Caspian Sea Mycosands: The Variety and Abundance of Medically Important Fungi in Beach Sand and Water
Abstract
1. Introduction
2. Materials and Methods
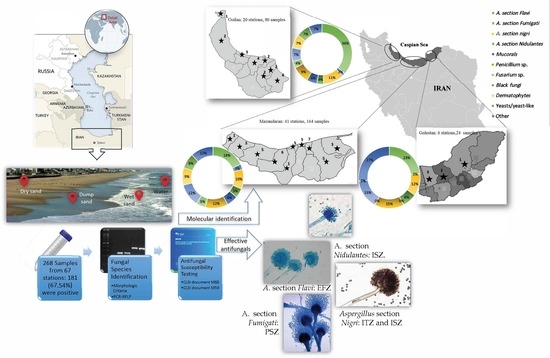
2.1. Study Design
2.2. Sample Collection
2.3. Detection and Identification
2.4. Antifungal Susceptibility Tests
2.5. Statistical Analyses
3. Results
3.1. Species Identification
3.2. Susceptibility Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmoudi, N.; Robeson, M.S.; Castro, H.F.; Fortney, J.L.; Techtmann, S.M.; Joyner, D.C.; Paradis, C.J.; Pfiffner, S.M.; Hazen, T.C. Microbial community composition and diversity in Caspian Sea sediments. FEMS Microbiol. Ecol. 2015, 91, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I.; Pletcher, S.D.; Goldberg, A.N.; Barker, B.M.; Cope, E.K. Fungal microbiota in chronic airway inflammatory disease and emerging relationships with the host immune response. Front. Microbiol. 2017, 8, 2477. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines on Recreational Water Quality. Volume 1: Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive 2006/7/EC of 15 February, Concerning the Management of Bathing Water Quality and Repealing Directive 76/160/EEC; European Union: Brussels, Belgium, 2006. [Google Scholar]
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Vogel, C.; Rogerson, A.; Schatz, S.; Laubach, H.; Tallman, A.; Fell, J. Prevalence of yeasts in beach sand at three bathing beaches in South Florida. Water Res. 2007, 41, 1915–1920. [Google Scholar] [CrossRef]
- Brandão, J.; Wergikosky, B.; Rosado, C.; Noronha, G.; Rosado, L.; Veríssimo, C.; Falcão, M.L.; Giraldes, A.; Simões, M.; Rebelo, H. Qualidade Microbiológica de Areias de Praias Litorais: Relatório Final; Agência Portuguesa do Ambiente: Alfragida, Portugal, 2002. [Google Scholar]
- Heaney, C.D.; Sams, E.; Wing, S.; Marshall, S.; Brenner, K.; Dufour, A.P.; Wade, T.J. Contact with beach sand among beachgoers and risk of illness. Am. J. Epidemiol. 2009, 170, 164–172. [Google Scholar] [CrossRef]
- Brandão, J.; Albergaria, I.; Albuquerque, J.; José, S.; Grossinho, J.; Ferreira, F.; Raposo, A.; Rodrigues, R.; Silva, C.; Jordao, L. Untreated sewage contamination of beach sand from a leaking underground sewage system. Sci. Total Environ. 2020, 740, 140237. [Google Scholar] [CrossRef] [PubMed]
- Candan, A.Y.; Katılmış, Y.; Ergin, Ç. First report of Fusarium species occurrence in loggerhead sea turtle (Caretta caretta) nests and hatchling success in Iztuzu Beach, Turkey. Biologia 2021, 76, 565–573. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Cunha, M.A.; Wergikoski, B.; Ferreira, F.C.; Rodrigues, R.; Parada, H.; Falcão, L.; Rosado, L.; Pinheiro, C. Pathogenic fungi: An unacknowledged risk at coastal resorts? New insights on microbiological sand quality in Portugal. Mar. Pollut. Bull. 2011, 62, 1506–1511. [Google Scholar] [CrossRef]
- Shah, A.; Abdelzaher, A.; Phillips, M.; Hernandez, R.; Solo-Gabriele, H.; Kish, J.; Scorzetti, G.; Fell, J.; Diaz, M.; Scott, T. Indicator microbes correlate with pathogenic bacteria, yeasts and helminths in sand at a subtropical recreational beach site. J. App. Microbiol. 2011, 110, 1571–1583. [Google Scholar] [CrossRef]
- Bik, H.; Halanych, K.; Sharma, J.; Thomas, W. Dramatic Shifts in Benthic Microbial Eukaryote Communities Following the Deepwater Horizon Oil. PLoS ONE 2012, 7, e38550. [Google Scholar] [CrossRef]
- Gomes, D.; Cavalcanti, M.; Fernandes, M.; Lima, D.; Passavante, J. Filamentous fungi isolated from sand and water of “Bairro Novo” and “Casa Caiada” beaches, Olinda, Pernambuco, Brazil. Braz. J. Biol. 2008, 68, 577–582. [Google Scholar] [CrossRef]
- del Carmen González, M.; Hanlin, R.T.; Herrera, T.; Ulloa, M. Fungi colonizing hair-baits from three coastal beaches of Mexico. Mycoscience 2000, 41, 259–262. [Google Scholar] [CrossRef]
- Kakroodi, A.; Kroonenberg, S.; Beni, A.N.; Noehgar, A. Short-and Long-Term Development of the Miankaleh Spit, Southeast Caspian Sea, Iran. J. Coast. Res. 2014, 30, 1236–1242. [Google Scholar] [CrossRef]
- Pong, D.L.; Marom, T.; Makishima, T. Phialemonium infection complicating chronic suppurative otitis media. Med. Mycol. Case Rep. 2014, 4, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Brandão, J.; Gangneux, J.; Arikan-Akdagli, S.; Barac, A.; Bostanaru, A.; Brito, S.; Bull, M.; Çerikçioğlu, N.; Chapman, B.; Efstratiou, M. Mycosands: Fungal diversity and abundance in beach sand and recreational waters—Relevance to human health. Sci. Total Environ. 2021, 781, 146598. [Google Scholar] [CrossRef] [PubMed]
- Käärik, A.; Keller, M.; Kiffer, M.; Perreau, J.; Reisinger, M. Atlas of Airborne Fungal Spores in Europe; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Campbell, C.K.; Johnson, E.M. Identification of Pathogenic Fungi; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Nejati, B.; Sayedi, S.J.; Shokohi, T.; Hedayati, M.T.; Nabili, M.; Mousavi, S.J.; Esmaeeli, M.; Moazeni, M. Pediatric Catheters Infectivity and Identification of Candida Species Isolated from Hospitalized Patients in Mashhad Pediatric Hospital. J. Mazand. Univ. Med. Sci. 2021, 31, 48–59. [Google Scholar]
- Davari, A.; Haghani, I.; Hassanmoghadam, F.; Nabili, M.; Shokohi, T.; Hedayati, M.T.; Shabanzadeh, S.; Moazeni, M. Echinocandin resistance in Candida parapsilosis sensu stricto: Role of alterations in CHS3, FKS1 and Rho gene expression. J. Glob. Antimic. Resist. 2020, 22, 685–688. [Google Scholar] [CrossRef]
- Nasri, T.; Hedayati, M.T.; Abastabar, M.; Pasqualotto, A.C.; Armaki, M.T.; Hoseinnejad, A.; Nabili, M. PCR-RFLP on β-tubulin gene for rapid identification of the most clinically important species of Aspergillus. J. Microbiol. Met. 2015, 117, 144–147. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mirhendi, H.; Rezaei-Matehkolaei, A.; Ghahri, M.; Shidfar, M.R.; Jalalizand, N.; Makimura, K. Molecular identification and distribution profile of Candida species isolated from Iranian patients. Med. Mycol. 2013, 51, 657–663. [Google Scholar] [CrossRef]
- Rezaei-Matehkolaei, A.; Makimura, K.; Shidfar, M.; Zaini, F.; Eshraghian, M.; Jalalizand, N.; Nouripour-Sisakht, S.; Hosseinpour, L.; Mirhendi, H. Use of single-enzyme PCR-restriction digestion barcode targeting the internal transcribed spacers (ITS rDNA) to identify dermatophyte species. Iran J. Pub. Health 2012, 41, 82. [Google Scholar]
- CLSI. Performance Standard for Antifungal Susceptibility Testing of Yeasts, 1st ed.; CLSI supplement M60; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; CLSI Standard M38; Clinical and Laboratory Standards Institute: Wayne PA, USA, 2017. [Google Scholar]
- CLSI. Epidemiological Cutoff Values for Antifungal Susceptibility Testing, 2nd ed.; CLSI Supplement M59; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Sato, M.I.Z.; Di Bari, M.; Lamparelli, C.C.; Truzzi, A.C.; Coelho, M.C.L.; Hachich, E.M. Sanitary quality of sands from marine recreational beaches of São Paulo, Brazil. Braz. J. Microbiol. 2005, 36, 321–326. [Google Scholar] [CrossRef]
- Burton, G.A., Jr.; Gunnison, D.; Lanza, G.R. Survival of pathogenic bacteria in various freshwater sediments. App. Environ. Microbiol. 1987, 53, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.; Nascimento, M.; Oliveira, J. Preliminary characterisation and proposal of microbiological quality standard of sand beaches. Water Sci. Technol. 1993, 27, 453–456. [Google Scholar] [CrossRef]
- Sabino, R.; Rodrigues, R.; Costa, I.; Carneiro, C.; Cunha, M.; Duarte, A.; Faria, N.l.; Ferreira, F.; Gargaté, M.J.o.; Júlio, C. Routine screening of harmful microorganisms in beach sands: Implications to public health. Sci. Total Environ. 2014, 472, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Safe Recreational Water Environments: Coastal and Fresh Waters; WHO: Geneva, Switzerland, 2003; Volume 1. [Google Scholar]
- Weiskerger, C.J.; Brandão, J.; Ahmed, W.; Aslan, A.; Avolio, L.; Badgley, B.D.; Boehm, A.B.; Edge, T.A.; Fleisher, J.M.; Heaney, C.D. Impacts of a changing earth on microbial dynamics and human health risks in the continuum between beach water and sand. Water Res. 2019, 162, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Mancini, L.; D’angelo, A.; Pierdominici, E.; Ferrari, C.; Anselmo, A.; Venturi, L.; Fazzo, L.; Formichetti, P.; Iaconelli, M.; Pennelli, B. Microbiological quality of Italian beach sands. Microchem. J. 2005, 79, 257–261. [Google Scholar] [CrossRef]
- Chadeganipour, M.; Mohammadi, R. A 9-Year Experience of Aspergillus Infections from Isfahan, Iran. Infect. Drug Resist. 2020, 13, 2301. [Google Scholar] [CrossRef]
- Khodavaisy, S.; Badali, H.; Rezaie, S.; Nabili, M.; Moghadam, K.G.; Afhami, S.; Hagen, F.; Aala, F.; Hashemi, S.J.; Meis, J.F. Genotyping of clinical and environmental Aspergillus flavus isolates from Iran using microsatellites. Mycoses 2016, 59, 220–225. [Google Scholar] [CrossRef]
- Hedayati, M.T.; Mayahi, S.; Denning, D.W. A study on Aspergillus species in houses of asthmatic patients from Sari City, Iran and a brief review of the health effects of exposure to indoor Aspergillus. Environ. Monit Assess. 2010, 168, 481–487. [Google Scholar] [CrossRef]
- Gago, S.; Denning, D.W.; Bowyer, P. Pathophysiological aspects of Aspergillus colonization in disease. Medmycol 2019, 57, S219–S227. [Google Scholar]
- Nabili, M.; Shokohi, T.; Moazeni, M.; Khodavaisy, S.; Aliyali, M.; Badiee, P.; Zarrinfar, H.; Hagen, F.; Badali, H. High prevalence of clinical and environmental triazole-resistant Aspergillus fumigatus in Iran: Is it a challenging issue? J. Med. Microbiol. 2016, 65, 468–475. [Google Scholar] [CrossRef]
- Badali, H.; Shokohi, T.; Khodavaisy, S.; Moazeni, M.; Farhadi, M.; Nabili, M. Molecular typing of clinical and environmental Aspergillus fumigatus isolates from Iran using microsatellites. Curr. Med. Mycol. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Ahangarkani, F.; Puts, Y.; Nabili, M.; Khodavaisy, S.; Moazeni, M.; Salehi, Z.; Laal Kargar, M.; Badali, H.; Meis, J.F. First azole-resistant Aspergillus fumigatus isolates with the environmental TR46/Y121F/T289A mutation in Iran. Mycoses 2020, 63, 430–436. [Google Scholar] [CrossRef]
- Ahangarkani, F.; Badali, H.; Abbasi, K.; Nabili, M.; Khodavaisy, S.; de Groot, T.; Meis, J.F. Clonal expansion of environmental triazole resistant Aspergillus fumigatus in Iran. J. Fungi 2020, 6, 199. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.-P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Cadena, J.; Thompson, G.R.; Patterson, T.F. Aspergillosis: Epidemiology, diagnosis, and treatment. Infect. Dis. Clin. 2021, 35, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Pinto, K.; Hachich, E.; Sato, M.; Di Bari, M.; Coelho, M.; Matte, M.H.; Lamparelli, C.; Razzolini, M.T.P. Microbiological quality assessment of sand and water from three selected beaches of South Coast, Sao Paulo State, Brazil. Water Sci. Technol. 2012, 66, 2475–2482. [Google Scholar] [CrossRef] [PubMed]

| Fungal Strain | Primer | Sequence (5′–3′) | Related Restriction Enzyme | Reference |
|---|---|---|---|---|
| Aspergillus sp. | Bt-F Bt-R | GGTAACCAAATCGGTGCTGCTTTC- ACCCTCAGTGTAGTGACCCTTGGC- | Alw1 | [23] |
| Dermatophytes | ITS-F ITS-R | GCACCTTCAGTCGTAGAGACG- GCACCTTCAGTCGTAGAGACG- | Mva1 | [25] |
| Yeast sp. | ITS-F ITS-R | GCACCTTCAGTCGTAGAGACG- GCACCTTCAGTCGTAGAGACG- | Msp1 | [24] |
| Number of Isolates Collected from Each Sample | |||||
|---|---|---|---|---|---|
| Number of Fungal Species | Dry Sand | Wet Sand | Water Sand | Water | |
| Hyaline filamentous fungi | Aspergillus section Flavi (44) | 15 | 18 | 5 | 6 |
| Aspergillus section Nigri (19) | 7 | 5 | 3 | 4 | |
| Aspergiilus section Fumigati (4) | 3 | 1 | 0 | 0 | |
| Aspergillus section Nidulantes (9) | 2 | 5 | 2 | 0 | |
| Penicillium sp. (21) | 7 | 5 | 4 | 5 | |
| Mucor sp. (5) | 2 | 3 | 0 | 0 | |
| Rhizopus sp. (4) | 1 | 2 | 0 | 1 | |
| Trichoderma (18) | 5 | 2 | 5 | 6 | |
| Fusarium sp. (7) | 2 | 4 | 1 | 0 | |
| Geothricom sp. (2) | 0 | 2 | 0 | 0 | |
| Acromonium (3) | 2 | 1 | 0 | 0 | |
| Black filamentous fungi | Cladosporium sp. (5) | 3 | 2 | 0 | 0 |
| Bipolaris (2) | 1 | 1 | 0 | 0 | |
| Alternaria (3) | 3 | 0 | 0 | 0 | |
| Other black fungi (10) | 4 | 3 | 1 | 2 | |
| Yeast/yeast-like fungi | Thrichosporon (3) | 2 | 1 | 0 | 0 |
| Candida sp. (5) | 3 | 1 | 0 | 1 | |
| Other yeast species (4) | 2 | 1 | 0 | 1 | |
| Dermatophytes | Trichophyton mentagrophytes/interdigitale (10) | 6 | 3 | 0 | 1 |
| Microsporum canis (1) | 1 | 0 | 0 | 0 | |
| Microsporum gypseum (2) | 1 | 1 | 0 | 0 | |
| Total (181) | 72 | 61 | 21 | 27 | |
| Aspergillus Isolate | ** Antifungal Agent | |||||||
|---|---|---|---|---|---|---|---|---|
| AMB | VRZ | ITZ | PSZ | ISZ | EFZ | |||
| Aspergillus section Flavi (n = 44) | * MICs (µg/mL) | MIC50 | 0.5 | 0.25 | 0.25 | 0.25 | 0.5 | 0.25 |
| MIC90 | 0.5 | 0.5 | 2 | 1 | 2 | 2 | ||
| GM | 0.3372 | 0.2704 | 0.3267 | 0.3067 | 0.3161 | 0.1684 | ||
| MIC range | 0.125–1 | 0.062–2 | 0.06–1 | 0.062–2 | 0.031–2 | |||
| Aspergiilus section Nigri (n = 19) | MIC50 | 0.125 | 0.5 | 0.25 | 0.5 | 0.5 | 0.5 | |
| MIC90 | 0.5 | 2 | 2 | 0.5 | 2 | 4 | ||
| GM | 0.1798 | 0.5785 | 0.3471 | 0.6943 | 0.3594 | 0.6000 | ||
| MIC range | 0.031–0.5 | 0.25–2 | 0.125–2 | 0.25–4 | 0.031–4 | 0.125–4 | ||
| Aspergiilus section Nidulantes (n = 9) | MIC50 | 0.5 | 0.25 | 0.25 | 0.125 | 0.062 | 0.125 | |
| MIC90 | 1 | 0.5 | 2 | 0.5 | 0.5 | 0.25 | ||
| GM | 0.6299 | 0.25 | 0.3149 | 0.1573 | 0.0986 | 0.1348 | ||
| MIC range | 0.25–1 | 0.125–0.5 | 0.125–2 | 0.062–0.5 | 0.062–0.5 | 0.031–0.25 | ||
| Aspergiilus section Fumigati (n = 4) | MIC50 | ND ♣ | ND | ND | ND | ND | ND | |
| MIC90 | ND | ND | ND | ND | ND | ND | ||
| GM | 1 | 0.4994 | 0.5946 | 0.2494 | 0.4989 | 0.5 | ||
| MIC range | 0.5–8 | 0.062–4 | 0.125–8 | 0.062–0.5 | 0.062–1 | 0.125–4 | ||
| Candida sp. (n = 5) | MIC50 | ND | ND | ND | ND | ND | - | |
| MIC90 | ND | ND | ND | ND | ND | |||
| GM | 0.6771 | 0.1764 | 0.25 | 0.6898 | 0.0364 | |||
| MIC range | 0.125–2 | 0.031–1 | 0.062–1 | 0.125–4 | 0.016–1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moazeni, M.; Hedayati, M.T.; Haghani, I.; Abastabar, M.; Jahantigh, A.S.; Kheshteh, M.; Nabili, M.; Brandão, J. Caspian Sea Mycosands: The Variety and Abundance of Medically Important Fungi in Beach Sand and Water. Int. J. Environ. Res. Public Health 2023, 20, 459. https://doi.org/10.3390/ijerph20010459
Moazeni M, Hedayati MT, Haghani I, Abastabar M, Jahantigh AS, Kheshteh M, Nabili M, Brandão J. Caspian Sea Mycosands: The Variety and Abundance of Medically Important Fungi in Beach Sand and Water. International Journal of Environmental Research and Public Health. 2023; 20(1):459. https://doi.org/10.3390/ijerph20010459
Chicago/Turabian StyleMoazeni, Maryam, Mohammad Taghi Hedayati, Iman Haghani, Mahdi Abastabar, Abolfazl Saravani Jahantigh, Maryam Kheshteh, Mojtaba Nabili, and João Brandão. 2023. "Caspian Sea Mycosands: The Variety and Abundance of Medically Important Fungi in Beach Sand and Water" International Journal of Environmental Research and Public Health 20, no. 1: 459. https://doi.org/10.3390/ijerph20010459
APA StyleMoazeni, M., Hedayati, M. T., Haghani, I., Abastabar, M., Jahantigh, A. S., Kheshteh, M., Nabili, M., & Brandão, J. (2023). Caspian Sea Mycosands: The Variety and Abundance of Medically Important Fungi in Beach Sand and Water. International Journal of Environmental Research and Public Health, 20(1), 459. https://doi.org/10.3390/ijerph20010459