Inequalities in Temporal Effects on Cervical Cancer Mortality in States in Different Geographic Regions of Brazil: An Ecological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Study Population, and Location Characterization
2.2. Study Variables
- (i)
- (ii)
- the proportional redistribution classified as incomplete diagnosis among all cancers and the deaths classified as incomplete diagnosis of female genital tract cancer, by age group, year of death and state (184, 195, 196, 197, 198, 199, C57, C76, C77, C78, C79, C80, and C97);
- (iii)
- the proportional redistribution of deaths classified as unspecified uterine cancer relating to cancers of the uterus (179, and C55), cervical cancer (189 and C53) and endometrial cancer (182 and C54), according to age group, year of death and state.
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations and Strengths of the Study
5. Conclusions
Policy Implication
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/330745 (accessed on 22 November 2021).
- Dickinson, J.A.; Stankiewicz, A.; Popadiuk, C.; Pogany, L.; Onysko, J.; Miller, A.B. Reduced cervical cancer incidence and mortality in Canada: National data from 1932 to 2006. BMC Public Health 2012, 12, 992. [Google Scholar] [CrossRef] [PubMed]
- Sasieni, P.; Adams, J. Effect of screening on cervical cancer mortality in England and Wales: Analysis of trends with an age period cohort model. BMJ 1999, 318, 1244–1245. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Lortet-Tieulent, J.; Plummer, M.; Franceschi, S.; Bray, F. Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. Eur. J. Cancer 2013, 49, 3262–3273. [Google Scholar] [CrossRef]
- Wang, J.; Lv, H.; Xue, Z.; Wang, L.; Bai, Z. Temporal trends of common female malignances on breast, cervical, and ovarian cancer mortality in Japan, Republic of Korea, and Singapore: Application of the age-period-cohort model. BioMed Res. Int. 2018, 2018, 5307459. [Google Scholar] [CrossRef]
- Arbyn, M.; Raifu, A.O.; Weiderpass, E.; Bray, F.; Anttila, A. Trends of cervical cancer mortality in the member states of the European Union. Eur. J. Cancer 2009, 45, 2640–2648. [Google Scholar] [CrossRef]
- Bergman, H.; Buckley, B.S.; Villanueva, G.; Petkovic, J.; Garritty, C.; Lutje, V.; Riveros-Balta, A.X.; Low, N.; Henschke, N. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males. Cochrane Database Syst. Rev. 2019, 22, CD013479. [Google Scholar] [CrossRef]
- Fidler, M.M.; Bray, F.; Soerjomataram, I. The global cancer burden and human development: A review. Scand. J. Public Health 2018, 46, 27–36. [Google Scholar] [CrossRef]
- Tsuchiya, C.; Lawrence, T.; Klen, M.; Fernandes, R.; Alves, M.R. O câncer de colo do útero no Brasil: Uma retrospectiva sobre as políticas públicas voltadas à saúde da mulher. J. Bras. Econ. Saúde 2017, 9, 137–147. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Diretoria de Pesquisas, Coordenação de Trabalho e Rendimento, Pesquisa Nacional de Saúde. 2019. Available online: https://www.ibge.gov.br/estatisticas/sociais/saude/9160-pesquisa-nacional-de-saude.html (accessed on 15 December 2021).
- Ministério da Saúde; Instituto Nacional de Câncer (INCA). Estimativa 2020 Incidência do Câncer no Brasil. Rio de Janeiro: INCA. 2020. Available online: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//estimativa-2020-incidencia-de-cancer-no-brasil.pdf (accessed on 15 December 2021).
- Gamarra, C.J.; Valente, J.G.; E. Silva, G.A. Magnitude of mortality from cervical cancer in the Brazilian Northeast and socioeconomic factors. Rev. Panam. Salud Pública 2010, 28, 100–106. [Google Scholar] [CrossRef]
- Girianelli, V.R.; Gamarra, C.J.; E. Silva, G.A. Disparities in cervical and breast cancer mortality in Brazil. Rev. Saúde Pública 2014, 48, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.G.; Girianelli, V.R.; Guamarra, C.J.; Bustamante-Teixeira, M.T. Cervical cancer mortality trends in Brazil, 1981–2006. Cad Saude Publica. 2010, 26, 2339–2407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guimarães, R.M.; Muzi, C.D.; Teixeira, M.P.; Pinheiro, S.S. Cancer’s transition in Brazil and strategical decision-making in women´s public health policies. R Pol Públ. 2016, 20, 33–50. [Google Scholar] [CrossRef][Green Version]
- Paim, J.; Travassos, C.; Almeida, C.; Bahia, L.; Macinko, J. The Brazilian system: History, advances and challegens. Lancet 2011, 21, 1778–1797. [Google Scholar] [CrossRef]
- Viacava, F.; Bellido, J.G. Health, access to services and sources of payment, according to household surveys. Ciênc Saúde Coletiva 2016, 21, 351–370. [Google Scholar] [CrossRef]
- De Oliveira, M.M.; Andrade, S.S.C.D.A.; De Oliveira, P.P.V.; E. Silva, G.A.; Da Silva, M.M.A.; Malta, D.C. Cobertura de exame Papanicolaou em mulheres de 25 a 64 anos, segundo a Pesquisa Nacional de Saúde e o Sistema de Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico, 2013. Rev. Bras. Epidemiol. 2018, 21, e180014. [Google Scholar] [CrossRef][Green Version]
- Meira, K.C.; Silva, G.W.D.S.; Dos Santos, J.; Guimarães, R.M.; De Souza, D.L.B.; Ribeiro, G.P.C.; Dantas, E.S.O.; De Carvalho, J.B.L.; Jomar, R.T.; Simões, T.C. Analysis of the effects of the age-period-birth cohort on cervical cancer mortality in the Brazilian Northeast. PLoS ONE 2020, 15, e0226258. [Google Scholar] [CrossRef]
- Malta, D.C.; Jorge, A.D.O. Análise de tendência de citologia oncótica e mamografia das capitais brasileiras. Ciência Cult. 2014, 66, 25–29. [Google Scholar] [CrossRef][Green Version]
- Filha, M.M.T.; Leal, M.D.C.; De Oliveira, E.F.V.; Esteves-Pereira, A.P.; Da Gama, S.G.N. Regional and social inequalities in the performance of Pap test and screening mammography and their correlation with lifestyle: Brazilian national health survey, 2013. Int. J. Equity Health 2016, 15, 136. [Google Scholar] [CrossRef]
- Rena-Júnior, N.L.; Silva, G.A. Temporal trend and associated factors to advanced stage at diagnosis of cervical cancer: Analy-sis of data from hospital based cancer registries in Brazil, 2000–2012. Epidemiol. Serv. Saude 2018, 27, e2017285. [Google Scholar] [CrossRef]
- Oliveira, N.P.D. Desigualdades No Diagnóstico e Mortalidade por Câncer de Mama e Colo do Útero no Brasil; Tese (Doutorado em Saúde Coletiva), Centro de Ciências da Saúde, Universidade Federal do Rio Grande do Norte: Natal, Brazil, 2020; Available online: https://repositorio.ufrn.br/jspui/handle/123456789/30744 (accessed on 16 December 2021).
- Albuquerque, M.V.; d’Ávila, A.L.; Lima, L.D.; Ferreira, M.P.; Fusaro, E.R.; Iozzi, F.L. Desigualdades regionais na saúde: Mudanças observadas no Brasil de 2000 a 2016. Ciência Saúde Coletiva 2017, 22, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Araújo, T.B. Tendências do desenvolvimento regional recente no Brasil. In Pacto Federativo, Integração Nacional e Desenvolvimento Regional; Editora Fundação Perseu Abramo: São Paulo, Brazil, 2013; pp. 39–51. [Google Scholar]
- Granato, L.; Batista, I.R. Heterogeneidade estrutural nas relações internacionais da América Latina: Um olhar através dos paradigmas de integração regional. Cad. PROLAM/USP 2018, 16, 5–29. [Google Scholar] [CrossRef]
- Holford, T.R. The estimation of age, period and cohort effects for vital rates. Biometrics 1983, 39, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Land, K.C. Age-Period-Cohort Analysis. New Models, Methods, and Empirical Applications; Interdisciplinary Statistics Series; Chapman & Hall/CRC: Boca Raton, FL, USA, 2013. [Google Scholar]
- Holford, T.R. Approaches to fitting age-period-cohort models with unequal intervals. Stat. Med. 2006, 25, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.; Boyle, P. Age, period and cohort models: The use of individual records. Stat. Med. 1986, 5, 527–538. [Google Scholar] [CrossRef]
- Stevens, G.A.; Alkema, L.; Black, R.E.; Boerma, J.T.; Collins, G.S.; Ezzati, M.; Grove, J.T.; Hogan, D.R.; Hogan, M.C.; Horton, R.; et al. Guidelines for accurate and transparent health estimates reporting: The gather statement. PLoS Med. 2016, 13, e1002056. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Sistema IBGE de Recuperação Automática; de Janeiro, R., Ed.; IBGE: Rio de Janeiro, Brazil, 2019. Available online: http://ftp.ibge.gov.br (accessed on 15 October 2021).
- Municipal Health Development Index (IFDM-Saúde/2016) State Median. Available online: https://www.firjan.com.br/ifdm/downloads (accessed on 15 October 2021).
- Departamento de Informática do Sistema Único de Saúde, Ministério da Saúde, Brasil. Sistema de Informação Sobre Mortalidade; Ministério da Saúde: Brasília, Brazil, 2021. Available online: http://www2.datasus.gov.br/DATASUS/index.php?area=0205 (accessed on 30 November 2021).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.R-project.org/ (accessed on 15 January 2022).
- Szwarcwald, C.L.; Morais-Neto, O.L.; Frias, P.G.; Souza Junior, P.R.B.; Escalante, J.J.C.; Lima, R.B.; Viola, R.C. Busca ativa de óbitos e nascimentos no Nordeste e na Amazonia Legal: Estimação das coberturas do SIM e do Sinasc nos municípios brasileiros. In Saúde Brasil 2010: Uma Análise da Situação de Saúde e de Evidências Selecionadas de Impacto de Ações de Vigilância em Saúde, 1st ed.; Ministério da Saúde—Secretaria de Vigilância em Saúde—Departamento de Análise de Situação em Saúde, Coordenação Geral de Informação e Análise epidemiológica CGIAE, Núcleo de Comunicação, Eds.; Ministério da Saúde: Brasília, Brazil, 2011; pp. 79–98. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/saude_brasil_2010.pdf (accessed on 25 November 2021).
- Mello, J.M.H.; Gotlieb, S.L.D.; Laurenti, R. The national mortality information system: Problems and proposals for solving them I—Deaths by natural causes. Rev. Bras. Epidemiol. 2002, 5, 197–211. [Google Scholar] [CrossRef]
- Queiroz, B.L.; Freire, F.H.M.D.A.; Gonzaga, M.R.; Lima, E. Completeness of death-count coverage and adult mortality (45q15) for Brazilian states from 1980 to 2010. Rev. Bras. Epidemiol. 2017, 20, 21–33. [Google Scholar] [CrossRef]
- Barbosa, I.R.; Souza, D.L.B.; Bernak, M.M.; Costa, I.C.C. Regional inequalities in cervical cancer mortality in Brazil: Trends and projections through to 2030. Ciênc Saúde Coletiva 2016, 21, 253–262. [Google Scholar] [CrossRef]
- de Sousa, A.M.V.; Teixeira, C.C.A.; Medeiros, S.d.; Nunes, S.J.C.; Salvador, P.T.C.d.; de Barros, R.M.B.; de Lima, F.F.S.; Nascimento, G.G.C.d.; Santos, J.d.; de Souza, D.L.B.; et al. Mortlidade por cancer do colo de útero no estado do Rio Grande do Norte, no período de 1996 a 2010: Tendência temporal e projeções até 2030. Epidemiol. Serv. Saúde 2016, 25, 311–322. [Google Scholar] [CrossRef]
- Mathers, C.D.; Bernard, C.; Iburg, K.M.; Inoue, M.; Fat, D.M.; Shibuya, K.; Stein, C.; Tomijima, N.; Xu, H. Global burden of disease in 2002: Data sources, methods and results. In Global Programme on Evidence for Health Policy Discussion Paper; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Doll, R.; Payne, P.M.; Waterhouse, J.A.H. Cancer incidence in five countries. In International Union Against Cancer; Springer: Berlin/Heidelberg, Germany, 1966. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2021. Available online: http://qgis.osgeo.org (accessed on 20 January 2022).
- Lima, E.; Queiroz, B. Evolution of the deaths registry system in Brazil: Associations with changes in the mortality profile, under-registration of death counts, and ill-defined causes of death. Cad. Saúde Pública 2014, 30, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Vale, D.B.; Sauvaget, C.; Muwonge, R.; Ferlay, J.; Zeferino, L.C.; Murillo, R.; Sankaranarayanan, R. Disparities in time trends of cervical cancer mortality rates in Brazil. Cancer Causes Control. 2016, 27, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Loos, A.; Bray, F.; McCarron, P.; Weiderpass, E.; Hakama, M.; Parkin, D. Sheep and goats: Separating cervix and corpus uteri from imprecisely coded uterine cancer deaths, for studies of geographical and temporal variations in mortality. Eur. J. Cancer 2004, 40, 2794–2803. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Coutinho, J.V.A.; Muzi, C.D.; Guimarães, R.M. Reasons for never receiving a pap test among Brazilian women: National health survey. Public Health Nurs. 2021, 38, 963–977. [Google Scholar] [CrossRef]
- Travassos, C.; De Oliveira, E.X.G.; Viacava, F. Desigualdades geográficas e sociais no acesso aos serviços de saúde no Brasil: 1998 e 2003. Ciência Saúde Coletiva 2006, 11, 975–986. [Google Scholar] [CrossRef]
- Barreto, M.L. Desigualdades em Saúde: Uma perspectiva global. Ciência Saúde Coletiva 2017, 22, 2097–2108. [Google Scholar] [CrossRef]
- Meira, K.C.; E. Silva, G.A.; Da Silva, C.M.F.P.; Valente, J.G. Age-period-cohort effect on mortality from cervical cancer. Rev. De Saúde Pública 2013, 47, 274–282. [Google Scholar] [CrossRef]
- Meira, K.C.; Ferreira, A.A.; Silva, C.M.P.F.; Valente, J.G.; Santos, J. Mortalidade por câncer do colo do útero no estado de Minas Gerais, análise do efeito da idade-período-coorte de nascimento. Cad. Saúde Colet 2012, 20, 381–388. [Google Scholar]
- Wang, J.; Bai, Z.; Wang, Z.; Yu, C. Comparison of secular trends in cervical cancer mortality in China and the United States: An Age-period-cohort analysis. Int. J. Environ. Res. Public Health 2016, 13, 1148. [Google Scholar] [CrossRef]
- Peto, J.; Gilham, C.; Fletcher, O.; Matthews, F. The cervical cancer epidemic that screening has prevented in the UK. Lancet 2004, 364, 249–256. [Google Scholar] [CrossRef]
- Small, W.; Bacon, M.A.; Bajaj, A.; Chuang, L.T.; Fisher, B.J.; Harkenrider, M.M.; Jhingran, A.; Kitchener, H.C.; Mileshkin, L.R.; Viswanathan, A.N.; et al. Cervical cancer: A global health crisis. Cancer 2017, 123, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Ojamaa, K.; Innos, K.; Baburin, A.; Everaus, H.; Veerus, P. Trends in cervical cancer incidence and survival in Estonia from 1995 to 2014. BMC Cancer 2018, 18, 1075. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Lortet-Tieulent, J.; Znaor, A.; Brotons, M.; Poljak, M.; Arbyn, M. Patterns and trends in human papillomavirus-related Diseases in central and eastern Europe and central Asia. Vaccine 2013, 31, H32–H45. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.R.; Bustamante-Teixeira, M.T.; Corrêa, C.S.L.; De Abreu, D.M.X.; Curado, M.P.; Mooney, M.; Naghavi, M.; Teixeira, R.; França, M.R.; Malta, D.C. Magnitude e variação da carga da mortalidade por câncer no Brasil e Unidades da Federação, 1990 e 2015. Rev. Bras. Epidemiol. 2017, 20, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Löwy, I. Imperfect tools for a difficult job: Colposcopy, “colpocytogy” and screening for cervical cancer in Brazil. Soc. Stud. Sci. 2011, 41, 586–608. [Google Scholar] [CrossRef]
- Lago, T.G. Políticas Nacionais de Rastreamento do Câncer do Colo Uterino no Brasil. Análise do Período 1998 a 2002; Tese de Doutorado, UNICAMP Universidade Estadual de Campinas: Campinas, Brazil, 2004. [Google Scholar]
- Costa, R.F.A.; Longatto-Filho, A.; Pinheiro, C.; Zeferino, L.C.; Fregnani, J.H. Historical analysis of the brazilian cervical cancer screening program from 2006 to 2013: A time for reflection. PLoS ONE 2015, 10, e0138945. [Google Scholar] [CrossRef]
- Dias, M.B.K.; Tomazelli, J.G.; Assis, M. Cervix cancer screening in Brazil: Analysis of Siscolo data from 2002 to 2006. Epidemiol. Serv Saúde 2010, 19, 293–306. [Google Scholar] [CrossRef]
- Nascimento, M.I.D.; E. Silva, G.A. Waiting time for radiotherapy in women with cervical cancer. Rev. Saúde Pública 2015, 49, 1–8. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Silva, G.A. Assessment of the production of cervical cancer care procedures in the Brazilian National Health System in 2015. Epidemiol. Serv. Saúde 2018, 27, e20172124. [Google Scholar] [CrossRef]
- Meira, K.C.; Jesus, J.C.; Aiquoc, K.M. Mortes por Câncer do Colo do Útero: Um Reflexo do Racism Estrutural. Available online: https://demografiaufrn.net/2021/11/09/cancer-racismo/ (accessed on 13 January 2022).
- Müller, E.V.; Biazevic, M.G.H.; Antunes, J.L.F.; Crosato, E.M. Tendência e diferenciais socioeconômicos da mortalida-de por câncer de colo de útero no Estado do Paraná (Brasil), 1980–2000. Cien Saude Colet 2011, 16, 2495–2500. [Google Scholar] [CrossRef]
- Fonseca, L.A.M.; Ramacciotti, A.S.; Eluf Neto, J. Tendência da mortalidade por câncer do útero no Município de São Paulo entre 1980 e 1999. Cad. Saude Publica 2004, 20, 136–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moon, E.-K.; Oh, C.-M.; Won, Y.-J.; Lee, J.-K.; Jung, K.-W.; Cho, H.; Jun, J.K.; Lim, M.C.; Ki, M. Trends and age-period-cohort effects on the incidence and mortality rate of cervical cancer in Korea. Cancer Res. Treat. 2017, 49, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Q.; Jin, S.-Q.; Xu, H.-X.; Thomas, D.B. The decline in the mortality rates of cervical cancer and a plausible explanation in Shandong, China. Int. J. Epidemiol. 2000, 29, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.X.G.; Melo, E.C.P.; Pinheiro, R.S.; Noronha, C.P.; Carvalho, M.S. Access to cancer care: Mapping hospital admissions and high-complexity outpatient care flows. The case of breast cancer. Cad. Saúde Pública 2011, 27, 317–326. [Google Scholar] [CrossRef]
- Constante, H.M.; Marinho, G.L.; Bastos, J. The door is open, but not everyone may enter: Racial inequities in healthcare access across three Brazilian surveys. Ciência Saúde Coletiva 2021, 26, 3981–3990. [Google Scholar] [CrossRef]
- Pinto, L.F.; Quesada, L.A.; D’Avila, O.P.; Hauser, L.; Gonçalves, M.E.; Harzheim, E. Primary Care Assessment Tool: Regional differences based on the National Health Survey from Instituto Brasileiro de Geografia e Estatística. Ciência e Saúde Coletiva 2021, 26, 3965–3979. [Google Scholar] [CrossRef]
- González, J.R.; Llorca, F.L.; Moreno, V. Algunos aspectos metodológicos sobre los modelos edad-período-cohorte. Aplicación a las tendências de mortalidad por câncer. Gac Sanit 2002, 16, 267–273. [Google Scholar] [CrossRef]
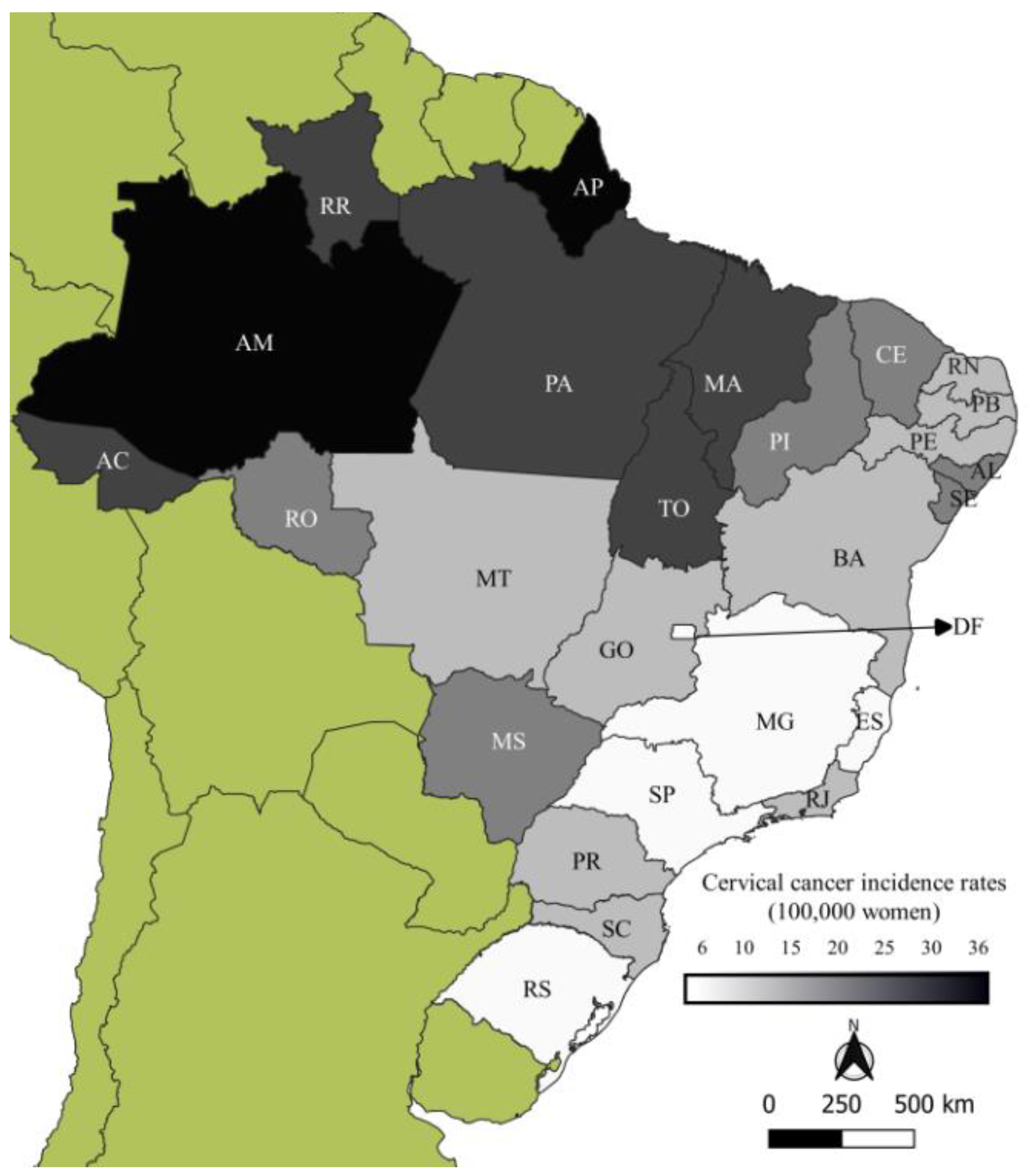



| South and Southeast | 1980–1984 | 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2014 | 2015–2019 | SMR * |
| Paraná | 12.81 | 13.41 | 14.28 | 14.93 | 12.94 | 10.87 | 10.35 | 11.03 | 14.38 |
| Rio Grande do Sul | 18.45 | 18.40 | 18.36 | 17.64 | 14.04 | 11.51 | 9.94 | 10.19 | 13.73 |
| Santa Catarina | 13.32 | 13.83 | 15.71 | 15.77 | 13.34 | 11.66 | 10.45 | 10.56 | 12.33 |
| Espírito Santo | 15.55 | 18.42 | 18.40 | 19.86 | 16.43 | 14.15 | 12.10 | 11.23 | 14.67 |
| Minas Gerais | 16.15 | 14.90 | 15.17 | 13.46 | 11.46 | 10.31 | 8.63 | 8.20 | 11.22 |
| Rio de Janeiro | 16.48 | 15.24 | 16.91 | 16.20 | 15.24 | 12.59 | 11.83 | 11.19 | 13.91 |
| São Paulo | 16.64 | 14.06 | 15.23 | 13.96 | 11.72 | 8.91 | 7.42 | 6.88 | 10.53 |
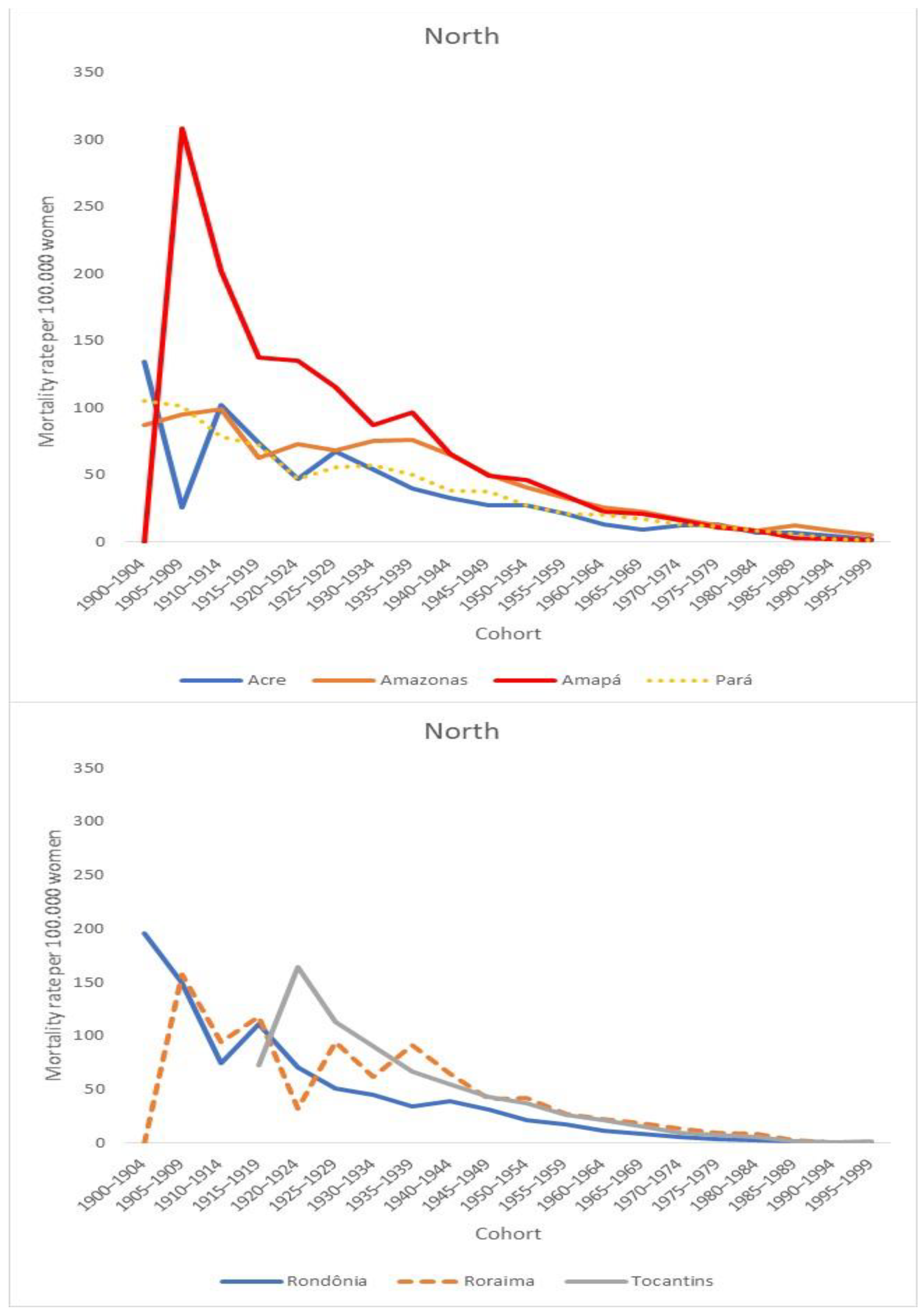
| North | 1980–1984 | 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2014 | 2015–2019 | SMR |
| Acre | 21.48 | 20.32 | 27.71 | 21.77 | 19.86 | 17.40 | 25.23 | 24.75 | 22.57 |
| Amazonas | 28.71 | 33.92 | 25.79 | 30.89 | 31.45 | 37.67 | 40.14 | 36.66 | 35.03 |
| Amapá | 40.88 | 49.16 | 46.07 | 38.44 | 29.94 | 30.29 | 33.02 | 29.96 | 35.02 |
| Pará | 28.25 | 28.60 | 25.56 | 22.99 | 26.02 | 26.20 | 24.89 | 26.65 | 26.05 |
| Rondônia | 23.80 | 30.70 | 20.66 | 19.44 | 20.28 | 16.37 | 14.18 | 15.76 | 17.91 |
| Roraima | 33.44 | 15.94 | 31.87 | 24.81 | 28.46 | 39.48 | 25.19 | 32.35 | 30.02 |
| Tocantis | _ | _ | _ | 19.45 | 21.31 | 24.18 | 22.71 | 21.12 | 21.36 |
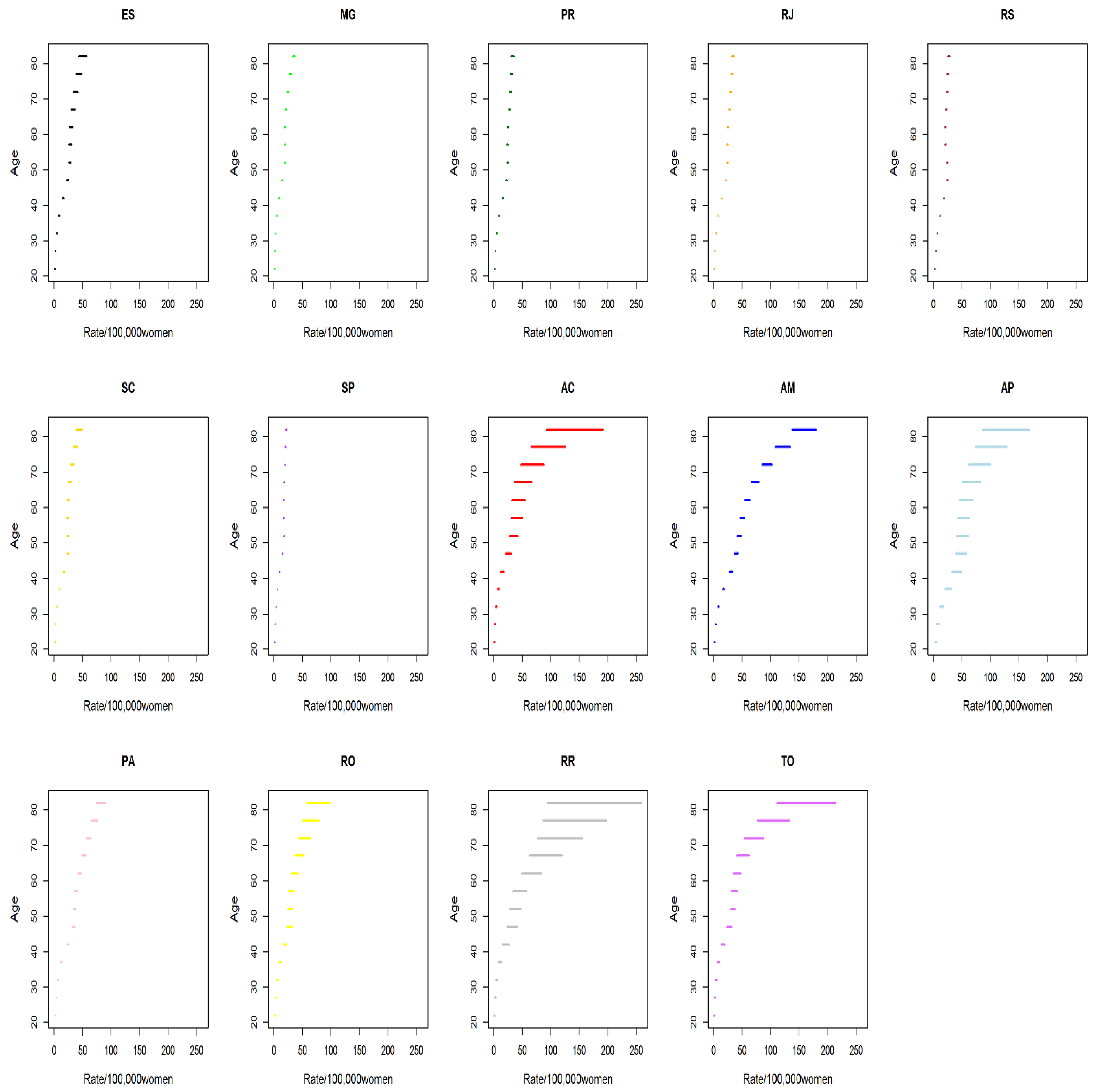
| Age Group | 20 to 24 | 25 to 29 | 30 to 34 | 35 to 39 | 40 to 44 | 45 to 49 | 50 to 54 | 55 to 59 | 60 to 64 | 65 to 69 | 70 to 74 | 75 to 79 | 80 and More Years | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| States * | AC | 0.76 | 1.04 | 7.23 | 11.12 | 18.63 | 24.34 | 33.06 | 42.92 | 45.79 | 42.21 | 55.16 | 70.38 | 104.28 |
| AM | 1.03 | 4.68 | 12.09 | 19.80 | 32.37 | 41.82 | 52.81 | 53.54 | 62.42 | 70.88 | 94.15 | 109.74 | 131.10 | |
| AP | 2.47 | 4.66 | 9.06 | 17.60 | 27.73 | 41.43 | 38.22 | 54.18 | 59.70 | 79.32 | 91.76 | 152.11 | 170.89 | |
| PA | 0.99 | 3.30 | 8.33 | 14.59 | 23.24 | 32.29 | 36.98 | 44.50 | 49.66 | 56.53 | 63.99 | 78.61 | 90.76 | |
| RO | 0.39 | 1.63 | 4.46 | 8.00 | 12.73 | 19.47 | 22.24 | 28.83 | 34.74 | 42.35 | 53.98 | 75.37 | 80.52 | |
| RR | 0.60 | 3.74 | 6.53 | 12.21 | 24.42 | 30.45 | 33.57 | 57.56 | 50.87 | 85.59 | 96.40 | 109.66 | 118.51 | |
| TO | 0.88 | 1.99 | 4.20 | 10.45 | 14.97 | 22.46 | 28.01 | 36.29 | 35.61 | 49.28 | 58.66 | 83.62 | 118.62 | |
| PR | 1.48 | 2.64 | 5.25 | 8.32 | 11.05 | 16.51 | 18.21 | 21.96 | 27.28 | 31.21 | 42.56 | 48.19 | 49.85 | |
| RS | 0.56 | 2.34 | 5.67 | 9.81 | 14.18 | 18.20 | 20.19 | 21.77 | 23.50 | 28.08 | 30.03 | 33.62 | 40.98 | |
| SC | 0.36 | 1.94 | 4.33 | 8.39 | 12.60 | 16.23 | 18.49 | 19.66 | 22.29 | 22.58 | 29.26 | 34.54 | 38.09 | |
| ES | 0.27 | 1.65 | 4.07 | 7.76 | 11.57 | 16.18 | 21.76 | 24.84 | 29.53 | 32.67 | 40.40 | 43.80 | 59.24 | |
| MG | 0.24 | 1.07 | 2.73 | 5.09 | 8.71 | 12.29 | 16.37 | 19.26 | 22.35 | 25.94 | 31.89 | 38.11 | 46.20 | |
| RJ | 0.44 | 2.05 | 4.58 | 8.09 | 12.54 | 17.38 | 20.69 | 23.05 | 26.32 | 30.07 | 34.15 | 38.63 | 47.23 | |
| SP | 0.31 | 1.21 | 2.72 | 5.00 | 8.44 | 12.00 | 15.57 | 18.86 | 20.96 | 24.63 | 28.89 | 33.91 | 36.50 | |
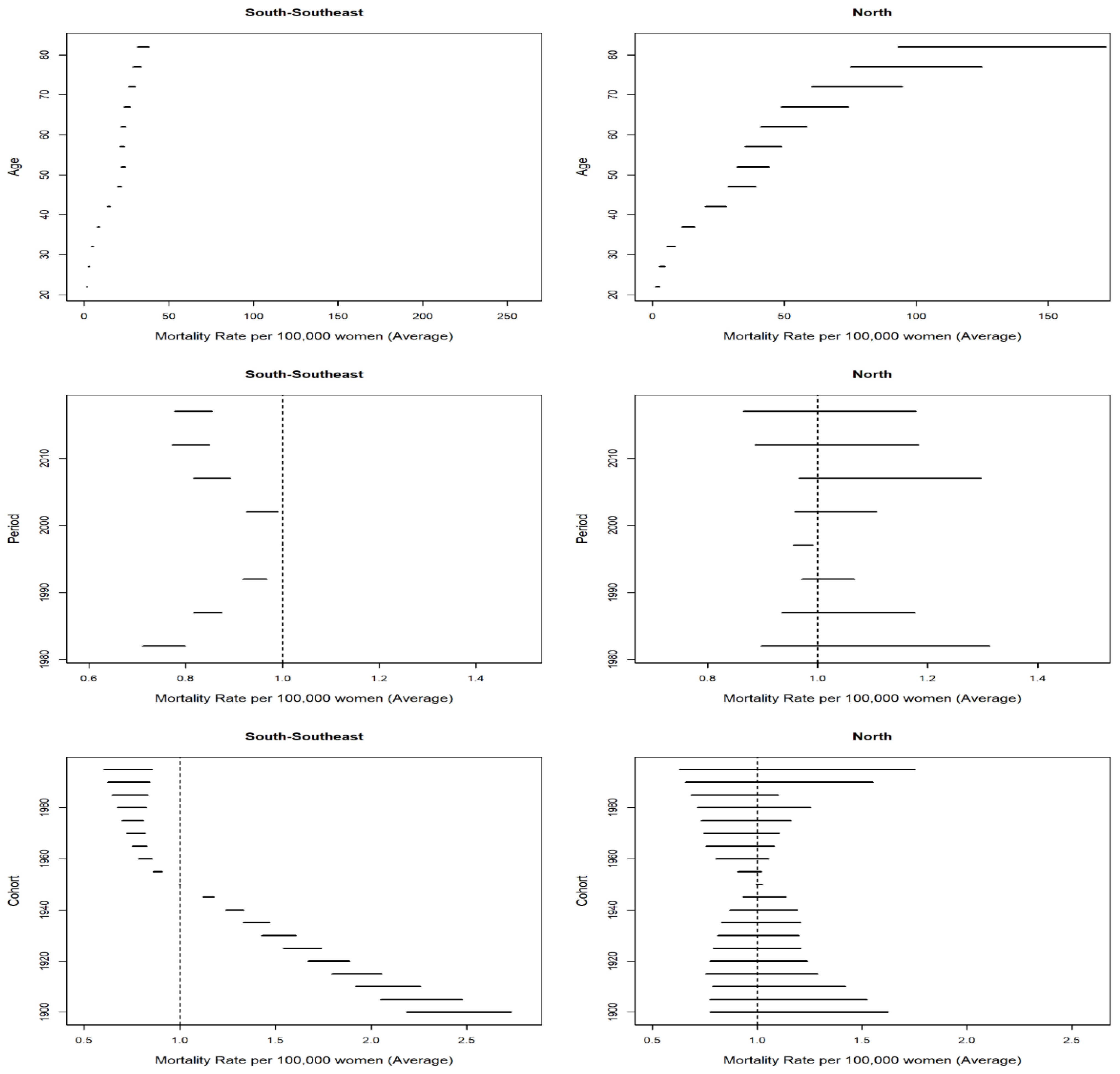
| State | Age–Drift | 95% CI | Trend |
|---|---|---|---|
| North | |||
| Acre | 1.005 | 0.998–1.010 | Stationary |
| Amazonas | 1.007 | 1.005–1.010 | Ascending |
| Amapá | 0.986 | 0.980–0.991 | Descending |
| Pará | 0.998 | 0.996–1.000 | Stationary |
| Rondônia | 0.982 | 0.978–0.986 | Descending |
| Roraima | 1.002 | 0.995–1.011 | Stationary |
| Tocantins | 1.003 | 0.996–1.011 | Stationary |
| South | |||
| Paraná | 0.980 | 0.979–0.981 | Descending |
| Rio Grande do Sul | 0.978 | 0.977–0.980 | Descending |
| Santa Catarina | 0.989 | 0.987–0.991 | Descending |
| Southeast | |||
| Espírito Santo | 0.984 | 0.982–0.987 | Descending |
| Minas Gerais | 0.981 | 0.980–0.982 | Descending |
| Rio de Janeiro | 0.987 | 0.986–0.988 | Descending |
| São Paulo | 0.975 | 0.973–0.975 | Descending |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meira, K.C.; Magnago, C.; Mendonça, A.B.; Duarte, S.F.S.; de Freitas, P.H.O.; dos Santos, J.; de Souza, D.L.B.; Simões, T.C. Inequalities in Temporal Effects on Cervical Cancer Mortality in States in Different Geographic Regions of Brazil: An Ecological Study. Int. J. Environ. Res. Public Health 2022, 19, 5591. https://doi.org/10.3390/ijerph19095591
Meira KC, Magnago C, Mendonça AB, Duarte SFS, de Freitas PHO, dos Santos J, de Souza DLB, Simões TC. Inequalities in Temporal Effects on Cervical Cancer Mortality in States in Different Geographic Regions of Brazil: An Ecological Study. International Journal of Environmental Research and Public Health. 2022; 19(9):5591. https://doi.org/10.3390/ijerph19095591
Chicago/Turabian StyleMeira, Karina Cardoso, Carinne Magnago, Angelo Braga Mendonça, Stephane Fernanda Soares Duarte, Pedro Henrique Oliveira de Freitas, Juliano dos Santos, Dyego Leandro Bezerra de Souza, and Taynãna César Simões. 2022. "Inequalities in Temporal Effects on Cervical Cancer Mortality in States in Different Geographic Regions of Brazil: An Ecological Study" International Journal of Environmental Research and Public Health 19, no. 9: 5591. https://doi.org/10.3390/ijerph19095591
APA StyleMeira, K. C., Magnago, C., Mendonça, A. B., Duarte, S. F. S., de Freitas, P. H. O., dos Santos, J., de Souza, D. L. B., & Simões, T. C. (2022). Inequalities in Temporal Effects on Cervical Cancer Mortality in States in Different Geographic Regions of Brazil: An Ecological Study. International Journal of Environmental Research and Public Health, 19(9), 5591. https://doi.org/10.3390/ijerph19095591








