19: A systematic review. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 1–13. [Google Scholar] [CrossRef]
- Chung, J.Y.; Lee, S.J.; Shin, B.S.; Kang, H.G. Myasthenia gravis following human papillomavirus vaccination: A case report. BMC Neurol. 2018, 18, 222. [Google Scholar] [CrossRef]
- Hoshina, Y.; Sowers, C.; Baker, V. Myasthenia Gravis Presenting after Administration of the mRNA-1273 Vaccine. Eur. J. Case Rep. Intern. Med. 2022, 9, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Mutair, A.A.; Hajissa, K.; Alfaraj, A.H.; Al-Jishi, J.M.; Alhajri, M.; Alwarthan, S.; Alsuliman, S.A.; Al-Najjar, A.H.; Al Zaydani, I.A.; et al. A Comprehensive Review on the Current Vaccines and Their Efficacies to Combat SARS-CoV-2 Variants. Vaccines 2022, 10, 1655. [Google Scholar] [CrossRef] [PubMed]
- Ishizuchi, K.; Takizawa, T.; Sekiguchi, K.; Motegi, H.; Oyama, M.; Nakahara, J.; Suzuki, S. Flare of myasthenia gravis induced by COVID-19 vaccines. J. Neurol. Sci. 2022, 436, 120225. [Google Scholar] [CrossRef]
- Li, H.Y.; Shao, L.Y.; Song, M.; Hu, S.M.; Yue, Y.X.; Li, H.F. Safety of inactivated SARS-CoV-2 vaccines in myasthenia gravis: A survey-based study. Front. Immunol. 2022, 13, 923017. [Google Scholar] [CrossRef] [PubMed]
- Sansone, G.; Bonifati, D.M. Vaccines and myasthenia gravis: A comprehensive review and retrospective study of SARS-CoV-2 vaccination in a large cohort of myasthenic patients. J. Neurol. 2022, 269, 3965–3981. [Google Scholar] [CrossRef] [PubMed]
- Doron, A.; Piura, Y.; Vigiser, I.; Kolb, H.; Regev, K.; Nesher, N.; Karni, A. BNT162b2 mRNA COVID-19 vaccine three-dose safety and risk of COVID-19 in patients with myasthenia gravis during the alpha, delta, and omicron waves. J. Neurol. 2022, 269, 6193–6201. [Google Scholar] [CrossRef]
- Lee, J.R.; Jaffry, M.; Mandava, K.; Rosario, S.; Jaffry, K.; Jedidi, K.; Souayah, N. Is COVID-19 Vaccination Associated with an Increased Reporting Rate of Myasthenia Gravis? A Vaccine Adverse Event Reporting System (VAERS) Study (P11-13.002). Neurology 2022, 98, 1795. [Google Scholar]
- Lupica, A.; Di Stefano, V.; Iacono, S.; Pignolo, A.; Quartana, M.; Gagliardo, A.; Fierro, B.; Brighina, F. Impact of COVID-19 in AChR Myasthenia Gravis and the Safety of Vaccines: Data from an Italian Cohort. Neurol. Int. 2022, 14, 406–416. [Google Scholar] [CrossRef]
- Tagliaferri, A.R.; Narvaneni, S.; Azzam, M.H.; Grist, W. A Case of COVID-19 Vaccine Causing a Myasthenia Gravis Crisis. Cureus 2021, 13, e15581. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Handunnetthi, L.; Saatci, D.; Pan, J.; Katikireddi, S.V.; Razvi, S.; Hunt, D.; Mei, X.W.; Dixon, S.; Zaccardi, F.; et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 2021, 27, 2144–2153. [Google Scholar] [CrossRef]
- Wraith, D.C.; Goldman, M.; Lambert, P.H. Vaccination and autoimmune disease: What is the evidence? Lancet 2003, 362, 1659–1666. [Google Scholar] [CrossRef]
- Caron, P. Autoimmune and inflammatory thyroid diseases following vaccination with SARS-CoV-2 vaccines: From etiopathogenesis to clinical management. Endocrine 2022, 78, 406–417. [Google Scholar] [CrossRef]
- Ramdas, S.; Hum, R.M.; Price, A.; Paul, A.; Bland, J.; Burke, G.; Farrugia, M.; Palace, J.; Storrie, A.; Ho, P.; et al. SARS-CoV-2 vaccination and new-onset myasthenia gravis: A report of 7 cases and review of the literature. Neuromuscul. Disord. 2022, 32, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Mirmosayyeb, O.; Moases Ghaffary, E.; Mazdak, M.; Bagheri, Z.; Bagherieh, S.; Shaygannejad, V. Is Myasthenia Gravis a Real Complication of the COVID-19 Vaccine? A Case Report-Based Systematic Review. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- Abicic, A.; Sitas, B.; Adamec, I.; Bilic, E.; Habek, M. New-Onset Ocular Myasthenia Gravis After Booster Dose of COVID-19 Vaccine. Cureus 2022, 14, e27213. [Google Scholar] [CrossRef] [PubMed]
- Fanella, G.; Baiata, C.; Candeloro, E.; Toscano, G.; Colnaghi, S.; Mauri, M.; Cariddi, L.P.; Rebecchi, V.; Solazzo, F.; Banfi, P.; et al. New-onset myasthenia gravis after mRNA SARS-CoV-2 vaccination: A case series. Neurol. Sci. 2022, 43, 5799–5802. [Google Scholar] [CrossRef] [PubMed]
- Galassi, G.; Rispoli, V.; Iori, E.; Ariatti, A.; Marchioni, A. Coincidental Onset of Ocular Myasthenia Gravis Following ChAdOx1 n-CoV-19 Vaccine against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Isr. Med. Assoc. J. 2022, 24, 9–10. [Google Scholar] [PubMed]
- Kang, M.C.; Park, K.A.; Min, J.H.; Oh, S.Y. Myasthenia gravis with ocular symptoms following a ChAdOx1 nCoV-19 vaccination: A case report. Am. J. Ophthalmol. Case Rep. 2022, 27, 101620. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Lee, C.; Park, J.H.; Lee, J.H. Early-Onset Myasthenia Gravis Following COVID-19 Vaccination. J. Korean Med. Sci. 2022, 37, e50. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Poli, K.; Poli, S.; Ziemann, U. Multiple Autoimmune Syndromes Including Acute Disseminated Encephalomyelitis, Myasthenia Gravis, and Thyroiditis Following Messenger Ribonucleic Acid-Based COVID-19 Vaccination: A Case Report. Front. Neurol. 2022, 13, 913515. [Google Scholar] [CrossRef]
- Slavin, E.; Fitzig, J.; Neubert, C.; Garcia-Lopez, F.; Cuevas-Trisan, R. New-onset myasthenia gravis confirmed by electrodiagnostic studies after a third dose of SARS-CoV-2 mRNA-1273 vaccine: A case report. Am. J. Phys. Med. Rehabil. 2022, 101, e176–e179. [Google Scholar] [CrossRef]
- Huang, B.-D.; Hsueh, H.-W.; Yang, S.-H.; Lin, C.-W. New-Onset Myasthenia Gravis After ChAdOx1 nCOV-19 Vaccine Inoculation. J. Neuro-Ophthalmol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Abna, Z.; Khanmoradi, Z.; Abna, Z. A new case of myasthenia gravis following COVID-19 Vaccination. Neuroimmunol. Rep. 2022, 2, 100128. [Google Scholar] [CrossRef]
- Most, D.; Tiem, M.; Ferrey, D.; Dunn-Pirio, A. Myasthenia Gravis Syndrome Following COVID-19 Vaccination and Subsequent Tolerance of Booster Vaccine: A Case Report (P4-1.005). Neurology 2022, 98, 3817. [Google Scholar]
- Bui, A.; Shrivastava, S. Progressive Ascending Weakness after the Pfizer/Biontech COVID-19 Vaccine. Chest 2022, 162, A69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virgilio, E.; Tondo, G.; Montabone, C.; Comi, C. COVID-19 Vaccination and Late-Onset Myasthenia Gravis: A New Case Report and Review of the Literature. Int. J. Environ. Res. Public Health 2023, 20, 467. https://doi.org/10.3390/ijerph20010467
Virgilio E, Tondo G, Montabone C, Comi C. COVID-19 Vaccination and Late-Onset Myasthenia Gravis: A New Case Report and Review of the Literature. International Journal of Environmental Research and Public Health. 2023; 20(1):467. https://doi.org/10.3390/ijerph20010467
Chicago/Turabian StyleVirgilio, Eleonora, Giacomo Tondo, Claudia Montabone, and Cristoforo Comi. 2023. "COVID-19 Vaccination and Late-Onset Myasthenia Gravis: A New Case Report and Review of the Literature" International Journal of Environmental Research and Public Health 20, no. 1: 467. https://doi.org/10.3390/ijerph20010467
APA StyleVirgilio, E., Tondo, G., Montabone, C., & Comi, C. (2023). COVID-19 Vaccination and Late-Onset Myasthenia Gravis: A New Case Report and Review of the Literature. International Journal of Environmental Research and Public Health, 20(1), 467. https://doi.org/10.3390/ijerph20010467







