Effects of Acute Hypobaric Hypoxia Exposure on Cardiovascular Function in Unacclimatized Healthy Subjects: A “Rapid Ascent” Hypobaric Chamber Study
Abstract
1. Introduction
2. Materials and Methods
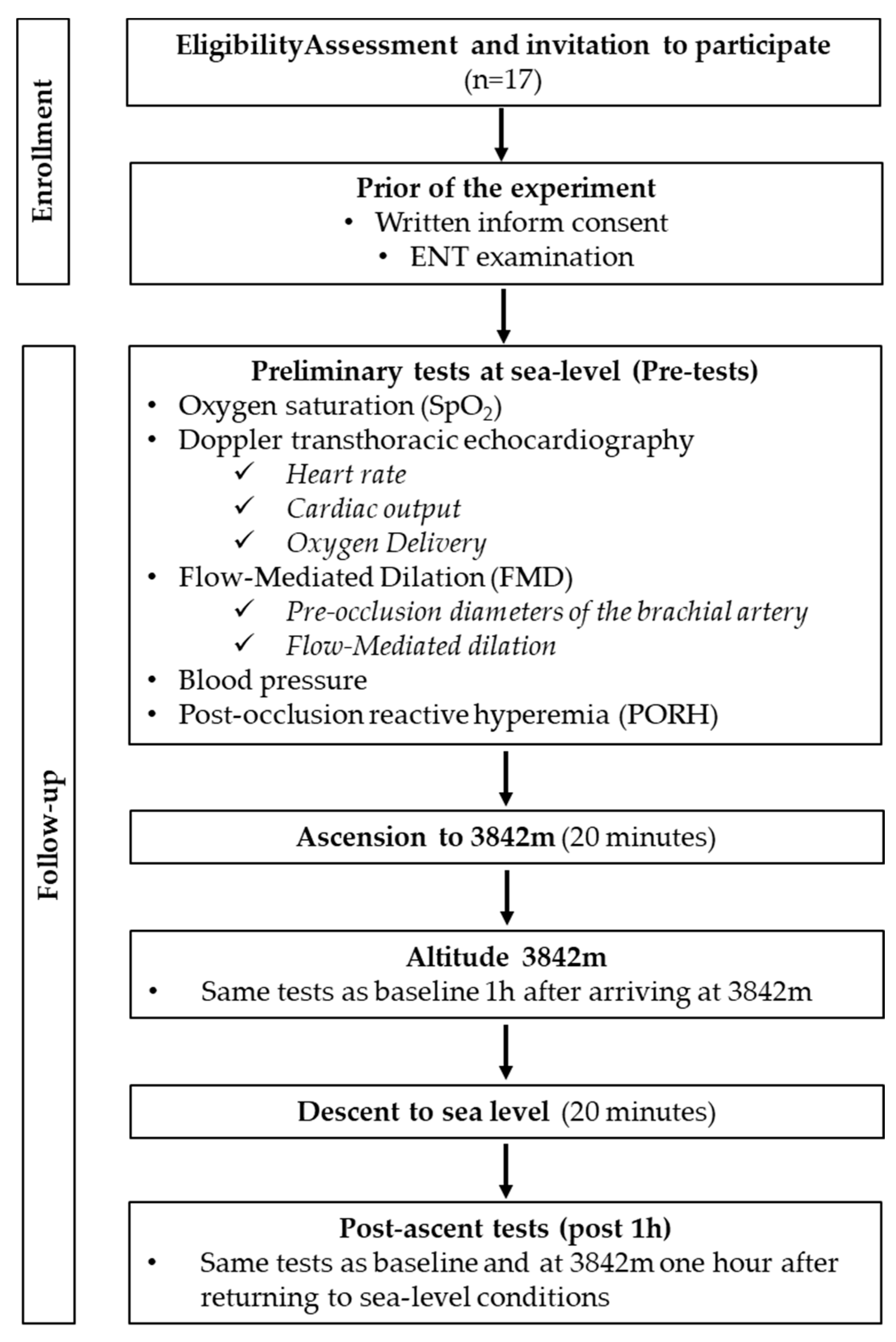
2.1. Study Population
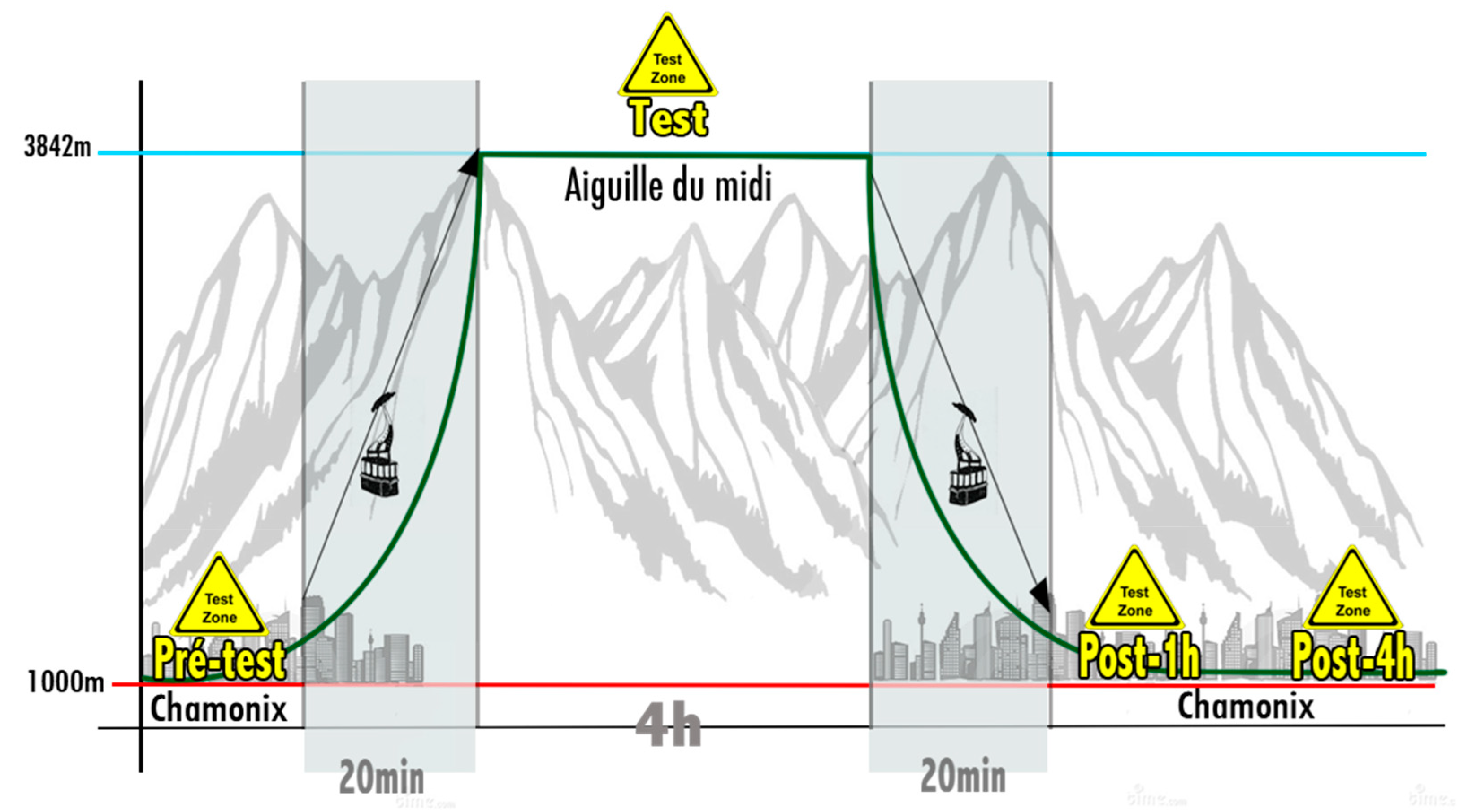
2.2. Experimental Protocol
2.3. Measurements
2.3.1. Oxygen Saturation and Heart Rate
2.3.2. Cardiac Parameters
Systemic Vascular Resistances
2.3.3. Vascular Parameters
Blood Pressure
Flow Mediated Dilation (FMD)
Post-Occlusive Hyperemia (PORH)
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Environmental Conditions
3.3. Hypoxia-Induced SpO2 Changes
3.4. Cardiac Parameters
3.5. Blood Pressure
3.6. Vascular Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CO or Q | Cardiac Output |
| CSA | Valve orifice Cross Sectional Aera |
| DO2index | Oxygen delivery index |
| ENTexamination | Ear, Nose and Throat examination |
| eNOS | Endothelial Nitric Oxide Synthase |
| FMD | Flow-Mediated Dilatation |
| HIF | Hypoxia-inductible Factor |
| HR | Heart rate |
| HVR | Hypoxia Ventilatory Response |
| ICU | Intensive Care Unit |
| iNOS | Inductible Nitric Oxide Synthase |
| LVOT | Left Ventricular Outflow Tract |
| NFκB | Nuclear transcription Factor kappa B |
| NO | Nitric Oxide |
| NRF2 | Nuclear Factor (erythroid-derived 2)-like 2 |
| PORH | Post Occlusive Reactive Hyperemia |
| QorCO | Cardiac Output |
| SpO2 | Oxygen saturation |
| SVR | Systemic vascular resistances |
| VTI | Blood Velocity Time Integral |
References
- Hochachka, P.W. Mechanism and evolution of hypoxia-tolerance in humans. J. Exp. Biol. 1998, 201, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Paralikar, S.J.; Paralikar, J.H. High-altitude medicine. Indian J. Occup. Environ. Med. 2010, 14, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, S.; Guerrero, F.; Sponsiello, N.; Cialoni, D.; Pieri, M.; Germonpre, P.; Obeid, G.; Tillmans, F.; Papadopoulou, V.; Hemelryck, W.; et al. Nitric oxide-related endothelial changes in breath-hold and scuba divers. Undersea Hyperb. Med. 2013, 40, 135–144. [Google Scholar] [PubMed]
- Clarke, C. Acute mountain sickness: Medical problems associated with acute and subacute exposure to hypobaric hypoxia. Postgrad. Med. J. 2006, 82, 748–753. [Google Scholar] [CrossRef]
- Pilmanis, A.A.; Balldin, U.I.; Fischer, J.R. Cognition Effects of Low-Grade Hypoxia. Aerosp Med. Hum. Perform. 2016, 87, 596–603. [Google Scholar] [CrossRef]
- Grittani, M.; Pellegrino, G.; Conte, S.; Morello, A.; Autore, A.; Cimmino, G.; Trimarco, B.; Morgagni, F.; Cirillo, P. Effects of Hypobaric Hypoxia on Endothelial Function and Adiponectin Levels in Airforce Aviators. High Alt. Med. Biol. 2019, 20, 165–170. [Google Scholar] [CrossRef]
- De Bels, D.; Pierrakos, C.; Bruneteau, A.; Reul, F.; Crevecoeur, Q.; Marrone, N.; Vissenaeken, D.; Borgers, G.; Balestra, C.; Honore, P.M.; et al. Variation of Cognitive Function During a Short Stay at Hypobaric Hypoxia Chamber (Altitude: 3842 M). Front. Physiol. 2019, 10, 806. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- O’Rourke, M.F. Vascular impedance in studies of arterial and cardiac function. Physiol. Rev. 1982, 62, 570–623. [Google Scholar] [CrossRef]
- Booth, J. A short history of blood pressure measurement. Proc. R Soc. Med. 1977, 70, 793–799. [Google Scholar] [CrossRef]
- Pyke, K.E.; Tschakovsky, M.E. The relationship between shear stress and flow-mediated dilatation: Implications for the assessment of endothelial function. J. Physiol. 2005, 568, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Areas, G.P.T.; Mazzuco, A.; Caruso, F.R.; Jaenisch, R.B.; Cabiddu, R.; Phillips, S.A.; Arena, R.; Borghi-Silva, A. Flow-mediated dilation and heart failure: A review with implications to physical rehabilitation. Heart Fail. Rev. 2019, 24, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.; Van Vlierberghe, E.; Knez, J.; Szczesny, G.; Thijs, L.; Jozeau, D.; Balestra, C.; D’Hooge, J.; Staessen, J.A. Association of digital vascular function with cardiovascular risk factors: A population study. BMJ Open 2014, 4, e004399. [Google Scholar] [CrossRef]
- Theunissen, S.; Schumacker, J.; Guerrero, F.; Tillmans, F.; Boutros, A.; Lambrechts, K.; Mazur, A.; Pieri, M.; Germonpre, P.; Balestra, C. Dark chocolate reduces endothelial dysfunction after successive breath-hold dives in cool water. Eur. J. Appl. Physiol. 2013, 113, 2967–2975. [Google Scholar] [CrossRef]
- Levenez, M.; Lambrechts, K.; Mrakic-Sposta, S.; Vezzoli, A.; Germonpré, P.; Pique, H.; Virgili, F.; Bosco, G.; Lafère, P.; Balestra, C. Full-Face Mask Use during SCUBA Diving Counters Related Oxidative Stress and Endothelial Dysfunction. Int. J. Environ. Res. Public Health 2022, 19, 965. [Google Scholar] [CrossRef]
- Balestra, C.; Lambrechts, K.; Mrakic-Sposta, S.; Vezzoli, A.; Levenez, M.; Germonpre, P.; Virgili, F.; Bosco, G.; Lafere, P. Hypoxic and Hyperoxic Breathing as a Complement to Low-Intensity Physical Exercise Programs: A Proof-of-Principle Study. Int. J. Mol. Sci. 2021, 22, 9600. [Google Scholar] [CrossRef]
- Siebenmann, C.; Lundby, C. Regulation of cardiac output in hypoxia. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S4), 53–59. [Google Scholar] [CrossRef]
- Allardet-Servent, J.; Sicard, G.; Metz, V.; Chiche, L. Benefits and risks of oxygen therapy during acute medical illness: Just a matter of dose! Rev. Med. Intern. 2019, 40, 670–676. [Google Scholar] [CrossRef]
- Beasley, R.; Chien, J.; Douglas, J.; Eastlake, L.; Farah, C.; King, G.; Moore, R.; Pilcher, J.; Richards, M.; Smith, S.; et al. Target oxygen saturation range: 92–96% Versus 94–98. Respirology 2017, 22, 200–202. [Google Scholar] [CrossRef]
- Schjørring, O.L.; Klitgaard, T.L.; Perner, A.; Wetterslev, J.; Lange, T.; Siegemund, M.; Bäcklund, M.; Keus, F.; Laake, J.H.; Morgan, M.; et al. Lower or Higher Oxygenation Targets for Acute Hypoxemic Respiratory Failure. N. Engl. J. Med. 2021, 384, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Durkin, C.; Romano, K.; Egan, S.; Lohser, J. Hypoxemia During One-Lung Ventilation: Does It Really Matter? Curr. Anesthesiol. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, T.L.; Schjørring, O.L.; Lange, T.; Møller, M.H.; Perner, A.; Rasmussen, B.S.; Granholm, A. Lower versus higher oxygenation targets in critically ill patients with severe hypoxaemia: Secondary Bayesian analysis to explore heterogeneous treatment effects in the Handling Oxygenation Targets in the Intensive Care Unit (HOT-ICU) trial. Br. J. Anaesth. 2022, 128, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.S.; Grocott, M.P. Oxygen therapy in critical illness: Precise control of arterial oxygenation and permissive hypoxemia. Crit. Care Med. 2013, 41, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Kawai, E.T.; Mitchell, K.; Martin, D.; Carlisle, J.; Grocott, M.P. Permissive hypoxaemia versus normoxaemia for mechanically ventilated critically ill patients. Cochrane Database Syst. Rev. 2014, 2014, Cd009931. [Google Scholar] [CrossRef]
- Guo, L.; Jin, Z.; Gan, T.J.; Wang, E. Silent Hypoxemia in Patients with COVID-19 Pneumonia: A Review. Med. Sci. Monit. 2021, 27, e930776. [Google Scholar] [CrossRef]
- Talbot, N.P.; Balanos, G.M.; Dorrington, K.L.; Robbins, P.A. Two temporal components within the human pulmonary vascular response to approximately 2 h of isocapnic hypoxia. J. Appl. Physiol 2005, 98, 1125–1139. [Google Scholar] [CrossRef]
- Puri, S.; Panza, G.; Mateika, J.H. A comprehensive review of respiratory, autonomic and cardiovascular responses to intermittent hypoxia in humans. Exp. Neurol. 2021, 341, 113709. [Google Scholar] [CrossRef]
- Takemoto, M.; Sun, J.; Hiroki, J.; Shimokawa, H.; Liao, J.K. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation 2002, 106, 57–62. [Google Scholar] [CrossRef]
- Pisarcik, S.; Maylor, J.; Lu, W.; Yun, X.; Undem, C.; Sylvester, J.T.; Semenza, G.L.; Shimoda, L.A. Activation of hypoxia-inducible factor-1 in pulmonary arterial smooth muscle cells by endothelin-1. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L549–L561. [Google Scholar] [CrossRef]
- Theunissen, S.; Balestra, C.; Boutros, A.; De Bels, D.; Guerrero, F.; Germonpre, P. The effect of pre-dive ingestion of dark chocolate on endothelial function after a scuba dive. Diving Hyperb. Med. 2015, 45, 4–9. [Google Scholar] [PubMed]
- Ali, S.S.; Hsiao, M.; Zhao, H.W.; Dugan, L.L.; Haddad, G.G.; Zhou, D. Hypoxia-adaptation involves mitochondrial metabolic depression and decreased ROS leakage. PLoS ONE 2012, 7, e36801. [Google Scholar] [CrossRef] [PubMed]
- Attaye, I.; Smulders, Y.M.; de Waard, M.C.; Oudemans-van Straaten, H.M.; Smit, B.; Van Wijhe, M.H.; Musters, R.J.; Koolwijk, P.; Spoelstra-de Man, A.M.E. The effects of hyperoxia on microvascular endothelial cell proliferation and production of vaso-active substances. Intensive Care Med. Exp. 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhilyaev, S.Y.; Moskvin, A.N.; Platonova, T.F.; Gutsaeva, D.R.; Churilina, I.V.; Demchenko, I.T. Hyperoxic vasoconstriction in the brain is mediated by inactivation of nitric oxide by superoxide anions. Neurosci. Behav. Physiol. 2003, 33, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.L.; Fletcher, N.M.; Diamond, M.P.; Abu-Soud, H.M.; Saed, G.M. Hypoxia regulates iNOS expression in human normal peritoneal and adhesion fibroblasts through nuclear factor kappa B activation mechanism. Fertil Steril 2009, 91, 616–621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moro, M.A.; De Alba, J.; Leza, J.C.; Lorenzo, P.; Fernández, A.P.; Bentura, M.L.; Boscá, L.; Rodrigo, J.; Lizasoain, I. Neuronal expression of inducible nitric oxide synthase after oxygen and glucose deprivation in rat forebrain slices. Eur. J. Neurosci. 1998, 10, 445–456. [Google Scholar] [CrossRef]
- Fratantonio, D.; Virgili, F.; Zucchi, A.; Lambrechts, K.; Latronico, T.; Lafere, P.; Germonpre, P.; Balestra, C. Increasing Oxygen Partial Pressures Induce a Distinct Transcriptional Response in Human PBMC: A Pilot Study on the “Normobaric Oxygen Paradox”. Int. J. Mol. Sci. 2021, 22, 458. [Google Scholar] [CrossRef]
- Fox, W.C.; Watson, R.; Lockette, W. Acute hypoxemia increases cardiovascular baroreceptor sensitivity in humans. Am. J. Hypertens 2006, 19, 958–963. [Google Scholar] [CrossRef][Green Version]
- Guerrero, F.; Lambrechts, K.; Wang, Q.; Mazur, A.; Theron, M.; Marroni, A. Endothelial function may be enhanced in the cutaneous microcirculation after a single air dive. Diving Hyperb. Med. 2020, 50, 214–219. [Google Scholar] [CrossRef]
- Connes, P.; Alexy, T.; Detterich, J.; Romana, M.; Hardy-Dessources, M.D.; Ballas, S.K. The role of blood rheology in sickle cell disease. Blood Rev. 2016, 30, 111–118. [Google Scholar] [CrossRef]
- Lambrechts, K.; Balestra, C.; Theron, M.; Henckes, A.; Galinat, H.; Mignant, F.; Belhomme, M.; Pontier, J.M.; Guerrero, F. Venous gas emboli are involved in post-dive macro, but not microvascular dysfunction. Eur. J. Appl. Physiol. 2017, 117, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Duncker, D.J.; Bache, R.J. Inhibition of nitric oxide production aggravates myocardial hypoperfusion during exercise in the presence of a coronary artery stenosis. Circ. Res. 1994, 74, 629–640. [Google Scholar] [CrossRef] [PubMed]

| Pre-Ascent | 3842 m | 1 h Post-Descent | n | |
|---|---|---|---|---|
| SpO2 (%) | 97.7 ± 0.9 | 83.1 ± 4.2 *** | 97.8 ± 0.9 ns | 17 |
| Cardiac parameters | ||||
| Heart rate (bpm) | 66 ± 15 | 81 ± 15 *** | 65 ± 12 ns | 17 |
| Oxygen delivery index | 301.2 ±104.4 | 329.6 ± 81.6 * | 266.3 ± 62.1 ns | 15 |
| Vascular parameters | ||||
| Pre occlusion diameter (cm) | 0.44 ± 0.04 | 0.46 ± 0.05 *** | 0.45 ± 0.04 ns | 17 |
| Systolic blood pressure (mmHg) | 127.2 ± 8.3 | 118.7 ± 12.8 ** | 122.8 ± 18.0 * | 17 |
| Diastolic blood pressure (mmHg) | 77.1 ± 7.6 | 71.4 ± 5.2 * | 74.1 ± 9.5 ns | 17 |
| Mean blood pressure (mmHg) | 93.9 ± 6.8 | 87.2 ± 5.4 ** | 90.2 ± 11.6 * | 17 |
| Systemic vascular resistance (dynes.sec.cm−5) | 2428 ± 1049 | 1786 ± 418.4 ** | 2726 ± 560 ns | 12 |
| PORH (%) | 133.4 ± 67.4 | 145.8 ± 77.2 ns | 116.1 ± 53.1 ns | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theunissen, S.; Balestra, C.; Bolognési, S.; Borgers, G.; Vissenaeken, D.; Obeid, G.; Germonpré, P.; Honoré, P.M.; De Bels, D. Effects of Acute Hypobaric Hypoxia Exposure on Cardiovascular Function in Unacclimatized Healthy Subjects: A “Rapid Ascent” Hypobaric Chamber Study. Int. J. Environ. Res. Public Health 2022, 19, 5394. https://doi.org/10.3390/ijerph19095394
Theunissen S, Balestra C, Bolognési S, Borgers G, Vissenaeken D, Obeid G, Germonpré P, Honoré PM, De Bels D. Effects of Acute Hypobaric Hypoxia Exposure on Cardiovascular Function in Unacclimatized Healthy Subjects: A “Rapid Ascent” Hypobaric Chamber Study. International Journal of Environmental Research and Public Health. 2022; 19(9):5394. https://doi.org/10.3390/ijerph19095394
Chicago/Turabian StyleTheunissen, Sigrid, Costantino Balestra, Sébastien Bolognési, Guy Borgers, Dirk Vissenaeken, Georges Obeid, Peter Germonpré, Patrick M. Honoré, and David De Bels. 2022. "Effects of Acute Hypobaric Hypoxia Exposure on Cardiovascular Function in Unacclimatized Healthy Subjects: A “Rapid Ascent” Hypobaric Chamber Study" International Journal of Environmental Research and Public Health 19, no. 9: 5394. https://doi.org/10.3390/ijerph19095394
APA StyleTheunissen, S., Balestra, C., Bolognési, S., Borgers, G., Vissenaeken, D., Obeid, G., Germonpré, P., Honoré, P. M., & De Bels, D. (2022). Effects of Acute Hypobaric Hypoxia Exposure on Cardiovascular Function in Unacclimatized Healthy Subjects: A “Rapid Ascent” Hypobaric Chamber Study. International Journal of Environmental Research and Public Health, 19(9), 5394. https://doi.org/10.3390/ijerph19095394







