Enhancing Human Biomonitoring Studies through Linkage to Administrative Registers–Status in Europe
Abstract
:1. Introduction
2. Methods
2.1. Setting
2.2. Questionnaire
3. Results
3.1. The Questionnaire
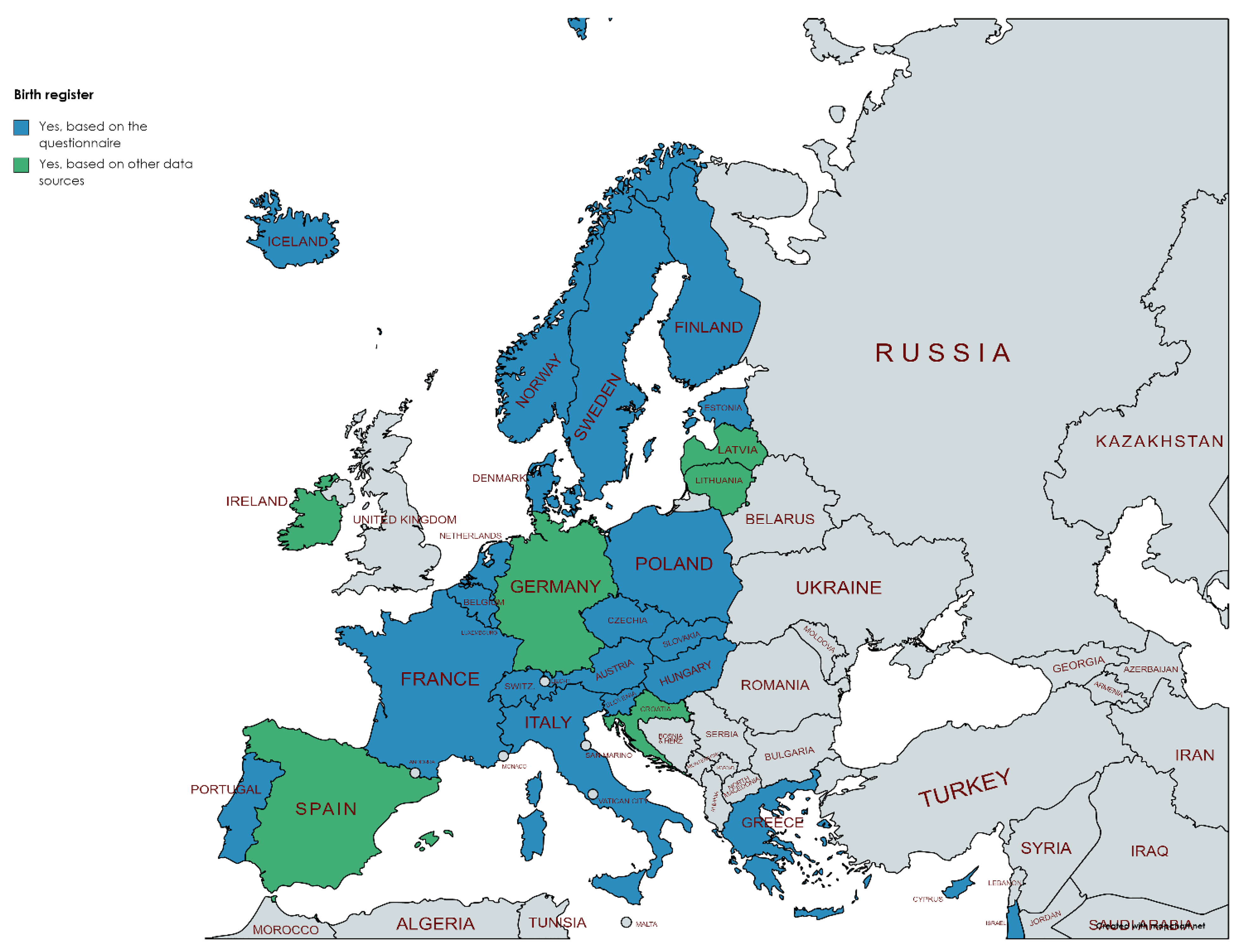
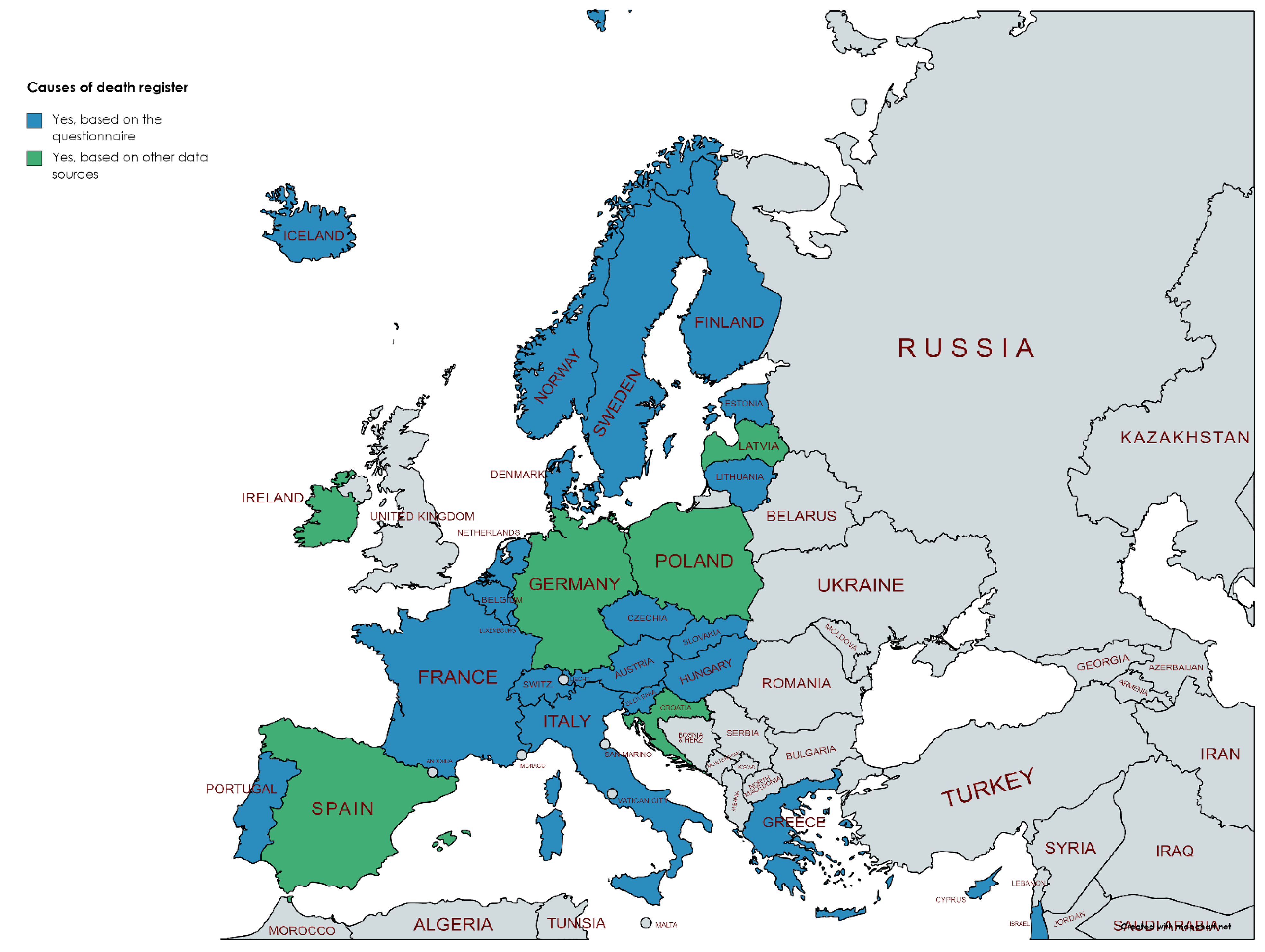
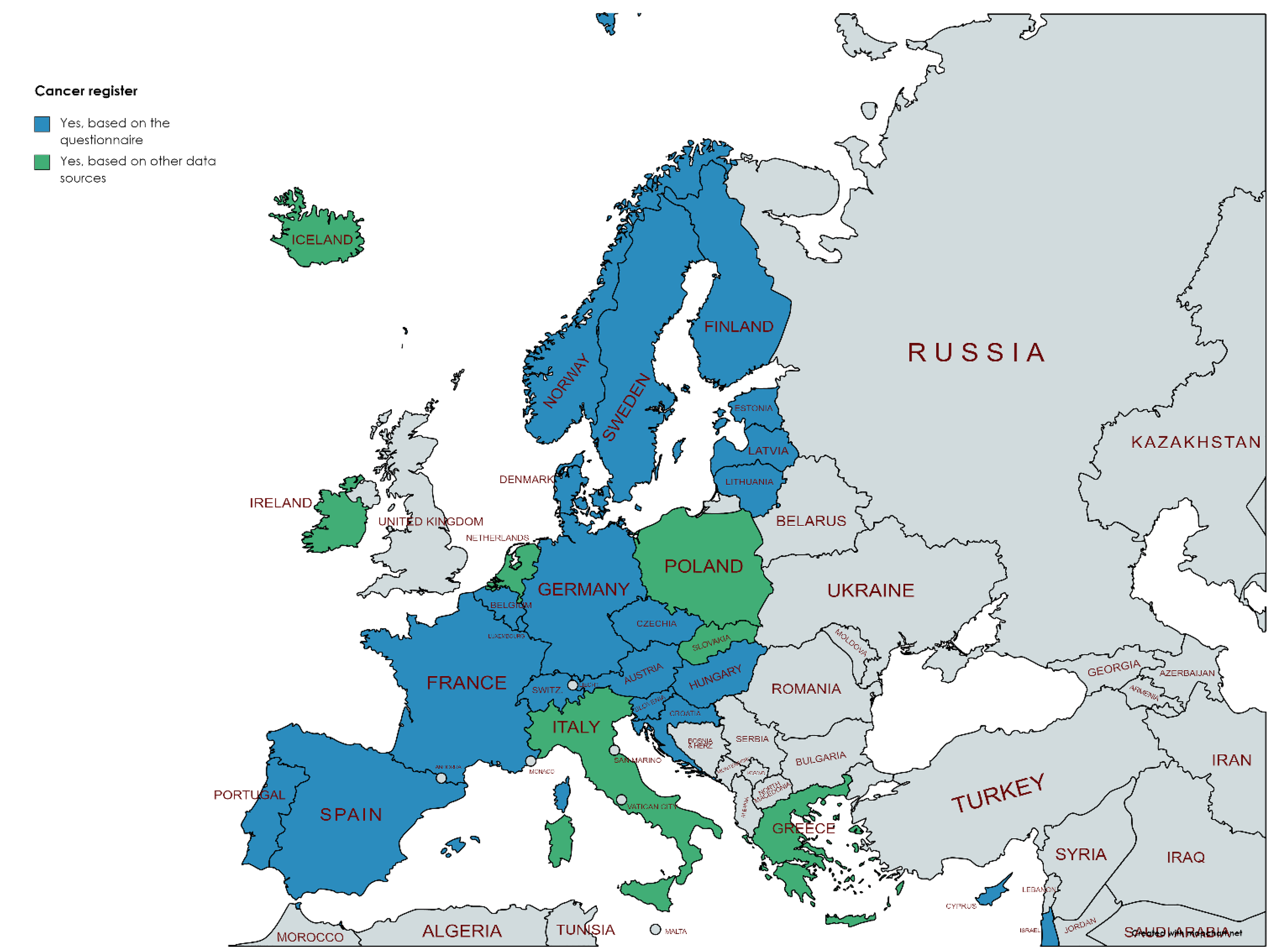
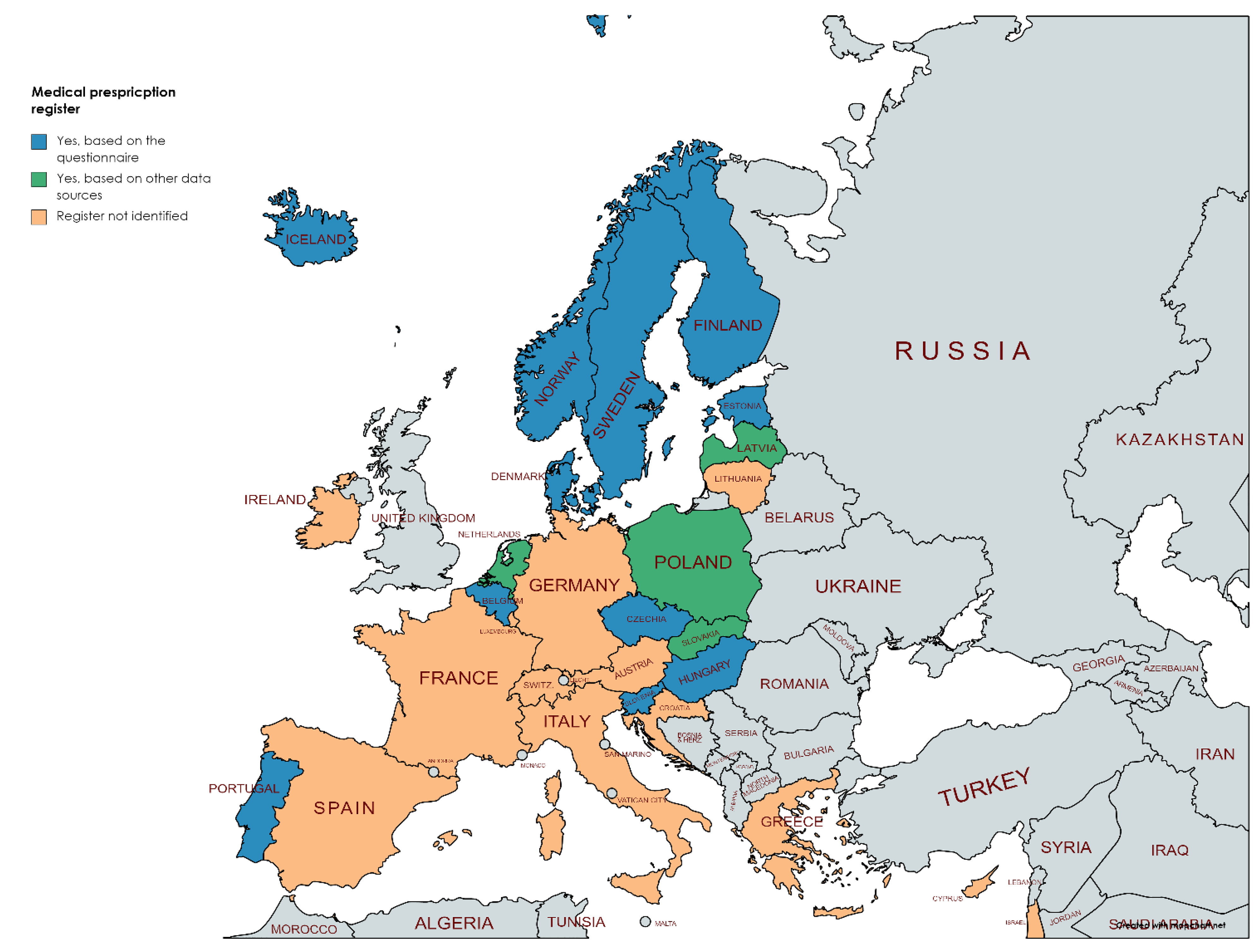
3.2. Availability of Different Registers
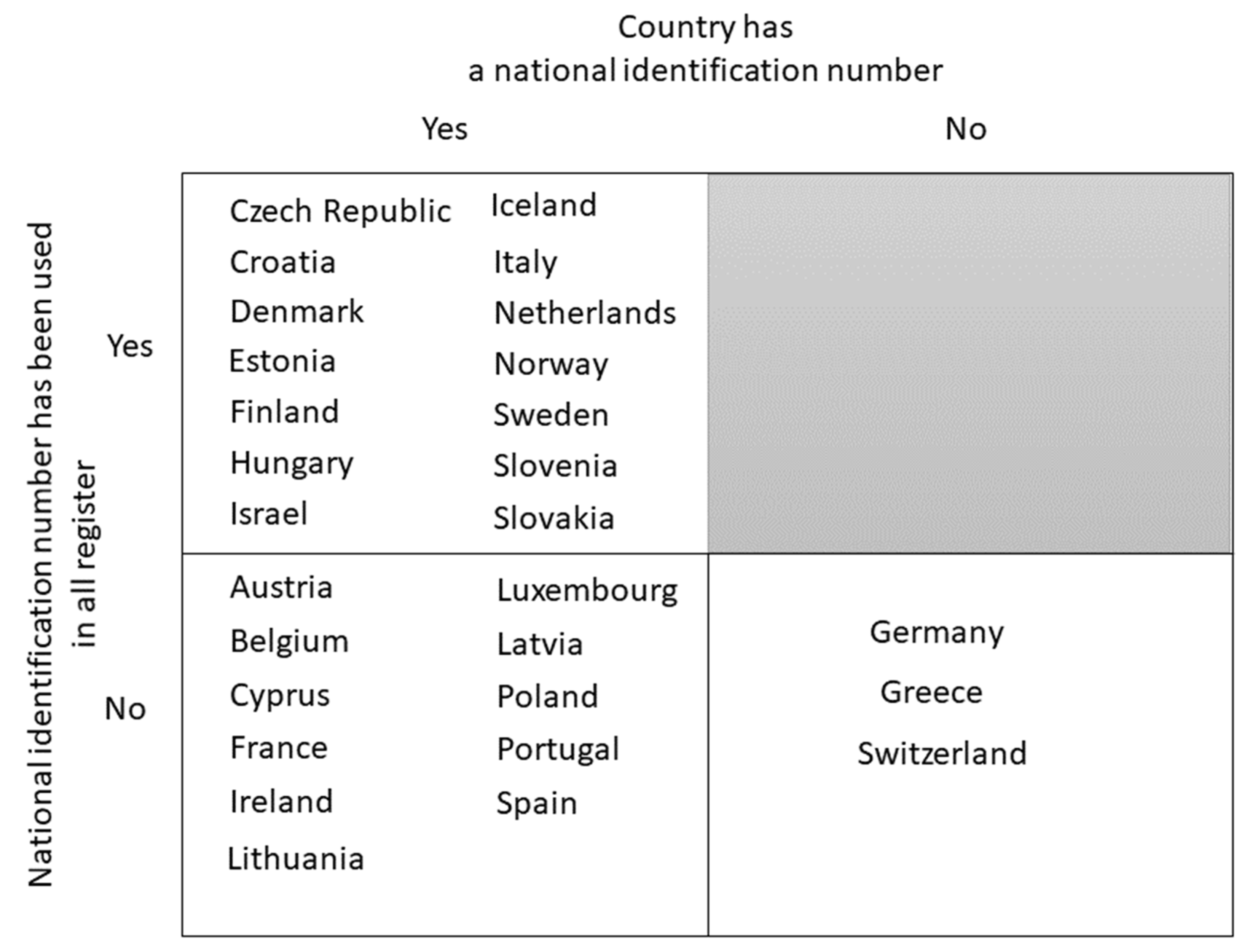
3.3. Availability of Personal Identifiers
3.4. Examples of Record Linkage Procedures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lermen, D.; Weber, T.; Goen, T.; Bartel-Steinbach, M.; Gwinner, F.; Mueller, S.C.; Conrad, A.; Ruther, M.; von Briesen, H.; Kolossa-Gehring, M. Long-term time trend of lead exposure in young German adults-Evaluation of more than 35 Years of data of the German Environmental Specimen Bank. Int. J. Hyg. Environ. Health 2021, 231, 113665. [Google Scholar] [CrossRef]
- Lynn, P.; Buck, N.; Burton, J.; Jäckle, A.; Laurie, H.; A Review of Methodological Research Pertinent to Longitudinal Survey Design and Data Collection. ISER Working Paper, (2005–2029). 2005. Available online: https://www.econstor.eu/bitstream/10419/91973/1/2005-29.pdf (accessed on 19 March 2022).
- Gilbert, R.; Lafferty, R.; Hagger-Johnson, G.; Harron, K.; Zhang, L.C.; Smith, P.; Dibben, C.; Goldstein, H. GUILD: GUidance for Information about Linking Data sets. J. Public Health 2018, 40, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haneef, R.; Delnord, M.; Vernay, M.; Bauchet, E.; Gaidelyte, R.; Van Oyen, H.; Or, Z.; Pérez-Gómez, B.; Palmieri, L.; Achterberg, P.; et al. Innovative use of data sources: A cross-sectional study of data linkage and artificial intelligence practices across European countries. Arch. Public Health 2020, 78, 55. [Google Scholar] [CrossRef] [PubMed]
- Májek, O.; Anttila, A.; Arbyn, M.; van Veen, E.B.; Engesæter, B.; Lönnberg, S. The legal framework for European cervical cancer screening programmes. Eur. J. Public Health 2019, 29, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Skerfving, S.; Löfmark, L.; Lundh, T.; Mikoczy, Z.; Strömberg, U. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology 2015, 49, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Sommar, J.N.; Svensson, M.K.; Björ, B.M.; Elmståhl, S.I.; Hallmans, G.; Lundh, T.; Schön, S.M.; Skerfving, S.; Bergdahl, I.A. End-stage renal disease and low level exposure to lead, cadmium and mercury; a population-based, prospective nested case-referent study in Sweden. Environ. Health 2013, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Ganzleben, C.; Antignac, J.P.; Barouki, R.; Castaño, A.; Fiddicke, U.; Klánová, J.; Lebret, E.; Olea, N.; Sarigiannis, D.; Schoeters, G.R.; et al. Human biomonitoring as a tool to support chemicals regulation in the European Union. Int. J. Hyg. Environ. Health 2017, 220, 94–97. [Google Scholar] [CrossRef]
- HBM4EU. About HBM4EU. Berlin. Available online: https://www.hbm4eu.eu/about-hbm4eu/ (accessed on 21 September 2021).
- Tolonen, H.; Holmboe, S.A.; Ancona, C.; Andersson, A.-M.; Alimonti, A.; Bergdahl, I.; Berglund, M.; Demarest, S.; Dias, C.; Dusek, L.; et al. Report on Opportunities and Obstacles of Combining HBM and Health Studies, Availability of Health Studies with Biological Samples, Availability of Administrative Registers, and Guidelines for Combining HBM and Health Studies (Deliverable Report D 11.1 (2.0)). Zenodo. 2018. Available online: https://zenodo.org/record/5956401#.YnSAq9NBxPZ (accessed on 22 February 2022). [CrossRef]
- Joint Action on Health Information, European Health Information Portal. Available online: https://www.healthinformationportal.eu/ (accessed on 4 March 2022).
- European Commission. European Network of Cancer Registries. Available online: https://www.encr.eu/ (accessed on 5 February 2022).
- Eurostat. Mortality (National Level) (demo_mor). Luxembourg. Available online: https://ec.europa.eu/eurostat/cache/metadata/en/demo_mor_esms.htm (accessed on 5 February 2022).
- OECD. OECD Health Statistics 2021. Definitions, Sources and Methods. OECD. Available online: https://www.oecd.org/els/health-systems/Table-of-Content-Metadata-OECD-Health-Statistics-2021.pdf (accessed on 5 February 2022).
- FamilySearch Research Wiki. Available online: https://www.familysearch.org/wiki/en/Main_Page (accessed on 5 February 2022).
- Milieu Ltd.-Time.lex. Overview of the National Laws on Electronic Health Records in the EU Member States and Their Interaction with the Provision of Cross-Border eHealth Services. Final Report and Recommendations. Brussels. Available online: https://ec.europa.eu/health/system/files/2019-02/laws_report_recommendations_en_0.pdf (accessed on 5 February 2022).
- Harron, K.; Goldstein, H.; Dibben, C. Methodological Developments in Data Linkage; Chapter 1, Introduction; Wiley: Hoboken, NJ, USA, 2015; Available online: https://www.wiley.com/en-us/Methodological+Developments+in+Data+Linkage-p-9781118745878 (accessed on 5 February 2022).
- Baghal, T.A.; Knies, G.; Burton, J. Linking Administrative Records to Surveys: Differences in the Correlates to Consent Decisions; (Understanding Society Working Paper Series No. 2014-09); University of Essex: Essex, UK, 2014; Available online: https://www.understandingsociety.ac.uk/sites/default/files/downloads/working-papers/2014-09.pdf (accessed on 5 February 2022).
- Davern, M.; Meyer, B.; Mittag, N. Creating Improved Survey Data Products Using Linked Administrative-Survey Data. J. Surv. Stat. Methodol. 2019, 7, 440–463. [Google Scholar] [CrossRef]
- Künn, S. The challenges of linking survey and administrative data. IZA World Lab. 2015. [Google Scholar] [CrossRef]
- Maret-Ouda, J.; Tao, W.; Wahlin, K.; Lagergren, J. Nordic registry-based cohort studies: Possibilities and pitfalls when combining Nordic registry data. Scand. J. Public Health 2017, 45, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Törmälehto, V.M. Social Statistics-Integrated Use of Survey and Administrative Data at Statistics Finland; Reshaping Official Statistics: Shanghai, China, 2008; Available online: https://www.iaos-isi.org/papers/CS_26_3_Tehto.pdf (accessed on 6 October 2021).
- Callahan, C.L.; Stewart, P.A.; Blair, A.; Purdue, M.P. Extended Mortality Follow-up of a Cohort of Dry Cleaners. Epidemiology 2019, 30, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Pitkäniemi, J.; Heikkinen, S.; Seppä, K.; Ryynänen, H.; Ylöstalo, T.; Eriksson, J.G.; Härkänen, T.; Jousilahti, P.; Knekt, P.; Koskinen, S.; et al. Pooling of Finnish population-based health studies: Lifestyle risk factors of colorectal and lung cancer. Acta Oncol. 2020, 59, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Pukkala, E.; Martinsen, J.I.; Lynge, E.; Gunnarsdottir, H.K.; Sparén, P.; Tryggvadottir, L.; Weiderpass, E.; Kjaerheim, K. Occupation and cancer-follow-up of 15 million people in five Nordic countries. Acta Oncol. 2009, 48, 646–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalsager, L.; Christensen, N.; Halekoh, U.; Timmermann, C.A.G.; Nielsen, F.; Kyhl, H.B.; Husby, S.; Grandjean, P.; Jensen, T.K.; Andersen, H.R. Exposure to perfluoroalkyl substances during fetal life and hospitalization for infectious disease in childhood: A study among 1,503 children from the Odense Child Cohort. Environ. Int. 2021, 149, 106395. [Google Scholar] [CrossRef]
- Evans, M.; Discacciati, A.; Quershi, A.R.; Åkesson, A.; Elinder, C.G. End-stage renal disease after occupational lead exposure: 20 years of follow-up. Occup. Environ. Med. 2017, 74, 396–401. [Google Scholar] [CrossRef]
- Villanger, G.D.; Ystrom, E.; Engel, S.M.; Longnecker, M.P.; Pettersen, R.; Rowe, A.D.; Reichborn-Kjennerud, T.; Aase, H. Neonatal thyroid-stimulating hormone and association with attention-deficit/hyperactivity disorder. Paediatr. Perinat. Epidemiol. 2020, 34, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Moshammer, H.; Neuberger, M. Lung cancer and dust exposure: Results of a prospective cohort study following 3260 workers for 50 years. Occup. Environ. Med. 2004, 61, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallner, P.; Kundi, M.; Moshammer, H.; Zimmerman, S.D.; Buchanich, J.M.; Marsh, G.M. Mortality Among Hardmetal Production Workers: A Retrospective Cohort Study in the Austrian Hardmetal Industry. J. Occup. Environ. Med. 2017, 59, e282–e287. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, R.W.; Shaji, K.; Hayward, A.C.; Abubakar, I. Accuracy of Probabilistic Linkage Using the Enhanced Matching System for Public Health and Epidemiological Studies. PLoS ONE 2015, 10, e0136179. [Google Scholar] [CrossRef]
- Avoundjian, T.; Dombrowski, J.C.; Golder, M.R.; Hughes, J.P.; Guthrie, B.L.; Baseman, J.; Sadinle, M. Comparing methods for record linkage for public health actions: Matching algorithm validation study. JMIR Public Health Surveill. 2020, 6, e15917. [Google Scholar] [CrossRef]
- Newgard, C.D.; Malveau, S.; Zive, D.; Lupton, J.; Lin, A. Building A Longitudinal Cohort From 9-1-1 to 1-Year Using Existing Data Sources, Probabilistic Linkage, and Multiple Imputation: A Validation Study. Acad. Emerg. Med. 2018, 25, 1268–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakshaug, J.W.; Couper, M.P.; Ofstedal, M.B.; Weir, D.R. Linking survey and administrative records: Mechanisms of consent. Sociol. Methods Res. 2012, 41, 535–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.R.; Hjelmborg, J.; Adami, H.O.; Czene, K.; Mucci, L.; Kaprio, J. The Nordic Twin Study on Cancer-NorTwinCan. Twin Res. Hum. Genet. 2019, 22, 817–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Wel, K.A.; Östergren, O.; Lundberg, O.; Korhonen, K.; Martikainen, P.; Nybo Andersen, A.M.; Kjaer Urhoj, S. A gold mine, but still no Klondike: Nordic register data in health inequality research. Scand. J. Public Health 2019, 47, 618–630. [Google Scholar] [CrossRef] [Green Version]

| Ethical Approval Including Research Plan | Approval from Data Protection Authority | Informed Consent | Permission by Register Owner/Controller | Identifier(s) Used for the Record Linkage | Methods of Data Linkage | To Which Registers HBM Data Can Be Linked | Who Performs the Record Linkage | |
|---|---|---|---|---|---|---|---|---|
| Austria | Required | Not required | Not required * | Required | Name and date of birth | Probabilistic | Mortality and cancer registers. Currently not possible to link to hospitalizations/patient data due to concerns over privacy issues | The register owner |
| Czech Republic | Required | Not required | Required | Required | National personal identification number | Deterministic | National health registers, potentially including reproductive health, hospitalizations, cancer, mortality, and cardiovascular surgery | The register owner |
| Denmark | Required | Required | Required | Required | National personal identification number | Deterministic | Both health-related and non-health related registers | The register owner |
| Finland | Required | Not required, evaluated as part of ethical approval process | Required | Required | National personal identification number | Deterministic | All health-related registers including birth, mortality, hospitalizations, medical prescriptions, cancer, malformation, infectious diseases, and vaccinations Several non-health registers such as marital status, education, and sociodemographic position | The register owner |
| Norway | Required | Required | Required | Required | National personal identification number | Deterministic | Health-related registers including birth, hospitalizations, cancer, medical prescriptions, and mortality | The registry owner |
| Sweden | Required | Required | Required | Required | National personal identification number | Deterministic | Both health-related and non-health related registers | The register owner |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meltzer, H.M.; Jensen, T.K.; Májek, O.; Moshammer, H.; Wennberg, M.; Åkesson, A.; Tolonen, H. Enhancing Human Biomonitoring Studies through Linkage to Administrative Registers–Status in Europe. Int. J. Environ. Res. Public Health 2022, 19, 5678. https://doi.org/10.3390/ijerph19095678
Meltzer HM, Jensen TK, Májek O, Moshammer H, Wennberg M, Åkesson A, Tolonen H. Enhancing Human Biomonitoring Studies through Linkage to Administrative Registers–Status in Europe. International Journal of Environmental Research and Public Health. 2022; 19(9):5678. https://doi.org/10.3390/ijerph19095678
Chicago/Turabian StyleMeltzer, Helle Margrete, Tina Kold Jensen, Ondřej Májek, Hanns Moshammer, Maria Wennberg, Agneta Åkesson, and Hanna Tolonen. 2022. "Enhancing Human Biomonitoring Studies through Linkage to Administrative Registers–Status in Europe" International Journal of Environmental Research and Public Health 19, no. 9: 5678. https://doi.org/10.3390/ijerph19095678
APA StyleMeltzer, H. M., Jensen, T. K., Májek, O., Moshammer, H., Wennberg, M., Åkesson, A., & Tolonen, H. (2022). Enhancing Human Biomonitoring Studies through Linkage to Administrative Registers–Status in Europe. International Journal of Environmental Research and Public Health, 19(9), 5678. https://doi.org/10.3390/ijerph19095678







