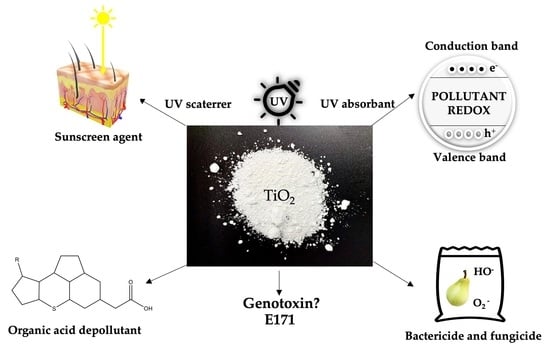
Titanium Dioxide: Structure, Impact, and Toxicity
Abstract
1. Introduction
1.1. Polymorphism
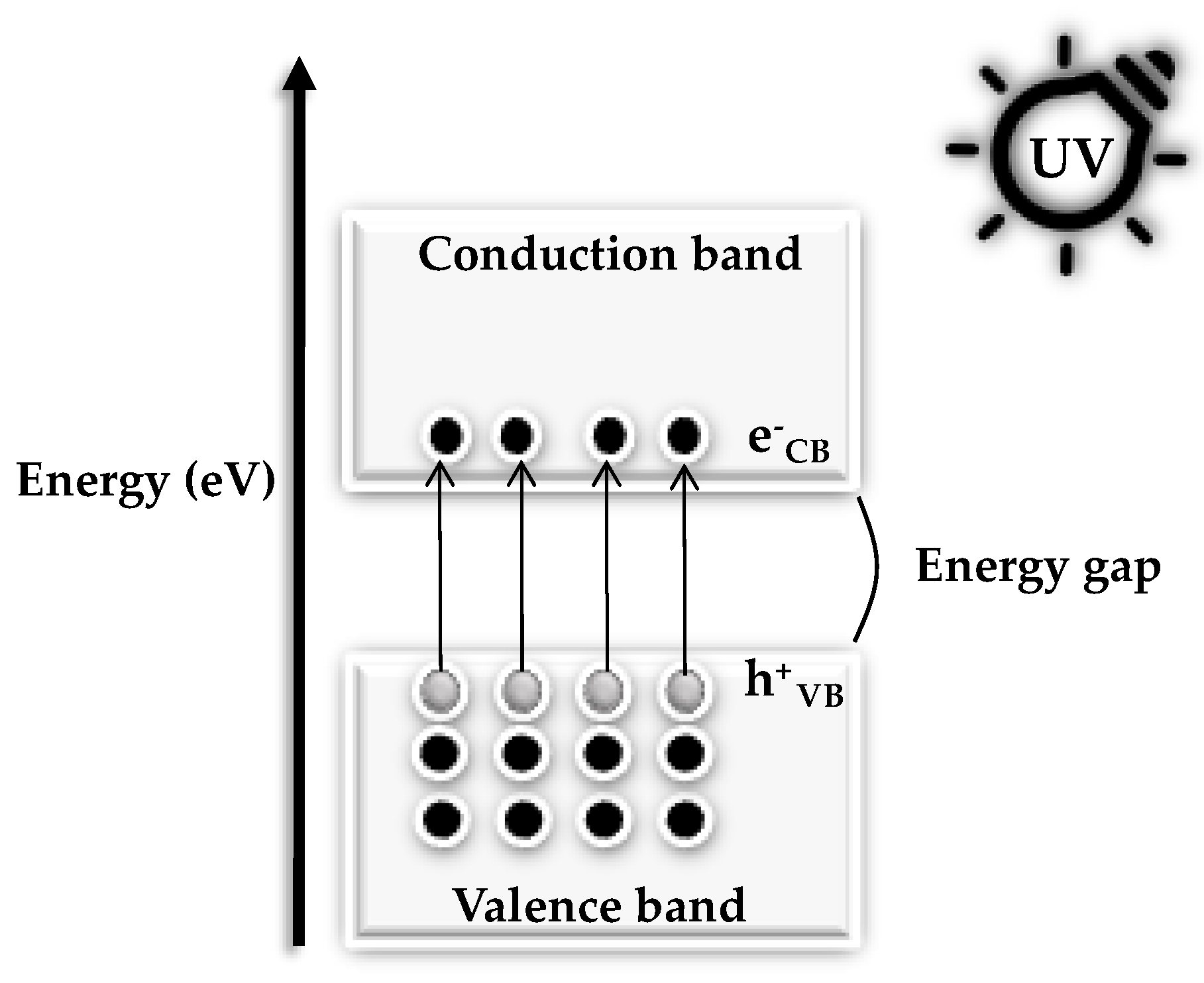
1.2. Optical Properties
1.3. Nanoarchitecture Achievements
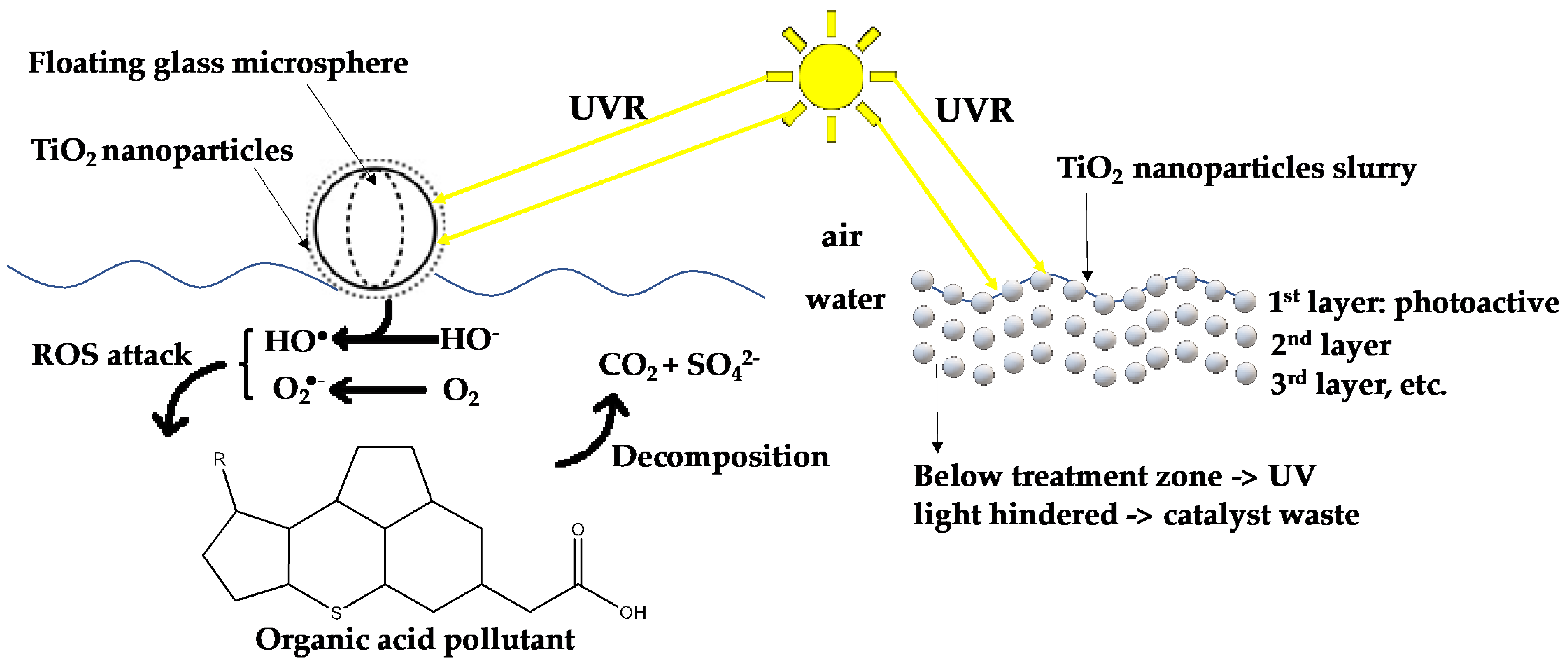
2. Environmental Decontaminant
2.1. Chemical and Biological Warfare Agents
2.2. Large-Scale Water Decontamination
3. Sunscreen Efficacy of TiO2
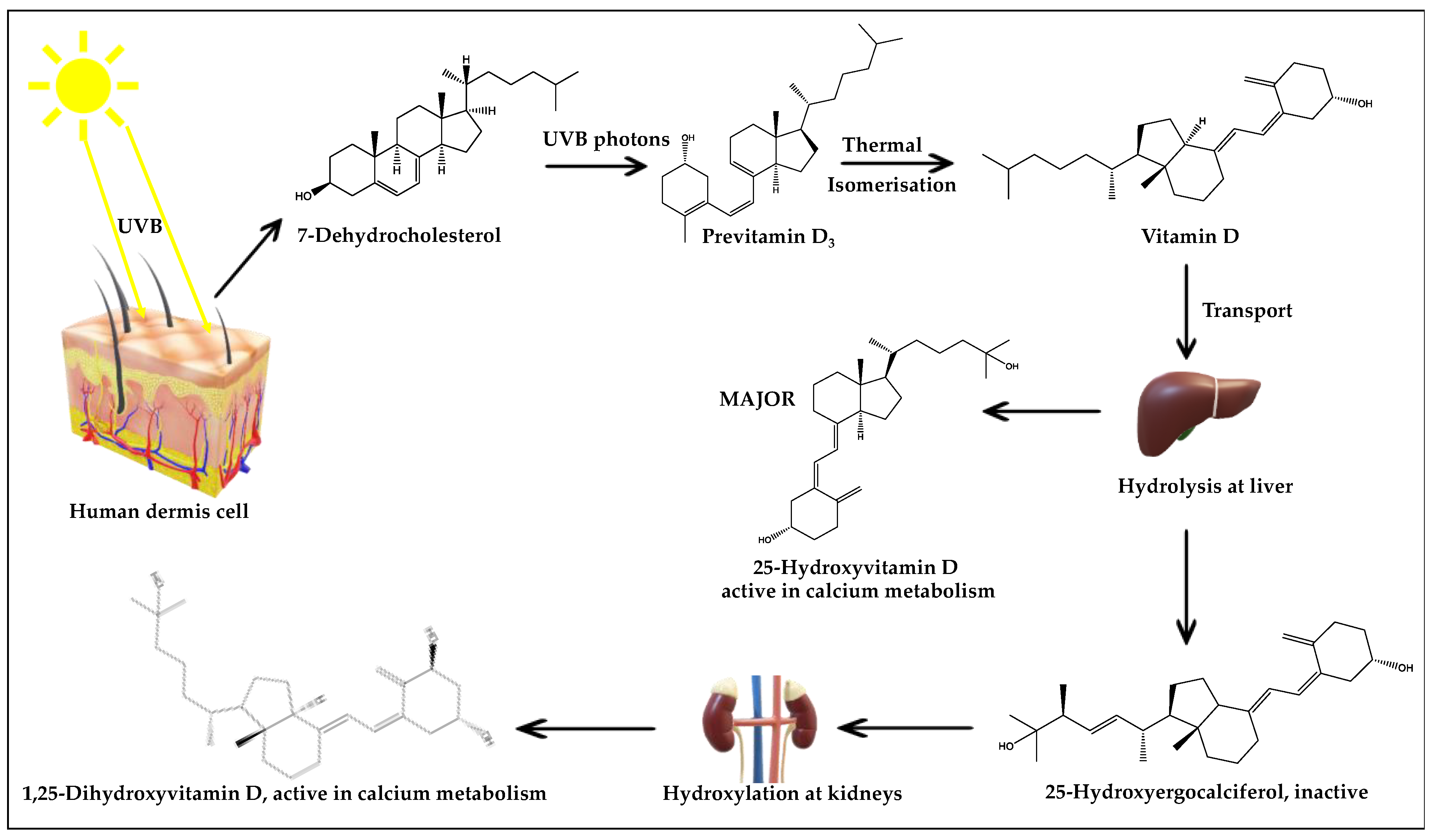
3.1. UV Radiation: Exposure Knowledge and Impact
3.2. TiO2 in Sun Cream Formulations
3.3. Silica-Coated, New-Generation TiO2
4. TiO2 Impact in the Food Industry
4.1. Smart Packaging Composite
4.2. Food-Grade TiO2
4.3. Emerging Controversies on E171
5. Titania Toxicity
5.1. Post-Inhalation Toxicity
5.2. Post-Ingestion Toxicity
5.2.1. Concentration Discrepancies
5.2.2. Human Toxicity Models in Different Concentrations
5.3. Toxicity after Dermal Exposure
6. Titania Toxicity Controversy
6.1. Parameters in Need of Improvement
6.2. The Dichlorofluorescein (DCF) Assay
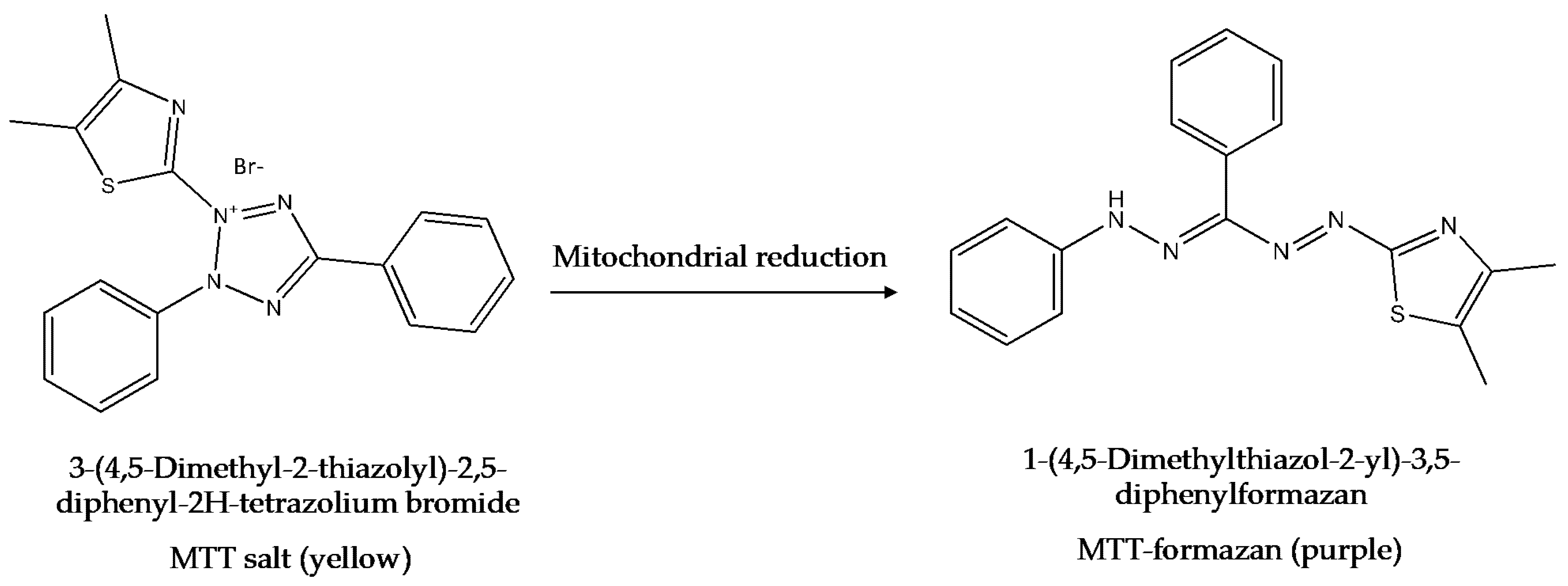
6.3. The MTT Assay
6.4. The Neutral Red (NR) Assay
6.5. Meta-Analysis as a Preventive Action
7. Conclusions and Outlook
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braun, J.H.; Baidins, A.; Marganski, R.E. TiO2 pigment technology: A review. Prog. Org. Coat. 1992, 20, 105–138. [Google Scholar] [CrossRef]
- Shand, M.; Anderson, A.J. Aqueous phase photocatalytic nitrate destruction using titania based materials: Routes to enhanced performance and prospects for visible light activation. Catal. Sci. Technol. 2013, 3, 879–899. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef] [PubMed]
- Cihlar, J.; Navarro, L.K.T.; Kasparek, V.; Michalicka, J.; Kastyl, J.; Castkova, K.; Celko, L. Influence of LA/Ti molar ratio on the complex synthesis of anatase/brookite nanoparticles and their hydrogen production. Int. J. Hydrogen Energy 2021, 46, 8578–8593. [Google Scholar] [CrossRef]
- Jing, L.; Zhou, W.; Tian, G.; Fu, H. Surface tuning for oxide-based nanomaterials as efficient photocatalysts. Chem. Soc. Rev. 2013, 42, 9509–9549. [Google Scholar] [CrossRef] [PubMed]
- Bichowsky, F.V. Titanium White—A new method for its preparation. Ind. Eng. Chem. 1929, 21, 1061–1063. [Google Scholar] [CrossRef]
- Li, W.; Elzatahry, A.; Aldhayan, D.; Zhao, D. Core–shell structured titanium dioxide nanomaterials for solar energy utilization. Chem. Soc. Rev. 2018, 47, 8203–8237. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Ireland, J.C.; Klostermann, P.; Rice, E.W.; Clark, R.M. Inactivation of Escherichia coli by titanium dioxide photocatalytic oxidation. Appl. Environ. Microbiol. 1993, 59, 1668–1670. [Google Scholar] [CrossRef] [PubMed]
- Fröschl, T.; Hörmann, U.; Kubiak, P.; Kučerová, G.; Pfanzelt, M.; Weiss, C.K.; Behm, R.J.; Hüsing, N.; Kaiser, U.; Landfester, K.; et al. High surface area crystalline titanium dioxide: Potential and limits in electrochemical energy storage and catalysis. Chem. Soc. Rev. 2012, 41, 5313–5360. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Shukla, R.; Sharma, V.; Pandey, A.K.; Singh, S.; Sultana, S.; Dhawan, A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol. In Vitro 2011, 25, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Lingaraju, K.; Basavaraj, R.; Jayanna, K.; Bhavana, S.; Devaraja, S.; Swamy, H.K.; Nagaraju, G.; Nagabhushana, H.; Naika, H.R. Biocompatible fabrication of TiO2 nanoparticles: Antimicrobial, anticoagulant, antiplatelet, direct hemolytic and cytotoxicity properties. Inorg. Chem. Commun. 2021, 127, 108505. [Google Scholar] [CrossRef]
- Çeşmeli, S.; Avci, C.B. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J. Drug Target. 2019, 27, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xiong, Z.; Xiong, H.; Cai, J. Synergistic effects of modified TiO2/multifunctionalized graphene oxide nanosheets as functional hybrid nanofiller in enhancing the interface compatibility of PLA/starch nanocomposites. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Golasik, M.; Herman, M.; Piekoszewski, W. Toxicological aspects of soluble titanium—A review of in vitro and in vivo studies. Metallomics 2016, 8, 1227–1242. [Google Scholar] [CrossRef]
- Wang, Y.; Sauvat, A.; Lacrouts, C.; Lebeau, J.; Grall, R.; Hullo, M.; Nesslany, F.; Chevillard, S. TiO2 nanomaterials non-controlled contamination could be hazardous for normal cells located in the field of radiotherapy. Int. J. Mol. Sci. 2020, 21, 940. [Google Scholar] [CrossRef]
- Kiss, B.; Br, T.; Czifra, G.; Tóth, B.I.; Kertész, Z.; Szikszai, Z.; Kiss, Á.Z.; Juhász, I.; Zouboulis, C.C.; Hunyadi, J. Investigation of micronized titanium dioxide penetration in human skin xenografts and its effect on cellular functions of human skin-derived cells. Exp. Dermatol. 2008, 17, 659–667. [Google Scholar] [CrossRef]
- Balachandran, K.; Mageswari, S.; Preethi, A. Photocatalytic decomposition of A549-lung cancer cells by TiO2 nanoparticles. Mater. Today Proc. 2021, 37, 1071–1074. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.A.; Nawaz, R.; Rehman, M.Z.U.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.; Kumar, A.; Kumar, D. Green synthesis of titanium dioxide nanoparticles using Azadirachta indica leaf extract and evaluation of their antibacterial activity. South Afr. J. Bot. 2019, 124, 223–227. [Google Scholar] [CrossRef]
- Rajendhiran, R.; Deivasigamani, V.; Palanisamy, J.; Masan, S.; Pitchaiya, S. Terminalia catappa and carissa carandas assisted synthesis of TiO2 nanoparticles—A green synthesis approach. Mater. Today Proc. 2021, 45, 2232–2238. [Google Scholar] [CrossRef]
- Abisharani, J.M.; Devikala, S.; Kumar, R.D.; Arthanareeswari, M.; Kamaraj, P. Green synthesis of TiO2 nanoparticles using Cucurbita pepo seeds extract. Mater. Today Proc. 2019, 14, 302–307. [Google Scholar] [CrossRef]
- de la Calle, I.; Menta, M.; Klein, M.; Séby, F. Screening of TiO2 and Au nanoparticles in cosmetics and determination of elemental impurities by multiple techniques (DLS, SP-ICP-MS, ICP-MS and ICP-OES). Talanta 2017, 171, 291–306. [Google Scholar] [CrossRef]
- Altsitsiadis, E.; Undheim, T.; de Vries, E.; Hinrichs, B.; Stockfleth, E.; Trakatelli, M.; on behalf of the EPIDERM Group. Health literacy, sunscreen and sunbed use: An uneasy association. Br. J. Dermatol. 2012, 167, 14–21. [Google Scholar] [CrossRef]
- Barnard, A.S. One-to-one comparison of sunscreen efficacy, aesthetics and potential nanotoxicity. Nat. Nanotechnol. 2010, 5, 271–274. [Google Scholar] [CrossRef]
- Veierød, M.B.; Weiderpass, E.; Thörn, M.; Hansson, J.; Lund, E.; Armstrong, B.; Adami, H.-O. A prospective study of pigmentation, sun exposure, and risk of cutaneous malignant melanoma in women. JNCI J. Natl. Cancer Inst. 2003, 95, 1530–1538. [Google Scholar] [CrossRef]
- Rodrigues, N.D.; Stavros, V.G. From fundamental science to product: A bottom-up approach to sunscreen development. Sci. Prog. 2018, 101, 8–31. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, S.; Feng, H.; Liu, Y.; Jia, G. Review of health safety aspects of titanium dioxide nanoparticles in food application. NanoImpact 2020, 18, 100224. [Google Scholar] [CrossRef]
- Li, J.; Singh, V.V.; Sattayasamitsathit, S.; Orozco, J.; Kaufmann, K.; Dong, R.; Gao, W.; Jurado-Sanchez, B.; Fedorak, Y.; Wang, J. Water-driven micromotors for rapid photocatalytic degradation of biological and chemical warfare agents. ACS Nano 2014, 8, 11118–11125. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, E.A.; Smirniotis, P.G.; Vorontsov, A.V. Comparative study on photocatalytic oxidation of four organophosphorus simulants of chemical warfare agents in aqueous suspension of titanium dioxide. J. Photochem. Photobiol. A 2004, 162, 503–511. [Google Scholar] [CrossRef]
- Chauhan, S.; D’Cruz, R.; Faruqi, S.; Singh, K.; Varma, S.; Singh, M.; Karthik, V. Chemical warfare agents. Environ. Toxicol. Pharmacol. 2008, 26, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Alexpandi, R.; Abirami, G.; Balaji, M.; Jayakumar, R.; Ponraj, J.G.; Cai, Y.; Pandian, S.K.; Ravi, A.V. Sunlight-active phytol-ZnO@TiO2 nanocomposite for photocatalytic water remediation and bacterial-fouling control in aquaculture: A comprehensive study on safety-level assessment. Water Res. 2022, 212, 118081. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhong, J.-Y.; Han, X.-Y.; Wang, L.-Y.; Cui, Y.; Chen, L.-K.; Zheng, Y.-C. Decontamination of chemical warfare agents on sensitive equipment materials using Zr4+ and Ge4+ co-doped TiO2 and hydrofluoroether suspension. Chem. Eng. J. 2016, 302, 111–119. [Google Scholar] [CrossRef]
- Shen, Z.; Zhong, J.-Y.; Yang, J.-C.; Cui, Y.; Zheng, H.; Wang, L.-Y.; Wang, J.-L. Decontamination of chemical warfare agents by Zn2+ and Ge4+ co-doped TiO2 nanocrystals at sub-zero temperatures: A solid-state NMR and GC study. Chem. Phys. Lett. 2018, 707, 31–39. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Silva, M.R.; Lourenço, M.A.; Tobaldi, D.M.; Silva, C.; Seabra, M.P.; Ferreira, P. Carbon-modified titanium oxide materials for photocatalytic water and air decontamination. Chem. Eng. J. 2020, 387, 124099. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Rinaldi, R. Titanium Dioxide (TiO2) and Its Applications; Parrino, F., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 373–393. [Google Scholar]
- Leshuk, T.; Krishnakumar, H.; Livera, D.D.O.; Gu, F. Floating photocatalysts for passive solar degradation of naphthenic acids in oil sands process-affected water. Water 2018, 10, 202. [Google Scholar] [CrossRef]
- Ollis, D.F.; Pelizzetti, E.; Serpone, N. Photocatalyzed destruction of water contaminants. Environ. Sci. Technol. 1991, 25, 1522–1529. [Google Scholar] [CrossRef]
- Sjogren, J.C.; Sierka, R.A. Inactivation of phage MS2 by iron-aided titanium dioxide photocatalysis. Appl. Environ. Microbiol. 1994, 60, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwar, K. Photoelectrochemistry and the environment. J. Appl. Electrochem. 1995, 25, 1067–1082. [Google Scholar] [CrossRef]
- Turchi, C. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Health and environmental impacts of dyes. Am. J. Environ. Sci. 2017, 1, 64–67. [Google Scholar]
- Treschev, S.Y.; Chou, P.-W.; Tseng, Y.-H.; Wang, J.-B.; Perevedentseva, E.V.; Cheng, C.-L. Photoactivities of the visible-light-activated mixed-phase carbon-containing titanium dioxide: The effect of carbon incorporation. Appl. Catal. B Environ. 2008, 79, 8–16. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D and bone health. J. Nutr. 1996, 126, 1159S–1164S. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The Role of Vitamin D in Cancer Prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef]
- Hansen, C.M.; Mäenpää, P.H. EB 1089, a novel vitamin D Analog with strong antiproliferative and differentiation-inducing effects on target cells. Biochem. Pharmacol. 1997, 54, 1173–1179. [Google Scholar] [CrossRef]
- Hansen, C.; Hamberg, K.; Binderup, E. Seocalcitol (EB 1089) A Vitamin D Analogue of Anti-cancer Potential. Background, Design, Synthesis, Pre-clinical and Clinical Evaluation. Curr. Pharm. Des. 2000, 6, 803–828. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Wang, M.H.; Zang, M.; Singh, R.K.; Siegal, G.P. 1 alpha,25-Dihydroxyvitamin D (calcitriol) inhibits the invasiveness of human prostate cancer cells. Cancer Epidemiol. Biomark. Prev. 1997, 6, 727–732. [Google Scholar]
- Koike, M.; Koshizuka, K.; Kawabata, H.; Yang, R.; E Taub, H.; Said, J.; Uskokovic, M.; Tsuruoka, N.; Koeffler, H.P. 20-Cyclopropyl-cholecalciferol vitamin D3 analogs: A unique class of potent inhibitors of proliferation of human prostate, breast and myeloid leukemia cell lines. Anticancer Res. 1999, 19, 1689–1697. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Arthey, S.; Clarke, V.A. Suntanning and sun protection: A review of the psychological literature. Soc. Sci. Med. 1995, 40, 265–274. [Google Scholar] [CrossRef]
- Ziegler, A.; Jonason, A.S.; Leffellt, D.J.; Simon, J.A.; Sharma, H.W.; Kimmelman, J.; Remington, L.; Jacks, T.; Brash, D.E. Sunburn and p53 in the onset of skin cancer. Nature 1994, 372, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Forestier, S. Rationale for sunscreen development. J. Am. Acad. Dermatol. 2008, 58, S133–S138. [Google Scholar] [CrossRef]
- Baker, L.A.; Grosvenor, L.C.; Ashfold, N.R.; Stavros, V.G. Ultrafast photophysical studies of a multicomponent sun-screen: Oxybenzone–titanium dioxide mixtures. Chem. Phys. Lett. 2016, 664, 39–43. [Google Scholar] [CrossRef][Green Version]
- Burnett, M.E.; Wang, S.Q. Current sunscreen controversies: A critical review. Photodermatol. Photoimmunol. Photomed. 2011, 27, 58–67. [Google Scholar] [CrossRef]
- Furusawa, T.; Honda, K.; Ukaji, E.; Sato, M.; Suzuki, N. The microwave effect on the properties of silica-coated TiO2 fine particles prepared using sol–gel method. Mater. Res. Bull. 2008, 43, 946–957. [Google Scholar] [CrossRef]
- Sohn, M.; Amorós-Galicia, L.; Krus, S.; Martin, K.; Herzog, B. Effect of emollients on UV filter absorbance and sunscreen efficiency. J. Photochem. Photobiol. B 2020, 205, 111818. [Google Scholar] [CrossRef]
- Schauder, S. Dermatological tolerance of UV absorbers, perfume components, and preservatives in sunscreen products. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2001, 44, 471–479. [Google Scholar] [CrossRef]
- El-Toni, A.M.; Yin, S.; Sato, T.; Ghannam, T.; Al-Hoshan, M.; Al-Salhi, M. Investigation of photocatalytic activity and UV-shielding properties for silica coated titania nanoparticles by solvothermal coating. J. Alloy. Compd. 2010, 508, L1–L4. [Google Scholar] [CrossRef]
- Osterwalder, U.; Sohn, M.; Herzog, B. Global state of sunscreens. Photodermatol. Photoimmunol. Photomed. 2014, 30, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N.; Salinaro, A.; Emeline, A. Deleterious effects of sunscreen titanium dioxide nanoparticles on DNA: Efforts to limit DNA damage by particle surface modification. SPIE Proc. 2001, 4258, 86–99. [Google Scholar] [CrossRef]
- Nekrashevich, S.S.; Gritsenko, V.A. Electronic structure of silicon dioxide (a review). Phys. Solid State 2014, 56, 207–222. [Google Scholar] [CrossRef]
- Siddiquey, I.A.; Furusawa, T.; Sato, M.; Honda, K.; Suzuki, N. Control of the photocatalytic activity of TiO2 nanoparticles by silica coating with polydiethoxysiloxane. Dyes Pigm. 2008, 76, 754–759. [Google Scholar] [CrossRef]
- Jaroenworaluck, A.; Sunsaneeyametha, W.; Kosachan, N.; Stevens, R. Characteristics of silica-coated TiO2 and its UV absorption for sunscreen cosmetic applications. Surf. Interface Anal. 2006, 38, 473–477. [Google Scholar] [CrossRef]
- Liang, C.; Gou, Y.; Wu, L.; Zhou, J.-G.; Kang, Z.; Shen, B.; Wang, Z. Nature of Electromagnetic-Transparent SiO2 Shell in Hybrid Nanostructure Enhancing Electromagnetic Attenuation. J. Phys. Chem. C 2016, 120, 12967–12973. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Hassan, E.A.; Abou-Zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef]
- Cao, C.; Wang, Y.; Zheng, S.; Zhang, J.; Li, W.; Li, B.; Guo, R.; Yu, J. Poly(butylene adipate-co-terephthalate)/titanium dioxide/silver composite biofilms for food packaging application. LWT-Food Sci. Technol. 2020, 132, 109874. [Google Scholar] [CrossRef]
- Mesgari, M.; Aalami, A.H.; Sahebkar, A. Antimicrobial activities of chitosan/titanium dioxide composites as a biological nanolayer for food preservation: A review. Int. J. Biol. Macromol. 2021, 176, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric Antimicrobial Food Packaging and Its Applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jeon, Y.; Han, T.; Kim, S.; Gwon, Y.; Kim, J. Nanoscale manufacturing as an enabling strategy for the design of smart food packaging systems. Food Packag. Shelf Life 2020, 26, 100570. [Google Scholar] [CrossRef]
- Sung, S.-Y.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Rahmat, A.R.; Rahman, W.; Tan, A.-C.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- de Chiara, M.L.V.; Pal, S.; Licciulli, A.; Amodio, M.L.; Colelli, G. Photocatalytic degradation of ethylene on mesoporous TiO2/SiO2 nanocomposites: Effects on the ripening of mature green tomatoes. Biosyst. Eng. 2015, 132, 61–70. [Google Scholar] [CrossRef]
- Saltveit, M.E. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Li, G.; Lv, L.; Fan, H.; Ma, J.; Li, Y.; Wan, Y.; Zhao, X.S. Effect of the agglomeration of TiO2 nanoparticles on their photocatalytic performance in the aqueous phase. J. Colloid Interface Sci. 2010, 348, 342–347. [Google Scholar] [CrossRef]
- Quintavalla, S.; Vicini, L. Antimicrobial food packaging in meat industry. Meat Sci. 2002, 62, 373–380. [Google Scholar] [CrossRef]
- European Food Safety Authority. Available online: https://www.efsa.europa.eu/en/topics/topic/food-contact-materials (accessed on 16 April 2021).
- Tang, Y.; Hu, X.; Zhang, X.; Guo, D.; Zhang, J.; Kong, F. Chitosan/titanium dioxide nanocomposite coatings: Rheological behavior and surface application to cellulosic paper. Carbohydr. Polym. 2016, 151, 752–759. [Google Scholar] [CrossRef]
- Lin, B.; Luo, Y.; Teng, Z.; Zhang, B.; Zhou, B.; Wang, Q. Development of silver/titanium dioxide/chitosan adipate nanocomposite as an antibacterial coating for fruit storage. LWT-Food Sci. Technol. 2015, 63, 1206–1213. [Google Scholar] [CrossRef]
- Lin, D.; Yang, Y.; Wang, J.; Yan, W.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Tu, Z. Preparation and characterization of TiO2-Ag loaded fish gelatin-chitosan antibacterial composite film for food packaging. Int. J. Biol. Macromol. 2020, 154, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xie, W.; Huang, X.; Chen, X.; Huang, N.; Wang, X.; Liu, J. The graphene oxide and chitosan biopolymer loads TiO2 for antibacterial and preservative research. Food Chem. 2017, 221, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhang, X.; Zhang, W.; Zhang, S.; Su, H.; Tan, T. Visible-light-mediated synergistic photocatalytic antimicrobial effects and mechanism of Ag-nanoparticles@chitosan–TiO2 organic–inorganic composites for water disinfection. Appl. Catal. B 2015, 170–171, 255–262. [Google Scholar] [CrossRef]
- BR PAT. Nanoparticulate Titanium Dioxide Nanomaterial Modified with Functional Groups and with Citric Extracts Adsorbed on the Surface, for the Removal of a Wide Range of Microorganisms. EP 3012225A1, 27 April 2016. Available online: https://patentimages.storage.googleapis.com/47/ec/07/027b1717b2d7f5/EP3012225A1.pdf (accessed on 16 April 2021).
- Cheng, L.; Hao, Y.; Song, X.; Chang, Q. Effects of chitosan and nano titanium dioxide on the mechanical, physicochemical and antibacterial properties of corn starch films. J. Macromol. Sci. Part B 2021, 60, 616–630. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Partovi, R.; Talebi, F.; Babaei, A. Chitosan/TiO2 nanoparticle/ Cymbopogon citratus essential oil film as food packaging material: Physico-mechanical properties and its effects on microbial, chemical, and organoleptic quality of minced meat during refrigeration. J. Food Process. Preserv. 2020, 44, 14536. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; AlMatrafi, M.; Jing, J.; Helal, M. Effect of titanium dioxide nanocomposite material and antimicrobial agents on mushrooms shelf-life preservation. Processes 2020, 8, 1632. [Google Scholar] [CrossRef]
- Zink, D.L. The impact of consumer demands and trends on food processing. Emerg. Infect. Dis. 1997, 3, 467–469. [Google Scholar] [CrossRef]
- Berardinelli, A.; Parisi, F. TiO2 in the food industry and cosmetics. In Titanium Dioxide (TiO2) and Its Applications; Parrino, F., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Peters, R.J.B.; van Bemmel, G.; Herrera-Rivera, Z.; Helsper, H.P.F.G.; Marvin, H.J.P.; Weigel, S.; Tromp, P.C.; Oomen, A.G.; Rietveld, A.G.; Bouwmeester, H. Characterization of titanium dioxide nanoparticles in food products: Analytical methods to define nanoparticles. J. Agric. Food Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef]
- Li, B.; Chua, S.L.; Yu, D.; Chan, S.H.; Li, A. Separation and size characterization of highly polydisperse titanium dioxide nanoparticles (E171) in powdered beverages by using Asymmetric Flow Field-Flow Fractionation hyphenated with Multi-Angle Light Scattering and Inductively Coupled Plasma Mass Spectrometry. J. Chromatogr. A 2021, 1643, 462059. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Winkler, H.C.; Notter, T.; Meyer, U.; Naegeli, H. Critical review of the safety assessment of titanium dioxide additives in food. J. Nanobiotechnol. 2018, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Ropers, M.-H.; Terrisse, H.; Mercier-Bonin, M.; Humbert, B. Titanium dioxide as food additive. In Application of Titanium Dioxide; Janus, M., Ed.; InTech: Rijeka, Croatia, 2017; pp. 3–4. [Google Scholar] [CrossRef]
- EFSA ANS Panel. Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016, 14, e04545. [Google Scholar] [CrossRef]
- Boutillier, S.; Fourmentin, S.; Laperche, B. Food additives and the future of health: An analysis of the ongoing controversy on titanium dioxide. Futures 2020, 122, 102598. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings; Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar]
- Moran, C.A.; Mullick, F.G.; Ishak, K.G.; Johnson, F.B.; Hummer, W.B. Identification of titanium in human tissues: Probable role in pathologic processes. Hum. Pathol. 1991, 22, 450–454. [Google Scholar] [CrossRef]
- Garabrant, D.H.; Fine, L.J.; Oliver, C.; Bernstein, L.; Peters, J.M. Abnormalities of pulmonary function and pleural disease among titanium metal production workers. Scand. J. Work. Environ. Health 1987, 13, 47–51. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 93, pp. 1–413. [Google Scholar]
- Baan, R.; Straif, K.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Cogliano, V.; WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol. 2006, 7, 295–296. [Google Scholar] [CrossRef]
- Bermudez, E.; Mangum, J.B.; Asgharian, B.; Wong, B.A.; Reverdy, E.E.; Janszen, D.B.; Hext, P.M.; Warheit, D.B.; Everitt, J.I. Long-term pulmonary responses of three laboratory rodent species to subchronic inhalation of pigmentary titanium dioxide particles. Toxicol. Sci. 2002, 70, 86–97. [Google Scholar] [CrossRef]
- Chen, H.-W.; Su, S.; Chien, C.; Lin, W.; Yu, S.-L.; Chou, C.; Chen, J.J.; Yang, P. Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. FASEB J. 2006, 20, 2393–2395. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Y.; Ye, H.; Huang, K.; Lv, Z.; Ke, Y. Different effects of titanium dioxide nanoparticles instillation in young and adult mice on DNA methylation related with lung inflammation and fibrosis. Ecotoxicol. Environ. Saf. 2019, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B. How to measure hazards/risks following exposures to nanoscale or pigment-grade titanium dioxide particles. Toxicol. Lett. 2013, 220, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Soutar, A.; Cherrie, J.; Granath, F.; Andersen, A.; Anttila, A.; Blettner, M.; Gaborieau, V.; Klug, S.J.; Langard, S.; et al. Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Causes Control 2004, 15, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Medina-Reyes, E.I.; Delgado-Buenrostro, N.L.; Díaz-Urbina, D.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; González, M.I.; Reyes, J.L.; Villamar-Duque, T.E.; Flores-Sánchez, M.L.; Hernández-Pando, R.; et al. Food-grade titanium dioxide (E171) induces anxiety, adenomas in colon and goblet cells hyperplasia in a regular diet model and microvesicular steatosis in a high fat diet model. Food Chem. Toxicol. 2020, 146, 111786. [Google Scholar] [CrossRef]
- Pele, L.C.; Thoree, V.; A Bruggraber, S.F.; Koller, D.; Thompson, R.P.H.; Lomer, M.; Powell, J.J. Pharmaceutical/food grade titanium dioxide particles are absorbed into the bloodstream of human volunteers. Part. Fibre Toxicol. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J.; et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef]
- Talamini, L.; Gimondi, S.; Violatto, M.B.; Fiordaliso, F.; Pedica, F.; Tran, N.L.; Sitia, G.; Aureli, F.; Raggi, A.; Nelissen, I.; et al. Repeated administration of the food additive E171 to mice results in accumulation in intestine and liver and promotes an inflammatory status. Nanotoxicology 2019, 13, 1087–1101. [Google Scholar] [CrossRef]
- Urrutia-Ortega, I.M.; Garduño-Balderas, L.G.; Delgado Buenrostro, N.L.; Freyre-Fonseca, V.; Flores-Flores, J.O.; González Robles, R.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Rodríguez Sosa, R.; León-Cabrera, S.; et al. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food Chem. Toxicol. 2016, 93, 20–31. [Google Scholar] [CrossRef]
- Gim-Krumm, M.; Donoso, P.; Zuñiga, R.N.; Estay, H.; Troncoso, E. A comprehensive study of glucose transfer in the human small intestine using an in vitro intestinal digestion system (i-IDS) based on a dialysis membrane process. J. Membr. Sci. 2018, 564, 700–711. [Google Scholar] [CrossRef]
- Dorier, M.; Béal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology 2017, 11, 751–761. [Google Scholar] [CrossRef]
- Dorier, M.; Tisseyre, C.; Dussert, F.; Béal, D.; Arnal, M.-E.; Douki, T.; Valdiglesias, V.; Laffon, B.; Fraga, S.; Brandão, F.; et al. Toxicological impact of acute exposure to E171 food additive and TiO2 nanoparticles on a co-culture of Caco-2 and HT29-MTX intestinal cells. Mutat. Res. Toxicol. Environ. Mutagen. 2018, 845, 402980. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.H.M.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles–known and unknown health risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Nonni, J. Medical makeup: The correction of hyperpigmentation disorders. Ann Dermatol. Venereol. 2012, 139, S170–S176. [Google Scholar] [CrossRef]
- Dunford, R.; Salinaro, A.; Cai, L.; Serpone, N.; Horikoshi, S.; Hidaka, H.; Knowland, J. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997, 418, 87–90. [Google Scholar] [CrossRef]
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Sadrieh, N.; Wokovich, A.M.; Gopee, N.V.; Zheng, J.; Haines, D.; Parmiter, D.; Siitonen, P.H.; Cozart, C.R.; Patri, A.R.K.; McNeil, S.E.; et al. Lack of Significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol. Sci. 2010, 115, 156–166. [Google Scholar] [CrossRef]
- Hidaka, H.; Horikoshi, S.; Serpone, N.; Knowland, J. In vitro photochemical damage to AND, ARN and their bases by an inorganic sunscreen agent on exposure UVA and UVB radiation. J. Photochem. Photobiol. A Chem. 1997, 111, 205–213. [Google Scholar] [CrossRef]
- Tan, M.-H.; A Commens, C.; Burnett, L.; Snitch, P.J. A pilot study on the percutaneous absorption of microfine titanium dioxide from sunscreens. Australas. J. Dermatol. 1996, 37, 185–187. [Google Scholar] [CrossRef]
- Ling, C.; An, H.; Li, L.; Wang, J.; Lu, T.; Wang, H.; Hu, Y.; Song, G.; Liu, S. Genotoxicity evaluation of titanium dioxide nanoparticles in vitro: A systematic review of the literature and meta-analysis. Biol. Trace Elem. Res. 2021, 199, 2057–2076. [Google Scholar] [CrossRef]
- Charles, S.; Jomini, S.; Fessard, V.; Bigorgne-Vizade, E.; Rousselle, C.; Michel, C. Assessment of the in vitro genotoxicity of TiO2 nanoparticles in a regulatory context. Nanotoxicology 2018, 12, 357–374. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Di Bucchianico, S.; Collins, A.; Dusinska, M. Can the comet assay be used reliably to detect nanoparticle-induced genotoxicity? Environ. Mol. Mutagen. 2014, 56, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Doak, S.; Griffiths, S.; Manshian, B.; Singh, N.; Williams, P.; Brown, A.; Jenkins, G. Confounding experimental considerations in nanogenotoxicology. Mutagenesis 2009, 24, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Kroll, A.; Pillukat, M.H.; Hahn, D.; Schnekenburger, J. Interference of engineered nanoparticles with in vitro toxicity assays. Arch. Toxicol. 2012, 86, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Kain, J.; Karlsson, H.L.; Moller, L. DNA damage induced by micro- and nanoparticles--interaction with FPG influences the detection of DNA oxidation in the comet assay. Mutagenesis 2012, 27, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. In Vitro 2013, 27, 954–963. [Google Scholar] [CrossRef]
- Guadagnini, R.; Halamoda Kenzaoui, B.; Walker, L.; Pojana, G.; Magdolenova, Z.; Bilanicova, D.; Saunders, M.; Juillerat-Jeanneret, L.; Marcomini, A.; Huk, A.; et al. Toxicity screenings of nanomaterials: Challenges due to interference with assay processes and components of classicin vitrotests. Nanotoxicology 2015, 9, 13–24. [Google Scholar] [CrossRef]
- Liu, Y.; Peterson, D.A.; Kimura, H.; Schubert, D. Mechanism of Cellular 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Reduction. J. Neurochem. 2002, 69, 581–593. [Google Scholar] [CrossRef]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Thirunavukkarasu, G.K.; Bacova, J.; Monfort, O.; Dworniczek, E.; Paluch, E.; Hanif, M.B.; Rauf, S.; Motlochova, M.; Capek, J.; Hensel, K.; et al. Critical comparison of aerogel TiO2 and P25 nanopowders: Cytotoxic properties, photocatalytic activity and photoinduced antimicrobial/antibiofilm performance. Appl. Surf. Sci. 2021, 579, 152145. [Google Scholar] [CrossRef]
- Gea, M.; Bonetta, S.; Iannarelli, L.; Giovannozzi, A.M.; Maurino, V.; Bonetta, S.; Hodoroaba, V.-D.; Armato, C.; Rossi, A.M.; Schilirò, T. Shape-engineered titanium dioxide nanoparticles (TiO2-NPs): Cytotoxicity and genotoxicity in bronchial epithelial cells. Food Chem. Toxicol. 2019, 127, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Di Bucchianico, S.; Cappellini, F.; Le Bihanic, F.; Zhang, Y.; Dreij, K.; Karlsson, H.L. Genotoxicity of TiO2 nanoparticles assessed by mini-gel comet assay and micronucleus scoring with flow cytometry. Mutagenesis 2017, 32, 127–137. [Google Scholar] [CrossRef]
- Borenfreund, E.; Babich, H.; Martin-Alguacil, N. Comparisons of two in vitro cytotoxicity assays—The neutral red (NR) and tetrazolium MTT tests. Toxicol. In Vitro 1988, 2, 1–6. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Pearce, J.M. Digital designs and scientific hardware. In Open-Source Lab; Pearce, J.M., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 165–252. [Google Scholar]
- Wang, J.; Fan, Y. Lung injury induced by TiO2 nanoparticles depends on their structural features: Size, shape, crystal phases, and surface coating. Int. J. Mol. Sci. 2014, 15, 22258–22278. [Google Scholar] [CrossRef]


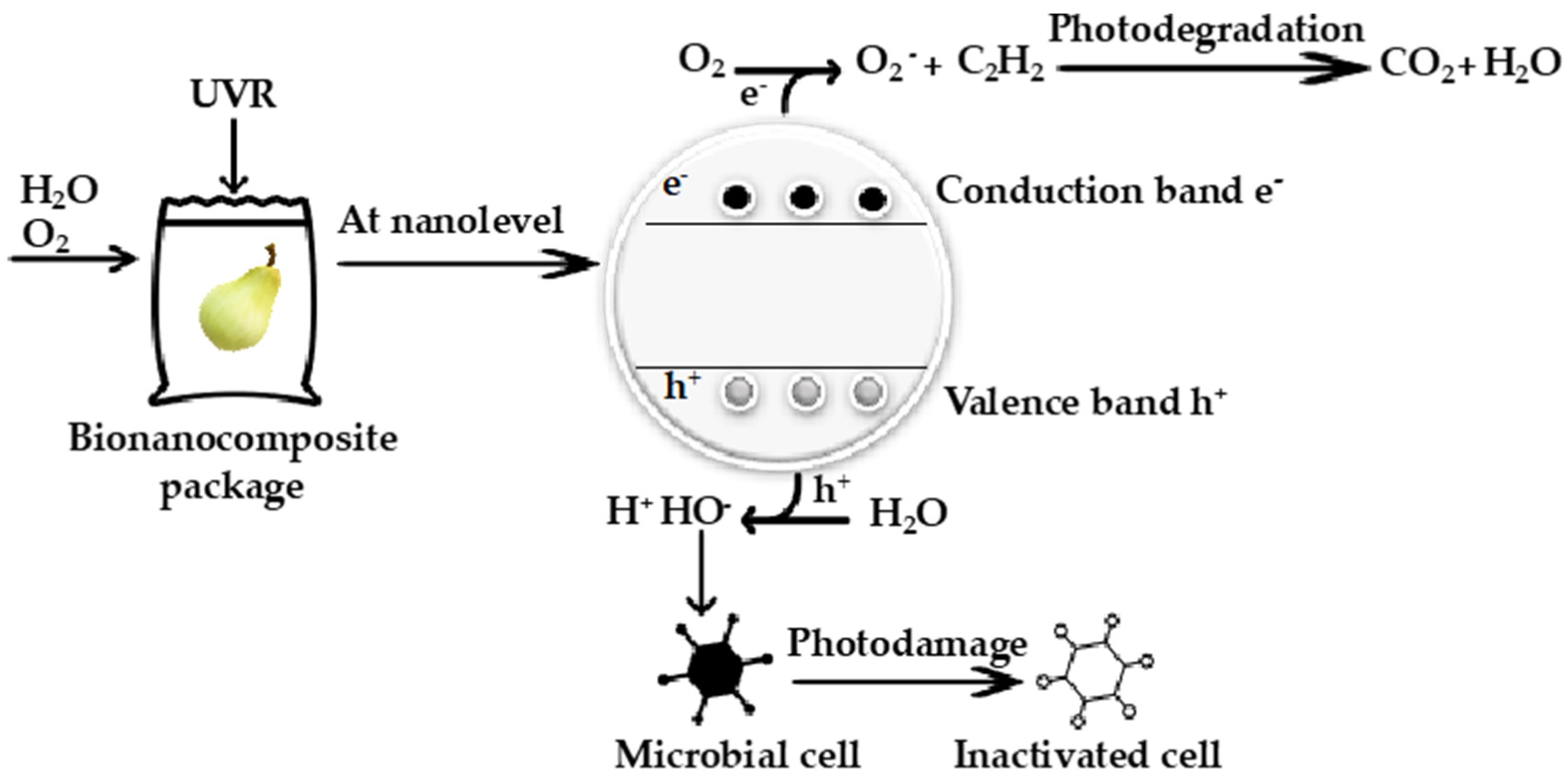

| Nanoparticle | Biocomposite | Antibacterial Activity | Antifungal Activity |
|---|---|---|---|
| TiO2 | Chitosan | Escherichia coli [16], Salmonella typhimurium [16], Staphylococcus aureus [16], Pseudomonas aeruginosa [16] | Aspergillus oryzae [16], Penicillium roqueforti [16] |
| TiO2 | Wheat gluten-cellulose | S. aureus [70], E. coli [70] | Saccharomyces cerevisiae [70] |
| TiO2 | Chitosan | E. coli [82] | N/A |
| TiO2 + Ag | Chitosan | E. coli [83] | N/A |
| TiO2 + Ag | Chitosan—fish gelatin | E. coli [84], S. aureus [83] | Botrytis cinerea [84] |
| TiO2 | Chitosan—graphene oxide | Bacillus subtilis [85] | Aspergillus niger [85] |
| TiO2 + Ag | Chitosan | E. coli [86], S. aureus [86] | Candida albicans [86] |
| TiO2 | Natural herbal and fruit extracts | E. coli [87], Salmonella paratyphi [87], B. subtilis [87], Vibrio cholerae [87], Listeria monocytogenes [87], Streptococcus faecalis [87], S. aureus [87], P. aeruginosa [87] | Saccharomyces diastaticus [87], A. niger [87] |
| TiO2 | Chitosan-starch | E. coli [88], S. aureus [88] | N/A |
| TiO2 | Chitosan-lemon grass oil | S. aureus [89], Enterobacteriaceae [89], psychrotrophic [89] and lactic acid bacteria [89] | N/A |
| TiO2 | Chitosan | N/A | Yeasts and moulds [90] |
| In Vitro Assay | Detection Target after Exposure to Nano-TiO2 |
|---|---|
| DCF (dichlorofluorescein) [128,129,130,131,132,133] | Cellular oxidative stress [128,129,130,131,132,133] |
| MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide) [12,117,129,133,134] | Cell viability through metabolic activity, once in contact with the MTT dye [12,117,129,133,134] |
| LDH (lactate dehydrogenase) [129] | Apoptosis [129] |
| IL-8 (interleukin-8) with ELISA kit [129,135] | Inflammatory immune response in pulmonary cells [129,135] |
| Comet [127,130] | DNA strand breaks and intracellular oxidative stress [127,130] |
| Micronucleus [127] | ROS-induced chromosome breakage and genetic abnormalities [127] |
| NR (neutral red) uptake [12,133,136] | Cell viability through functional lysosomal activity [133,136] |
| WST-1 [137] | Cell viability, recorded as mitochondrial dehydrogenase activity in the presence of the WST-1 reagent [137] |
| Glutathione assay [137] | Changes in glutathione expression as a response to toxin-induced oxidative stress, in the presence of fluorescent bimane dye [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racovita, A.D. Titanium Dioxide: Structure, Impact, and Toxicity. Int. J. Environ. Res. Public Health 2022, 19, 5681. https://doi.org/10.3390/ijerph19095681
Racovita AD. Titanium Dioxide: Structure, Impact, and Toxicity. International Journal of Environmental Research and Public Health. 2022; 19(9):5681. https://doi.org/10.3390/ijerph19095681
Chicago/Turabian StyleRacovita, Anca Diana. 2022. "Titanium Dioxide: Structure, Impact, and Toxicity" International Journal of Environmental Research and Public Health 19, no. 9: 5681. https://doi.org/10.3390/ijerph19095681
APA StyleRacovita, A. D. (2022). Titanium Dioxide: Structure, Impact, and Toxicity. International Journal of Environmental Research and Public Health, 19(9), 5681. https://doi.org/10.3390/ijerph19095681