Solvent-Free Synthesis of Magnetic Sewage Sludge-Derived Biochar for Heavy Metal Removal from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Magnetic Sewage Sludge-Derived Biochar
2.3. Characterization of Magnetic Sludge-Based Biochar
2.4. Batch Adsorption Experiments
2.5. Statistical Analysis
3. Results and Discussion
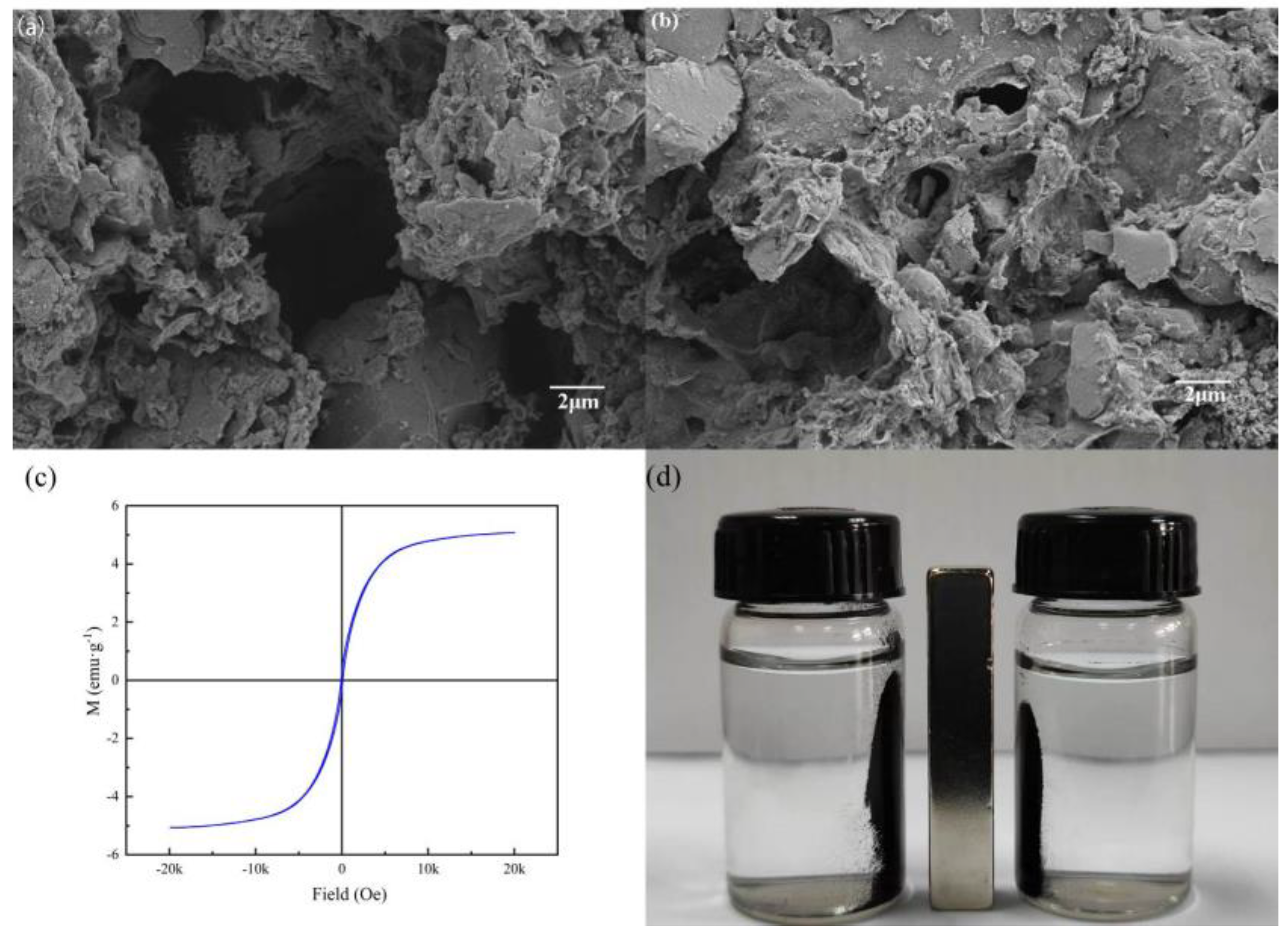
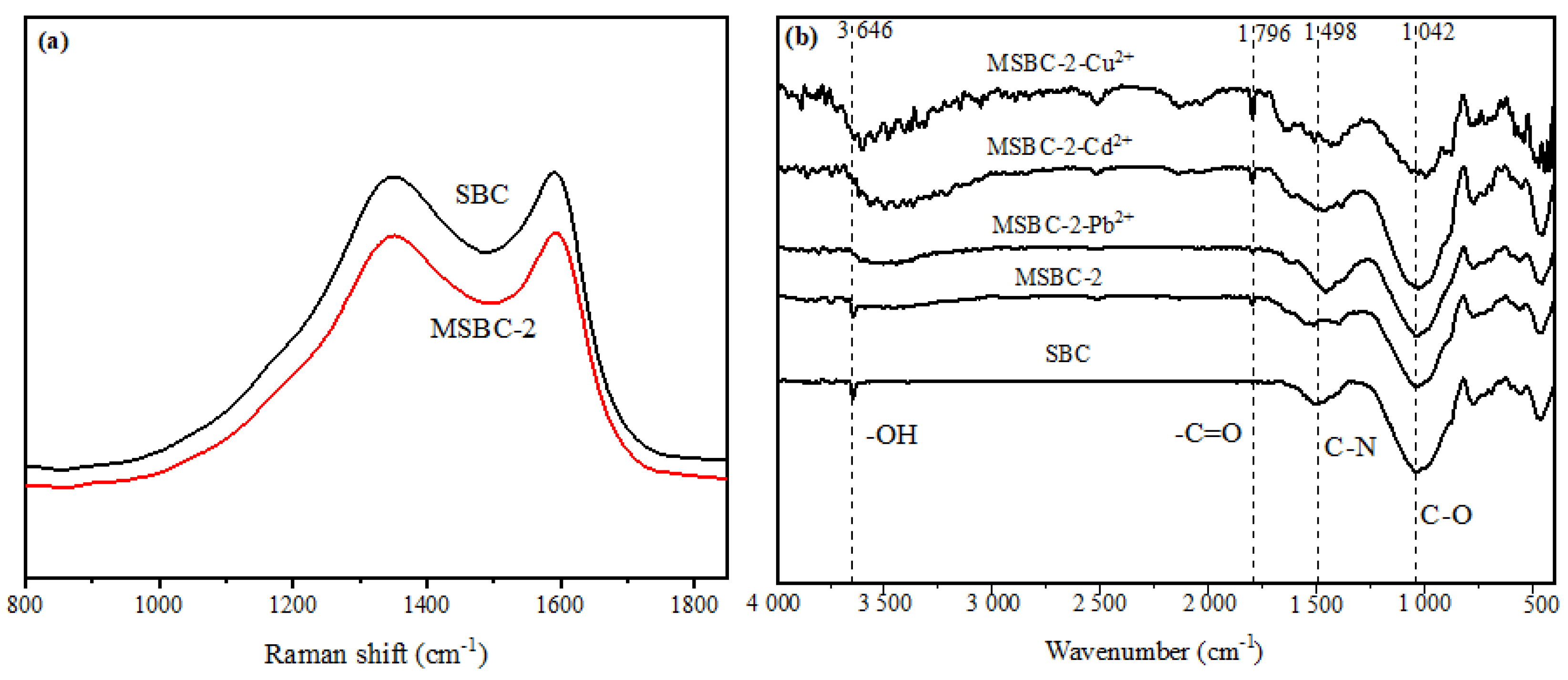
3.1. Characterization of MSBC-2
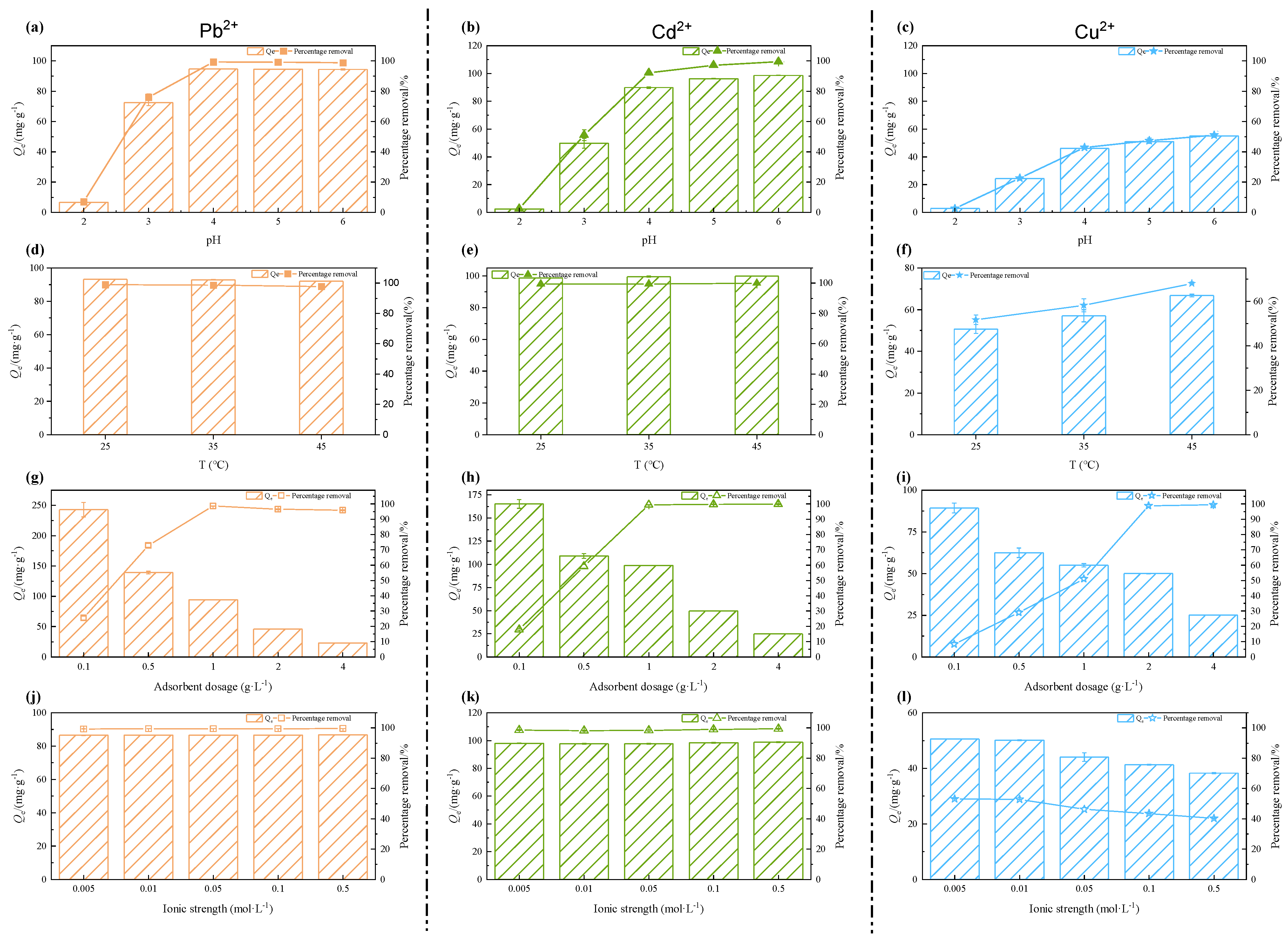
3.2. The Influence of Environmental Factors
3.2.1. pH
3.2.2. Temperature
3.2.3. Adsorbent Dosage
3.2.4. Ionic Strength
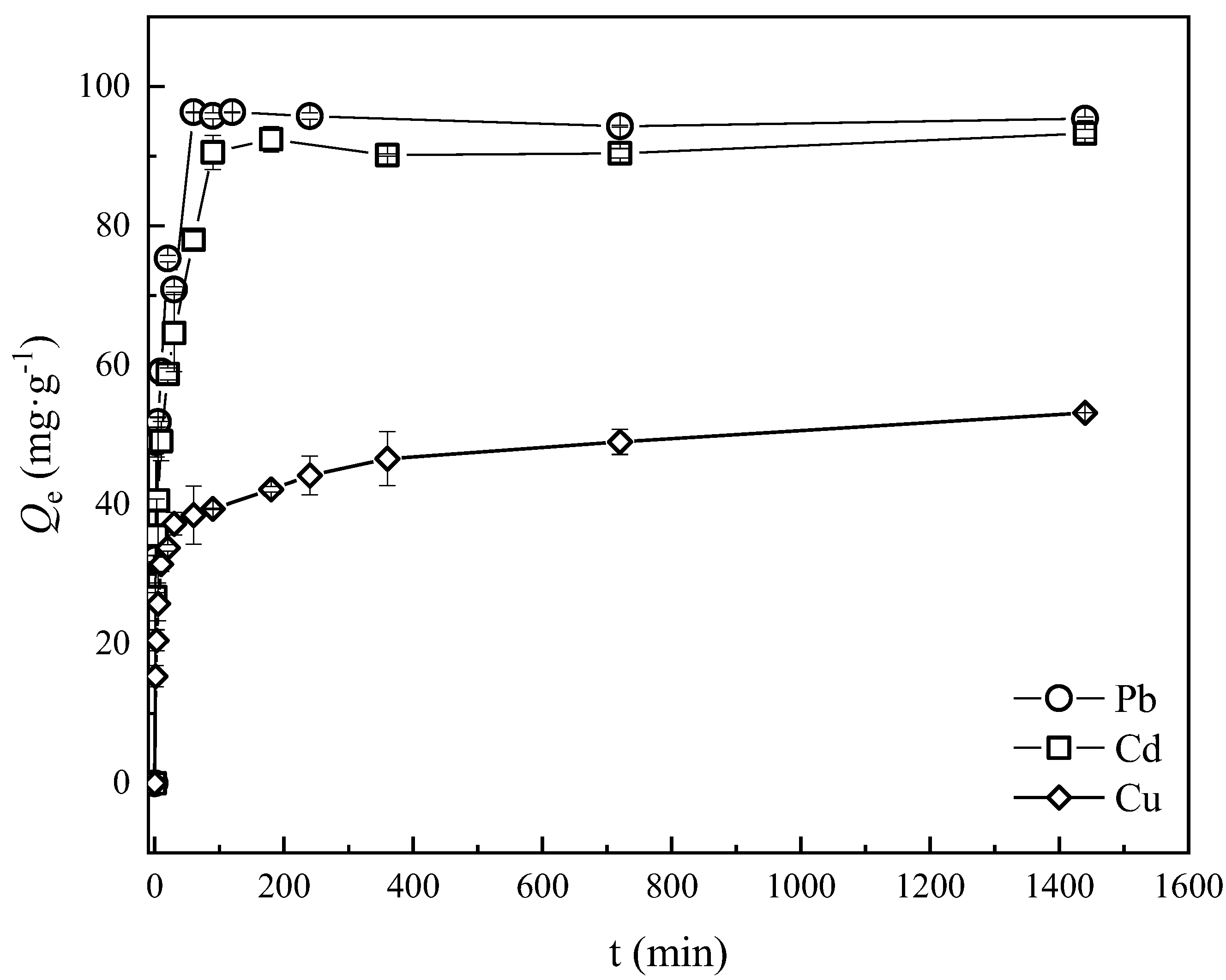
3.3. Adsorption Kinetics
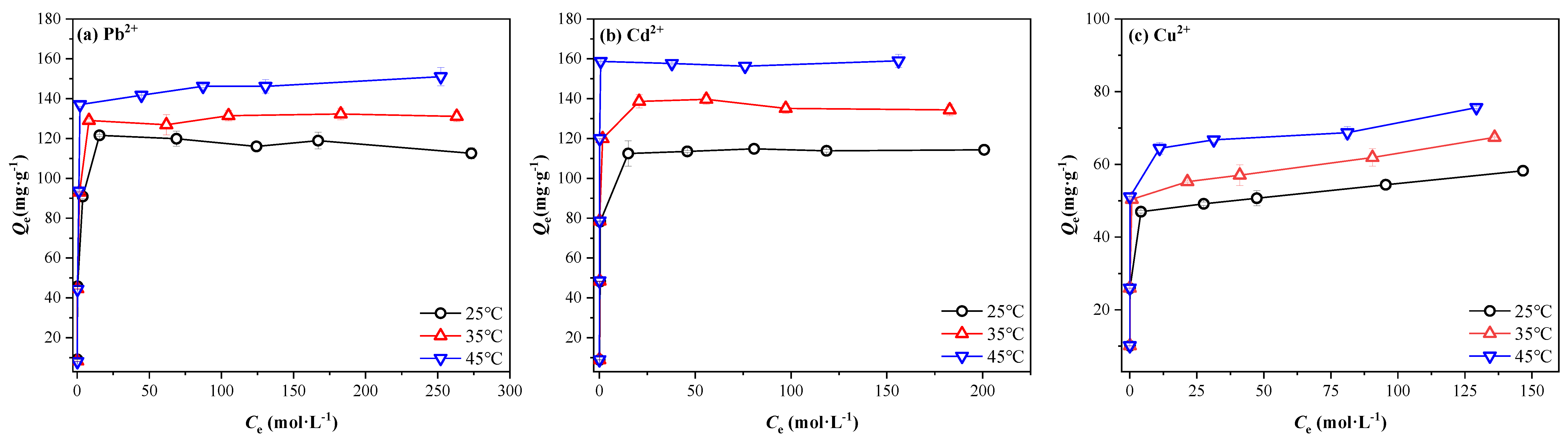
3.4. Adsorption Isotherms
3.5. Adsorption Thermodynamic Analysis
3.6. Adsorption Mechanisms
3.7. Comparison with Other Relevant Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, J.; Xie, H.; Liu, C.; Liang, S.; Ren, Y.; Wang, W. Heavy metal bioaccumulation and health hazard assessment for three fish species from Nansi Lake, China. Bull. Environ. Contam. Toxicol. 2015, 94, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Adsorption of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Wang, L.K.; Vaccari, D.A.; Li, Y.; Shammas, N.K. Chemical precipitation. In Physicochemical Treatment Processes; Springer: Berlin/Heidelberg, Germany, 2005; pp. 141–197. [Google Scholar]
- Zhang, Z.; Wu, Y.; Luo, L.; Li, G.; Li, Y.; Hu, H. Application of disk tube reverse osmosis in wastewater treatment: A review. Sci. Total Environ. 2021, 792, 148291. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.J.M.; Carrott, M.M.L.R. Lignin–from natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Bartoli, M.; Arrigo, R.; Malucelli, G.; Tagliaferro, A.; Duraccio, D. Recent advances in biochar polymer composites. Polymers 2022, 14, 2506. [Google Scholar] [CrossRef]
- Arif, M.; Liu, G.; Yousaf, B.; Ahmed, R.; Irshad, S.; Ashraf, A.; Zia-ur-Rehman, M.; Rashid, M.S. Synthesis, characteristics and mechanistic insight into the clays and clay minerals-biochar surface interactions for contaminants removal-A review. J. Clean. Prod. 2021, 310, 127548. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.-J.; Chang, Y.; Lee, Y.-J. Sludge treatment: Current research trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Kelessidis, A.; Stasinakis, A.S. Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag. 2012, 32, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Luo, J.; Chen, Y. Dilemma of sewage sludge treatment and disposal in China. Environ. Sci. Technol. 2015, 49, 4781–4782. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Walkiewicz, A.; Neugschwandtner, R.W.; Kopecký, M.; Zamanian, K.; Chen, W.-H.; Bucur, D. Feasibility of Biochar Derived from Sewage Sludge to Promote Sustainable Agriculture and Mitigate GHG Emissions—mdash;A Review. Int. J. Environ. Res. Public Health 2022, 19, 12983. [Google Scholar]
- Ifthikar, J.; Wang, J.; Wang, Q.; Wang, T.; Wang, H.; Khan, A.; Jawad, A.; Sun, T.; Jiao, X.; Chen, Z. Highly efficient lead distribution by magnetic sewage sludge biochar: Sorption mechanisms and bench applications. Bioresour. Technol. 2017, 238, 399–406. [Google Scholar] [CrossRef]
- Zahedifar, M.; Seyedi, N.; Shafiei, S.; Basij, M. Surface-modified magnetic biochar: Highly efficient adsorbents for removal of Pb (ΙΙ) and Cd (ΙΙ). Mater. Chem. Phys. 2021, 271, 124860. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, J.; Liu, J.; Liu, B.; Chen, G. Preparation and application of magnetic biochar in water treatment: A critical review. Sci. Total Environ. 2020, 711, 134847. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Kopecký, M.; Kolář, L. A meta-analysis on the impacts of different oxidation methods on the surface area properties of biochar. Land Degrad. Dev. 2022, 1–14. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef]
- Zhang, A.; Li, X.; Xing, J.; Xu, G. Adsorption of potentially toxic elements in water by modified biochar: A review. J. Environ. Chem. Eng. 2020, 8, 104196. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, J.; Saleem, M.; Yang, Y.; Zhang, Q. Enhanced adsorptive removal of carbendazim from water by FeCl3-modified corn straw biochar as compared with pristine, HCl and NaOH modification. J. Environ. Chem. Eng. 2022, 10, 107024. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, R.; Liu, H.; Li, M.; Chen, T.; Chen, D.; Zou, X.; Frost, R. Green preparation of magnetic biochar for the effective accumulation of Pb (II): Performance and mechanism. Chem. Eng. J. 2019, 375, 122011. [Google Scholar] [CrossRef]
- Dong, J.; Shen, L.; Shan, S.; Liu, W.; Qi, Z.; Liu, C.; Gao, X. Optimizing magnetic functionalization conditions for efficient preparation of magnetic biochar and adsorption of Pb(II) from aqueous solution. Sci. Total Environ. 2022, 806, 151442. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Shi, S.; Zhang, H.; Qiu, J.; Yu, W.; Li, M.; Gan, Q.; Yu, W.; Xiao, K.; Liu, B.; et al. One-pot solvothermal synthesis of magnetic biochar from waste biomass: Formation mechanism and efficient adsorption of Cr(VI) in an aqueous solution. Sci. Total Environ. 2019, 695, 133886. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Sridhar, K.; Chen, B.-H. Removal of polycyclic aromatic hydrocarbons from water by magnetic activated carbon nanocomposite from green tea waste. J. Hazard. Mater. 2021, 415, 125701. [Google Scholar] [CrossRef]
- Qu, J.; Dong, M.; Wei, S.; Meng, Q.; Hu, L.; Hu, Q.; Wang, L.; Han, W.; Zhang, Y. Microwave-assisted one pot synthesis of β-cyclodextrin modified biochar for concurrent removal of Pb (II) and bisphenol a in water. Carbohydr. Polym. 2020, 250, 117003. [Google Scholar] [CrossRef]
- Jack, J.; Huggins, T.M.; Huang, Y.; Fang, Y.; Ren, Z.J. Production of magnetic biochar from waste-derived fungal biomass for phosphorus removal and recovery. J. Clean. Prod. 2019, 224, 100–106. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- Guo, K.; Tian, J.; Sun, Y.; Lin, R.; Gong, Y.; Yang, T.; Chen, T.; Zhuo, Y. Adsorption performance of magnetic sludge-derived biochar towards Pb2+. Chin. J. Environ. Eng. 2022, 16, 1416–1428. [Google Scholar]
- Yin, W.; Zhao, C.; Xu, J. Enhanced adsorption of Cd (II) from aqueous solution by a shrimp bran modified Typha orientalis biochar. Environ. Sci. Pollut. Res. 2019, 26, 37092–37100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, J.; Liu, Y.; Guo, J.; Ren, J.; Zhou, F. Preparation of iminodiacetic acid-modified magnetic biochar by carbonization, magnetization and functional modification for Cd(II) removal in water. Fuel 2018, 233, 469–479. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Liu, T.; Liu, Y.; Yan, L.; Wei, Q.; Du, B.; Xu, W. EDTA functionalized magnetic biochar for Pb(II) removal: Adsorption performance, mechanism and SVM model prediction. Sep. Purif. Technol. 2019, 227, 115696. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B.; Lee, X.; Lehmann, J.; Gao, B. Sorption and desorption of Pb (II) to biochar as affected by oxidation and pH. Sci. Total Environ. 2018, 634, 188–194. [Google Scholar] [CrossRef]
- Shan, R.; Shi, Y.; Gu, J.; Wang, Y.; Yuan, H. Single and competitive adsorption affinity of heavy metals toward peanut shell-derived biochar and its mechanisms in aqueous systems. Chin. J. Chem. Eng. 2020, 28, 1375–1383. [Google Scholar] [CrossRef]
- Khan, Z.H.; Gao, M.; Qiu, W.; Islam, M.S.; Song, Z. Mechanisms for cadmium adsorption by magnetic biochar composites in an aqueous solution. Chemosphere 2020, 246, 125701. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Hong, M.; Li, H.; Ye, Z.; Gong, H.; Zhang, J.; Huang, Q.; Tan, Z. Contributions and mechanisms of components in modified biochar to adsorb cadmium in aqueous solution. Sci. Total Environ. 2020, 733, 139320. [Google Scholar] [CrossRef] [PubMed]
- Elaigwu, S.E.; Rocher, V.; Kyriakou, G.; Greenway, G.M. Removal of Pb2+ and Cd2+ from aqueous solution using chars from pyrolysis and microwave-assisted hydrothermal carbonization of Prosopis africana shell. J. Ind. Eng. Chem. 2014, 20, 3467–3473. [Google Scholar] [CrossRef]
- Semerciöz, A.S.; Göğüş, F.; Celekli, A.; Bozkurt, H. Development of carbonaceous material from grapefruit peel with microwave implemented-low temperature hydrothermal carbonization technique for the adsorption of Cu (II). J. Clean. Prod. 2017, 165, 599–610. [Google Scholar] [CrossRef]
- Gao, L.; Li, Z.; Yi, W.; Li, Y.; Zhang, P.; Zhang, A.; Wang, L. Impacts of pyrolysis temperature on lead adsorption by cotton stalk-derived biochar and related mechanisms. J. Environ. Chem. Eng. 2021, 9, 105602. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, R.; Chen, G.; Xing, B. Facile synthesis of multifunctional bone biochar composites decorated with Fe/Mn oxide micro-nanoparticles: Physicochemical properties, heavy metals sorption behavior and mechanism. J. Hazard. Mater. 2020, 399, 123067. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-L.; Wang, X.-Y.; Zeng, G.-M.; Chen, L.; Deng, J.-H.; Zhang, X.-R.; Niu, Q.-Y. Copper (II) removal by pectin–iron oxide magnetic nanocomposite adsorbent. Chem. Eng. J. 2012, 185, 100–107. [Google Scholar] [CrossRef]
- Pholosi, A.; Naidoo, E.B.; Ofomaja, A.E. Intraparticle diffusion of Cr(VI) through biomass and magnetite coated biomass: A comparative kinetic and diffusion study. South. Afr. J. Chem. Eng. 2020, 32, 39–55. [Google Scholar] [CrossRef]
- Abdelhafez, A.A.; Li, J. Removal of Pb(II) from aqueous solution by using biochars derived from sugar cane bagasse and orange peel. J. Taiwan Inst. Chem. Eng. 2016, 61, 367–375. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapiński, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Allen, S.J.; McKay, G.; Porter, J.F. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef]
- Zeng, H.; Qi, W.; Zhai, L.; Wang, F.; Zhang, J.; Li, D. Preparation and Characterization of Sludge-Based Magnetic Biochar by Pyrolysis for Methylene Blue Removal. Nanomaterials 2021, 11, 2473. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Czech, B.; Tyszczuk-Rotko, K.; Kończak, M.; Fakhrhoseini, S.M.; Yadav, R.; Naebe, M. Sustainable synthesis of rose flower-like magnetic biochar from tea waste for environmental applications. J. Adv. Res. 2021, 34, 13–27. [Google Scholar] [CrossRef]
- Pezoti Junior, O.; Cazetta, A.L.; Gomes, R.C.; Barizão, É.O.; Souza, I.P.A.F.; Martins, A.C.; Asefa, T.; Almeida, V.C. Synthesis of ZnCl2-activated carbon from macadamia nut endocarp (Macadamia integrifolia) by microwave-assisted pyrolysis: Optimization using RSM and methylene blue adsorption. J. Anal. Appl. Pyrolysis 2014, 105, 166–176. [Google Scholar] [CrossRef]
- Truong, Q.-M.; Ho, P.-N.-T.; Nguyen, T.-B.; Chen, W.-H.; Bui, X.-T.; Patel, A.K.; Singhania, R.R.; Chen, C.-W.; Dong, C.-D. Magnetic biochar derived from macroalgal Sargassum hemiphyllum for highly efficient adsorption of Cu(II): Influencing factors and reusability. Bioresour. Technol. 2022, 361, 127732. [Google Scholar] [CrossRef]
- Dou, D.; Wei, D.; Guan, X.; Liang, Z.; Lan, L.; Lan, X.; Liu, P.; Mo, H.; Lan, P. Adsorption of copper (II) and cadmium (II) ions by in situ doped nano-calcium carbonate high-intensity chitin hydrogels. J. Hazard. Mater. 2022, 423, 127137. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-X.; Chen, S.; You, N.; Fan, H.-T.; Sun, L.-N. Nanocomposites of zero-valent Iron@Activated carbon derived from corn stalk for adsorptive removal of tetracycline antibiotics. Chemosphere 2020, 255, 126917. [Google Scholar] [CrossRef] [PubMed]
- Khosa, M.A.; Ullah, A. Mechanistic insight into protein supported biosorption complemented by kinetic and thermodynamics perspectives. Adv. Colloid Interface Sci. 2018, 261, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Sag, Y.; Kutsal, T. Determination of the biosorption heats of heavy metal ions on Zoogloea ramigera and Rhizopus arrhizus. Biochem. Eng. J. 2000, 6, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Özsin, G.; Kılıç, M.; Apaydın-Varol, E.; Pütün, A.E. Chemically activated carbon production from agricultural waste of chickpea and its application for heavy metal adsorption: Equilibrium, kinetic, and thermodynamic studies. Appl. Water Sci. 2019, 9, 56. [Google Scholar] [CrossRef]
- Khan, Z.H.; Gao, M.; Qiu, W.; Song, Z. Properties and adsorption mechanism of magnetic biochar modified with molybdenum disulfide for cadmium in aqueous solution. Chemosphere 2020, 255, 126995. [Google Scholar] [CrossRef]
- Wen, T.; Wang, J.; Li, X.; Huang, S.; Chen, Z.; Wang, S.; Hayat, T.; Alsaedi, A.; Wang, X. Production of a generic magnetic Fe3O4 nanoparticles decorated tea waste composites for highly efficient sorption of Cu (II) and Zn (II). J. Environ. Chem. Eng. 2017, 5, 3656–3666. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Huang, X.; Wang, W.; Sun, P.; Li, Y.; Han, J. Functionalized biochar-supported magnetic MnFe2O4 nanocomposite for the removal of Pb (ii) and Cd (ii). RSC Adv. 2019, 9, 365–376. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.; Zhang, S.; Gong, Y.; Su, Y.; Cao, J.; Hu, H. Adsorption characteristics and mechanism of Pb (II) by agricultural waste-derived biochars produced from a pilot-scale pyrolysis system. Waste Manag. 2019, 100, 287–295. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Li, Y.; Liu, Y.; Chen, Y.; Li, H.; Li, L.; Xu, F.; Jiang, H.; Chen, L. Hydroxyapatite modified sludge-based biochar for the adsorption of Cu2+ and Cd2+: Adsorption behavior and mechanisms. Bioresour. Technol. 2021, 321, 124413. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, D.; Lu, L.; Li, Z.; Zhang, B.; Liu, Y.; Zhuang, L.; Yang, T.; Chen, T. The potential of ferrihydrite-synthetic humic-like acid composite to remove metal ions from contaminated water: Performance and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130771. [Google Scholar] [CrossRef]
- Feng, Z.; Feng, C.; Chen, N.; Lu, W.; Wang, S. Preparation of composite hydrogel with high mechanical strength and reusability for removal of Cu(II) and Pb(II) from water. Sep. Purif. Technol. 2022, 300, 121894. [Google Scholar] [CrossRef]
- Wang, W.; Wen, T.; Bai, H.; Zhao, Y.; Ni, J.; Yang, L.; Xia, L.; Song, S. Adsorption toward Cu(II) and inhibitory effect on bacterial growth occurring on molybdenum disulfide-montmorillonite hydrogel surface. Chemosphere 2020, 248, 126025. [Google Scholar] [CrossRef]
- Xue, S.; Fan, J.; Wan, K.; Wang, G.; Xiao, Y.; Bo, W.; Gao, M.; Miao, Z. Calcium-Modified Fe3O4 Nanoparticles Encapsulated in Humic Acid for the Efficient Removal of Heavy Metals from Wastewater. Langmuir 2021, 37, 10994–11007. [Google Scholar] [CrossRef]
- Islam, M.S.; Kwak, J.-H.; Nzediegwu, C.; Wang, S.; Palansuriya, K.; Kwon, E.E.; Naeth, M.A.; El-Din, M.G.; Ok, Y.S.; Chang, S.X. Biochar heavy metal removal in aqueous solution depends on feedstock type and pyrolysis purging gas. Environ. Pollut. 2021, 281, 117094. [Google Scholar] [CrossRef]
- Neeli, S.T.; Ramsurn, H.; Ng, C.Y.; Wang, Y.; Lu, J. Removal of Cr (VI), As (V), Cu (II), and Pb (II) using cellulose biochar supported iron nanoparticles: A kinetic and mechanistic study. J. Environ. Chem. Eng. 2020, 8, 103886. [Google Scholar] [CrossRef]
- Li, A.Y.; Deng, H.; Jiang, Y.H.; Ye, C.H.; Yu, B.G.; Zhou, X.L.; Ma, A.Y. Superefficient removal of heavy metals from wastewater by Mg-loaded biochars: Adsorption characteristics and removal mechanisms. Langmuir 2020, 36, 9160–9174. [Google Scholar] [CrossRef]
- Xiang, J.; Lin, Q.; Cheng, S.; Guo, J.; Yao, X.; Liu, Q.; Yin, G.; Liu, D. Enhanced adsorption of Cd (II) from aqueous solution by a magnesium oxide–rice husk biochar composite. Environ. Sci. Pollut. Res. 2018, 25, 14032–14042. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, M.; Kohout, T.; Lahtinen, M.; Garg, V.K.; Sillanpää, M. Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr (VI), Cu (II) and Cd (II) ions from aqueous solution. Water Resour. Ind. 2018, 20, 54–74. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Chitosan/CaCO3-silane nanocomposites: Synthesis, characterization, in vitro bioactivity and Cu (II) adsorption properties. Int. J. Biol. Macromol. 2018, 114, 149–160. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, P.; Sarswat, A.; Steele, P.H.; Pittman, C.U., Jr. Lead sorptive removal using magnetic and nonmagnetic fast pyrolysis energy cane biochars. J. Colloid Interface Sci. 2015, 448, 238–250. [Google Scholar] [CrossRef] [PubMed]

| Sample | BET (m2·g−1) | Total Pore Volume (cm3·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| MSBC-2 | 63.68 | 0.089 | 5.96 |
| SBC | 59.38 | 0.073 | 4.56 |
| Metal | Qe,exp (mg·g−1) | Pseudo-First-Order Model | Pseudo-Second-Order Model | Elovich Model | Intraparticle Diffusion Model | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe,cal (mg·g−1) | k1(min−1) | R2 | p | Qe,cal (mg·g−1) | k2 (g·(mg·min)−1) | R2 | p | a (mg·(g·min)−1) | b (g·mg−1) | R2 | p | Kid (mg·(g·min0.5)−1) | C (mg·g−1) | R2 | p | ||
| Pb | 95.75 | 44.35 | 4.80 × 10−3 | 0.8788 | 0.0006 | 95.24 | 3.33 × 10−3 | 0.9999 | <0.0001 | 6.75 × 102 | 0.104 | 0.8392 | <0.0001 | 1.333 | 61.94 | 0.4263 | 0.0213 |
| Cd | 93.22 | 44.33 | 4.60 × 10−3 | 0.7896 | 0.0006 | 93.46 | 1.27 × 10−3 | 0.9998 | <0.0001 | 1.53 × 102 | 0.095 | 0.9124 | <0.0001 | 1.638 | 49.41 | 0.5750 | 0.0043 |
| Cu | 53.17 | 24.39 | 3.00 × 10−3 | 0.7398 | 0.0002 | 52.91 | 8.60 × 10−3 | 0.9971 | <0.0001 | 6.75 × 102 | 0.201 | 0.9794 | <0.0001 | 0.862 | 26.87 | 0.7350 | 0.0002 |
| Metal | Temperature (°C) | Langmuir | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qm (mg·g−1) | KL (L·mg−1) | R2 | p | RL | Kf (mg·g−1·(L·mg−1)1/n) | n | R2 | p | ||
| Pb | 25 | 113.64 | 1.073 | 0.9990 | <0.0001 | 0.0011–0.0458 | 39.958 | 4.024 | 0.7527 | 0.0052 |
| 35 | 131.58 | 1.310 | 0.9999 | <0.0001 | 0.0020–0.0768 | 44.228 | 3.986 | 0.6709 | 0.0129 | |
| 45 | 151.52 | 0.617 | 0.9997 | <0.0001 | 0.0042–0.1501 | 49.511 | 3.849 | 0.6036 | 0.0233 | |
| Cd | 25 | 101.01 | 0.339 | 0.9992 | <0.0001 | 0.0073–0.2278 | 34.03 | 4.40 | 0.5379 | 0.0384 |
| 35 | 106.38 | 0.355 | 0.9997 | <0.0001 | 0.0070–0.2198 | 33.08 | 4.06 | 0.5890 | 0.0262 | |
| 45 | 109.89 | 0.387 | 0.9996 | <0.0001 | 0.0064–0.2053 | 35.08 | 4.13 | 0.5601 | 0.0327 | |
| Cu | 25 | 57.80 | 0.413 | 0.9968 | <0.0001 | 0.0060–0.1949 | 27.183 | 5.89 | 0.7695 | 0.0095 |
| 35 | 66.23 | 0.557 | 0.9958 | <0.0001 | 0.0045–0.1522 | 32.122 | 6.03 | 0.7258 | 0.0149 | |
| 45 | 74.07 | 0.808 | 0.9966 | <0.0001 | 0.0031–0.1101 | 41.04 | 6.89 | 0.7231 | 0.0153 | |
| Metal | T (K) | ΔG0 (kJ·mol−1) | ΔS0 (kJ·(mol·K)−1) | ΔH0 (kJ·mol−1) | R2 | p |
|---|---|---|---|---|---|---|
| Pb | 298.15 | −4.8321 | 0.3218 | 91.4139 | 0.9631 | <0.05 |
| 308.15 | −7.0649 | |||||
| 318.15 | −11.3108 | |||||
| Cd | 298.15 | −4.9783 | 0.6430 | 186.8869 | 0.9973 | <0.05 |
| 308.15 | −10.8810 | |||||
| 318.15 | −17.8607 | |||||
| Cu | 298.15 | −1.4316 | 0.1594 | 46.2807 | 0.9464 | <0.05 |
| 308.15 | −2.4190 | |||||
| 318.15 | −4.6460 |
| Raw Materials | k2 (g·(mg·min)−1) | Qm (mg·g−1) | Reference |
|---|---|---|---|
| Rice straw | 2.4 × 10−2 (Pb) 9.00 × 10−2 (Cd) 3.90 × 10−2 (Cu) | 133.3 (Pb) 42.7 (Cd) 19.6 (Cu) | [66] |
| Date leaves and stalks | 3.98 × 10−3 (Pb) 2.70 × 10−3 (Cd) | 103.1 (Pb) 106.4 (Cd) | [18] |
| Cellulose | 5.00 × 10−3 (Pb) 5.00 × 10−4 (Cu) | 17.3 (Pb) 42.2 (Cu) | [67] |
| Corn straw | 3.2 × 10−4 (Pb) 1.30 × 10−4 (Cd) 6.10 × 10−4 (Cu) | 54.5 (Pb) 66.2 (Cd) 84.8 (Cu) | [68] |
| Rice husk | 5.00 × 10−2 (Cd) | 21.7 (Cd) | [69] |
| Sunflower | 2.8 × 10−2 (Cd) 2.3 × 10−2 (Cu) | 2.9 (Cd) 2.7 (Cu) | [70] |
| Chitosan | 4.5 × 10−3 (Cu) | 33.9 (Cu) | [71] |
| Cane | 6.33 × 10−4 (Pb) | 40.6 (Pb) | [72] |
| Sludge | 3.33 × 10−3 (Pb) 1.27 × 10−3 (Cd) 8.60 × 10−4 (Cu) | 113.6 (Pb) 101.0 (Cd) 57.8 (Cu) | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Guo, K.; Sun, Y.; Lin, R.; Chen, T.; Zhang, B.; Liu, Y.; Yang, T. Solvent-Free Synthesis of Magnetic Sewage Sludge-Derived Biochar for Heavy Metal Removal from Wastewater. Int. J. Environ. Res. Public Health 2023, 20, 155. https://doi.org/10.3390/ijerph20010155
Tian J, Guo K, Sun Y, Lin R, Chen T, Zhang B, Liu Y, Yang T. Solvent-Free Synthesis of Magnetic Sewage Sludge-Derived Biochar for Heavy Metal Removal from Wastewater. International Journal of Environmental Research and Public Health. 2023; 20(1):155. https://doi.org/10.3390/ijerph20010155
Chicago/Turabian StyleTian, Jiayi, Kexin Guo, Yucan Sun, Ruoxi Lin, Tan Chen, Bing Zhang, Yifei Liu, and Ting Yang. 2023. "Solvent-Free Synthesis of Magnetic Sewage Sludge-Derived Biochar for Heavy Metal Removal from Wastewater" International Journal of Environmental Research and Public Health 20, no. 1: 155. https://doi.org/10.3390/ijerph20010155
APA StyleTian, J., Guo, K., Sun, Y., Lin, R., Chen, T., Zhang, B., Liu, Y., & Yang, T. (2023). Solvent-Free Synthesis of Magnetic Sewage Sludge-Derived Biochar for Heavy Metal Removal from Wastewater. International Journal of Environmental Research and Public Health, 20(1), 155. https://doi.org/10.3390/ijerph20010155







