Network Analysis of Neurobehavioral Symptom Patterns in an International Sample of Spanish-Speakers with a History of COVID-19 and Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Procedure
2.4. Data Analyses
3. Results
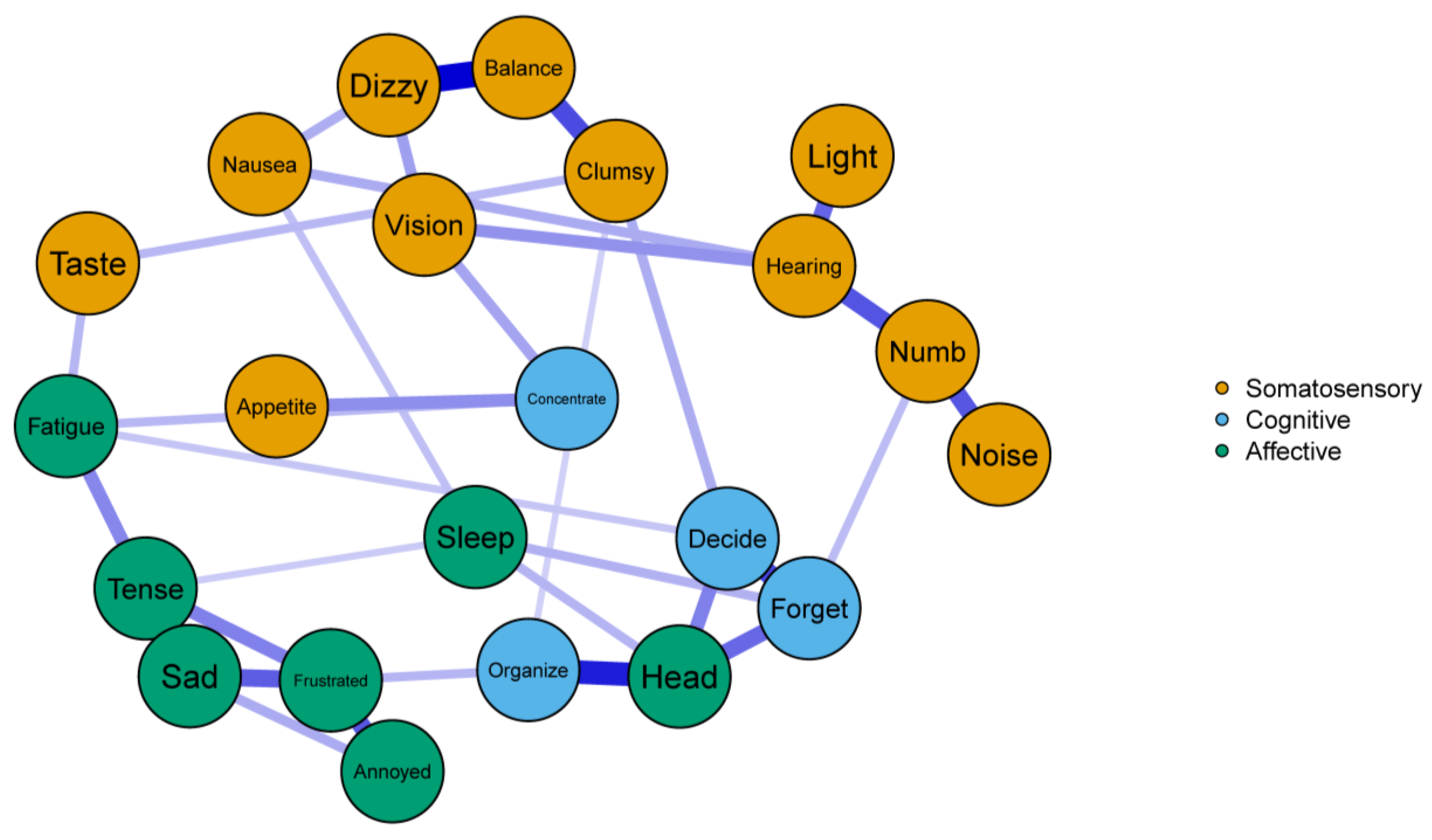
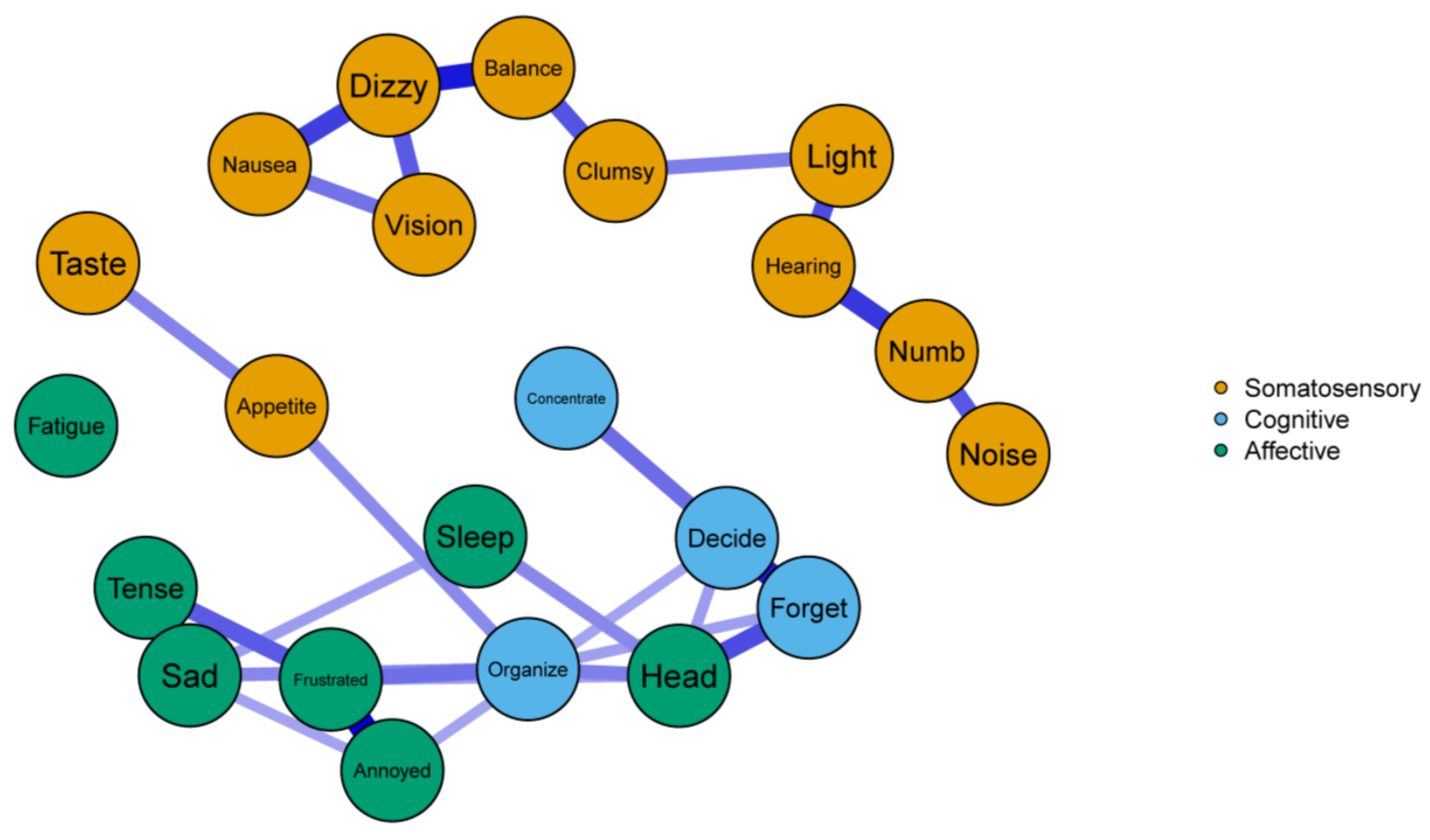
3.1. Network Structure
3.2. Global Network Comparisons
3.3. Edge Comparisons
3.4. Centrality Comparisons
4. Discussion
4.1. Connections with Previous Research and Implications
4.2. Study limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Callard, F.; Perego, E. How and why patients made Long Covid. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: What do we know about “long covid”? BMJ 2020, 370, m2815. [Google Scholar] [CrossRef]
- CDC. Post-COVID Conditions. Centers for Disease Control and Prevention. Published 17 September 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 7 January 2022).
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Hugon, J.; Msika, E.-F.; Queneau, M.; Farid, K.; Paquet, C. Long COVID: Cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J. Neurol. 2022, 269, 44–46. [Google Scholar] [CrossRef]
- Becker, J.H.; Lin, J.J.; Doernberg, M.; Stone, K.; Navis, A.; Festa, J.R.; Wisnivesky, J.P. Assessment of Cognitive Function in Patients After COVID-19 Infection. JAMA Netw. Open 2021, 4, e2130645. [Google Scholar] [CrossRef]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyère, F.; Stefic, K.; et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021, 27, 258–263. [Google Scholar] [CrossRef]
- Nehme, M.; Braillard, O.; Alcoba, G.; Perone, S.A.; Courvoisier, D.; Chappuis, F.; Guessous, I. COVID-19 Symptoms: Longitudinal Evolution and Persistence in Outpatient Settings. Ann. Intern. Med. 2021, 174, 723–725. [Google Scholar] [CrossRef]
- Chopra, V.; Flanders, S.A.; O’Malley, M.; Malani, A.N.; Prescott, H.C. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann. Intern. Med. 2021, 174, 576–578. [Google Scholar] [CrossRef]
- Kamal, M.; Omirah, M.A.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2021, 75, e13746. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef]
- de Freitas, R.F.; Torres, S.C.; Martín-Sánchez, F.J.; Carbó, A.V.; Lauria, G.; Nunes, J.P.L. Syncope and COVID-19 disease—A systematic review. Auton. Neurosci. 2021, 235, 102872. [Google Scholar] [CrossRef]
- Tu, T.M.; Seet, C.Y.H.; Koh, J.S.; Tham, C.H.; Chiew, H.J.; De Leon, J.A.; Chua, C.Y.K.; Hui, A.C.-F.; Tan, S.S.Y.; Vasoo, S.S.; et al. Acute Ischemic Stroke During the Convalescent Phase of Asymptomatic COVID-2019 Infection in Men. JAMA Netw. Open 2021, 4, e217498. [Google Scholar] [CrossRef]
- Zubair, A.S.; McAlpine, L.S.; Gardin, T.; Farhadian, S.; Kuruvilla, D.E.; Spudich, S. Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurol. 2020, 77, 1018–1027. [Google Scholar] [CrossRef]
- Mullaguri, N.; Sivakumar, S.; Battineni, A.; Anand, S.; Vanderwerf, J. COVID-19 Related Acute Hemorrhagic Necrotizing Encephalitis: A Report of Two Cases and Literature Review. Cureus 2021, 13, e14236. [Google Scholar] [CrossRef]
- Naz, S.; Hanif, M.; Haider, M.A.; Ali, M.J.; Ahmed, M.U.; Saleem, S. Meningitis as an Initial Presentation of COVID-19: A Case Report. Front. Public Health 2020, 8, 474. [Google Scholar] [CrossRef]
- Kuroda, N. Epilepsy and COVID-19: Updated evidence and narrative review. Epilepsy Behav. 2021, 116, 107785. [Google Scholar] [CrossRef]
- Khan, F.; Sharma, P.; Pandey, S.; Sharma, D.; V., V.; Kumar, N.; Shukla, S.; Dandu, H.; Jain, A.; Garg, R.K.; et al. COVID-19-associated Guillain-Barre syndrome: Postinfectious alone or neuroinvasive too? J. Med. Virol. 2021, 93, 6045–6049. [Google Scholar] [CrossRef]
- Crunfli, F.; Carregari, V.C.; Veras, F.P.; Vendramini, P.H.; Valença, A.G.F.; Antunes, A.S.L.M.; Brandão-Teles, C.; da Silva Zuccoli, G.; Reis-de-Oliveira, G.; Silva-Costa, L.C.; et al. SARS-CoV-2 infects brain astrocytes of COVID-19 patients and impairs neuronal viability. MedRxiv 2021. [Google Scholar] [CrossRef]
- Andrews, M.G.; Mukhtar, T.; Eze, U.C.; Simoneau, C.R.; Ross, J.; Parikshak, N.; Wang, S.; Zhou, L.; Koontz, M.; Velmeshev, D.; et al. Tropism of SARS-CoV-2 for developing human cortical astrocytes. Proc. Natl. Acad. Sci. USA 2022, 119, e2122236119. [Google Scholar] [CrossRef]
- Isacson, O. The Consequences of Coronavirus-Induced Cytokine Storm Are Associated With Neurological Diseases, Which May Be Preventable. Front. Neurol. 2020, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Hirunpattarasilp, C.; James, G.; Freitas, F.; Sethi, H.; Kittler, J.T.; Huo, J.; Owens, R.J.; Attwell, D. SARS-CoV-2 binding to ACE2 triggers pericyte-mediated angiotensin-evoked cerebral capillary constriction. bioRxiv 2021. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. Symptoms, complications and management of long COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Benzakour, L.; Assal, F.; Péron, J.A. Neuropsychological long-COVID: Neurologic or psychiatric origin? Rev. Med. Suisse 2021, 17, 822–826. [Google Scholar] [PubMed]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive deficits in people who have recovered from COVID-19. Eclinicalmedicine 2021, 39, 101044. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.; Johnsen, S.; Sattler, S.; Nielsen, S.; Kunalan, K.; Rungby, J.; Lapperre, T.; Porsberg, C. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021, 46, 39–48. [Google Scholar] [CrossRef]
- Frontera, J.A.; Lewis, A.; Melmed, K.; Lin, J.; Kondziella, D.; Helbok, R.; Yaghi, S.; Meropol, S.; Wisniewski, T.; Balcer, L.; et al. Prevalence and Predictors of Prolonged Cognitive and Psychological Symptoms following COVID-19 in the United States. Front. Aging Neurosci. 2021, 13, 357. [Google Scholar] [CrossRef]
- Bonizzato, S.; Ghiggia, A.; Ferraro, F.; Galante, E. Cognitive, behavioral, and psychological manifestations of COVID-19 in post-acute rehabilitation setting: Preliminary data of an observational study. Neurol. Sci. 2022, 43, 51–58. [Google Scholar] [CrossRef]
- Thye, A.Y.-K.; Law, J.W.-F.; Tan, L.T.-H.; Pusparajah, P.; Ser, H.-L.; Thurairajasingam, S.; Letchumanan, V.; Lee, L.-H. Psychological Symptoms in COVID-19 Patients: Insights into Pathophysiology and Risk Factors of Long COVID-19. Biology 2022, 11, 61. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Fried, E.I.; Haslbeck, J.M.B. Using network analysis to examine links between individual depression symptoms, inflammatory markers, and covariates. Psychol. Med. 2020, 50, 2682–2690. [Google Scholar] [CrossRef] [PubMed]
- Armour, C.; Fried, E.I.; Deserno, M.K.; Tsai, J.; Pietrzak, R.H. A network analysis of DSM-5 posttraumatic stress disorder symptoms and correlates in U.S. military veterans. J. Anxiety Disord. 2017, 45, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A. Review of the Neurobehavioral Symptom Inventory. Rehabil. Psychol. 2021, 66, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Cicerone, K.D.; Kalmar, K. Persistent post-concussive syndrome: Structure of subjective complaints after mild traumatic brain injury. Arch. Phys. Med. Rehabil. 1994, 75, 723. [Google Scholar] [CrossRef]
- Lequerica, A.H.; Arango-Lasprilla, J.C.; Krch, D. Factor analysis of the Neurobehavioral Symptom Inventory among a sample of Spanish-speakers. Neuropsychol. Rehabil. 2020, 32, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Epskamp, S.; Borsboom, D.; Fried, E.I. Estimating psychological networks and their accuracy: A tutorial paper. Behav. Res. Methods 2018, 50, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Epskamp, S.; Fried, E.I. A tutorial on regularized partial correlation networks. Psychol. Methods 2018, 23, 617–634. [Google Scholar] [CrossRef]
- Epskamp, S.; Cramer, A.O.J.; Waldorp, L.J.; Schmittmann, V.D.; Borsboom, D. qgraph: Network Visualizations of Relationships in Psychometric Data. J. Stat. Softw. 2012, 48, 1–18. [Google Scholar] [CrossRef]
- Golino, H.F.; Epskamp, S. Exploratory graph analysis: A new approach for estimating the number of dimensions in psychological research. PLoS ONE 2017, 12, e0174035. [Google Scholar] [CrossRef]
- van Borkulo, C.D.; van Bork, R.; Boschloo, L.; Kossakowski, J.J.; Tio, P.; Schoevers, R.A.; Borsboom, D.; Waldorp, L.J. Comparing network structures on three aspects: A permutation test. Psychol. Methods 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liu, H.; Roeder, K.; Lafferty, J.; Wasserman, L. The huge Package for High-dimensional Undirected Graph Estimation in R. J. Mach. Learn. Res. 2012, 13, 1059–1062. [Google Scholar] [PubMed]
- Gallus, R.; Melis, A.; Rizzo, D.; Piras, A.; De Luca, L.M.; Tramaloni, P.; Serra, A.; Longoni, E.; Soro, G.M.; Bussu, F. Audiovestibular symptoms and sequelae in COVID-19 patients. J. Vestib. Res. 2021, 31, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Badenoch, J.B.; Rengasamy, E.R.; Watson, C.; Jansen, K.; Chakraborty, S.; Sundaram, R.D.; Hafeez, D.; Burchill, E.; Saini, A.; Thomas, L.; et al. Persistent neuropsychiatric symptoms after COVID-19: A systematic review and meta-analysis. Brain Commun. 2022, 4, fcab297. [Google Scholar] [CrossRef] [PubMed]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.-M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef]
- Krishnan, K.; Miller, A.K.; Reiter, K.; Bonner-Jackson, A. Neurocognitive Profiles in Patients with Persisting Cognitive Symptoms Associated with COVID-19. Arch. Clin. Neuropsychol. 2022, 37, 729–737. [Google Scholar] [CrossRef]
- Mukaetova-Ladinska, E.B.; Kronenberg, G. Psychological and neuropsychiatric implications of COVID-19. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 271, 235–248. [Google Scholar] [CrossRef]
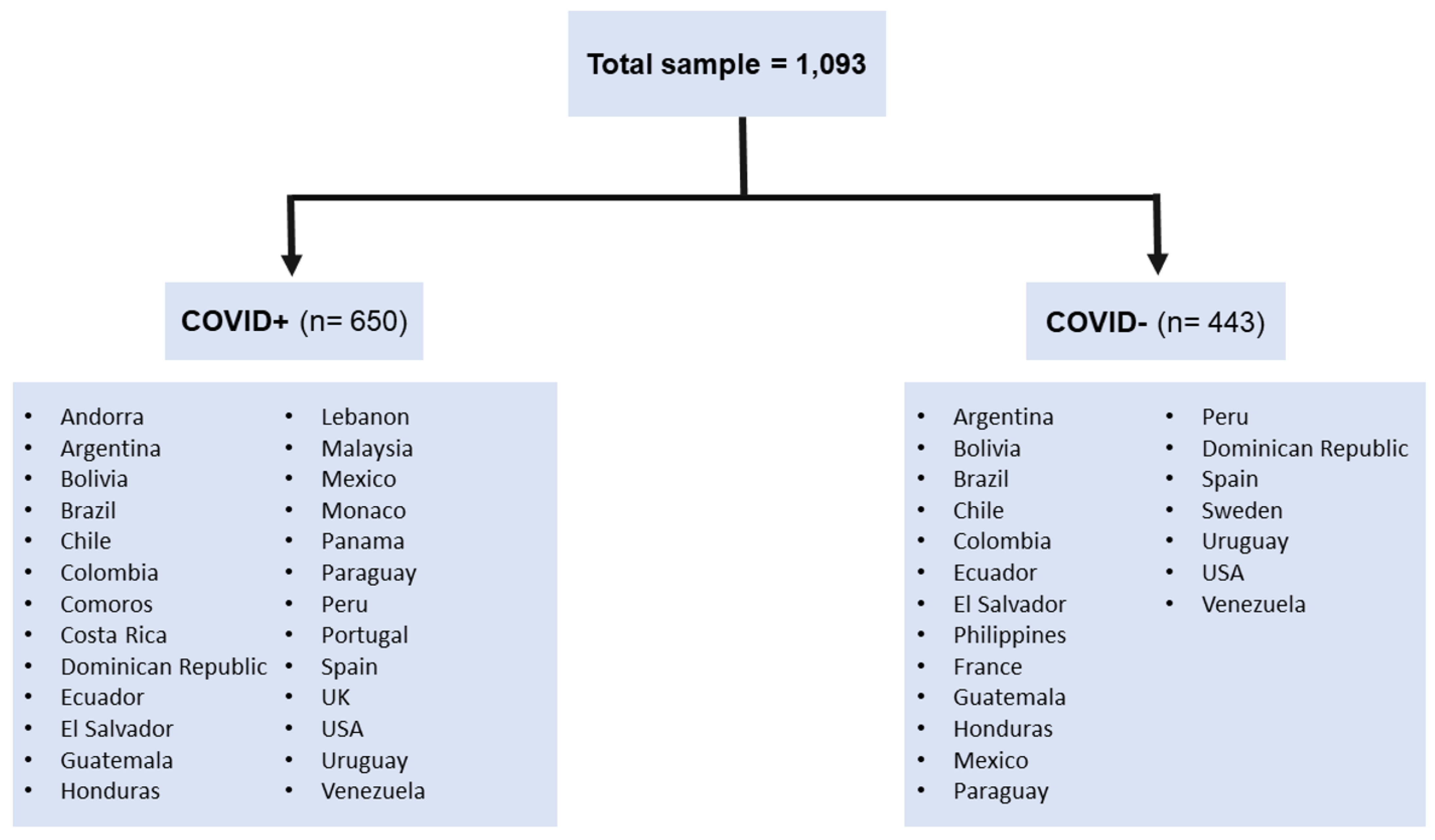
| Variable | COVID+ | COVID− | ||
|---|---|---|---|---|
| Age (years), M 1, SD 2 | 43.5 | 11.9 | 33.9 | 13.2 |
| Gender, N, % | ||||
| Man | 130 | 20.0 | 140 | 31.6 |
| Woman | 519 | 79.8 | 303 | 68.4 |
| Non-binary, transgender, or other | 1 | 0.2 | 0 | 0 |
| Highest level of education completed, N, % | ||||
| Some school education | 12 | 1.8 | 54 | 12.4 |
| Graduated high school | 31 | 4.9 | 40 | 9.1 |
| Some college/technical degree | 145 | 22.3 | 69 | 15.7 |
| Completed undergraduate education | 201 | 30.9 | 47 | 10.9 |
| Postgraduate education (some or completed) | 261 | 40.1 | 230 | 51.9 |
| Days from COVID-19 diagnosis, M, SD | 147.6 | 98.9 | - | - |
| Node | Symptom Group | COVID+ | COVID− |
|---|---|---|---|
| Annoyed | Affective | 0.46 | 0.61 |
| Appetite | Somatosensory | 0.21 | 0.34 |
| Balance | Somatosensory | 0.80 | 0.56 |
| Clumsy | Somatosensory | 0.70 | 0.42 |
| Concentrate | Cognitive | 0.51 | 0.21 |
| Decide | Cognitive | 0.83 | 0.59 |
| Dizzy | Somatosensory | 0.79 | 0.82 |
| Fatigue | Affective | 0.60 | 0.00 |
| Forget | Cognitive | 0.88 | 0.91 |
| Frustrated | Affective | 0.97 | 0.92 |
| Head | Affective | 1.06 | 0.87 |
| Hearing | Somatosensory | 0.95 | 0.53 |
| Light | Somatosensory | 0.28 | 0.43 |
| Nausea | Somatosensory | 0.42 | 0.46 |
| Noise | Somatosensory | 0.31 | 0.23 |
| Numb | Somatosensory | 0.75 | 0.51 |
| Organize | Cognitive | 0.64 | 0.85 |
| Sad | Affective | 0.78 | 0.76 |
| Sleep | Affective | 0.49 | 0.30 |
| Taste | Somatosensory | 0.27 | 0.17 |
| Tense | Affective | 0.88 | 0.55 |
| Vision | Somatosensory | 0.54 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrin, P.B.; Ramos-Usuga, D.; West, S.J.; Merced, K.; Klyce, D.W.; Lequerica, A.H.; Olabarrieta-Landa, L.; Alzueta, E.; Baker, F.C.; Iacovides, S.; et al. Network Analysis of Neurobehavioral Symptom Patterns in an International Sample of Spanish-Speakers with a History of COVID-19 and Controls. Int. J. Environ. Res. Public Health 2023, 20, 183. https://doi.org/10.3390/ijerph20010183
Perrin PB, Ramos-Usuga D, West SJ, Merced K, Klyce DW, Lequerica AH, Olabarrieta-Landa L, Alzueta E, Baker FC, Iacovides S, et al. Network Analysis of Neurobehavioral Symptom Patterns in an International Sample of Spanish-Speakers with a History of COVID-19 and Controls. International Journal of Environmental Research and Public Health. 2023; 20(1):183. https://doi.org/10.3390/ijerph20010183
Chicago/Turabian StylePerrin, Paul B., Daniela Ramos-Usuga, Samuel J. West, Kritzia Merced, Daniel W. Klyce, Anthony H. Lequerica, Laiene Olabarrieta-Landa, Elisabet Alzueta, Fiona C. Baker, Stella Iacovides, and et al. 2023. "Network Analysis of Neurobehavioral Symptom Patterns in an International Sample of Spanish-Speakers with a History of COVID-19 and Controls" International Journal of Environmental Research and Public Health 20, no. 1: 183. https://doi.org/10.3390/ijerph20010183
APA StylePerrin, P. B., Ramos-Usuga, D., West, S. J., Merced, K., Klyce, D. W., Lequerica, A. H., Olabarrieta-Landa, L., Alzueta, E., Baker, F. C., Iacovides, S., Cortes, M., & Arango-Lasprilla, J. C. (2023). Network Analysis of Neurobehavioral Symptom Patterns in an International Sample of Spanish-Speakers with a History of COVID-19 and Controls. International Journal of Environmental Research and Public Health, 20(1), 183. https://doi.org/10.3390/ijerph20010183








