Modeling and Predicting Pulmonary Tuberculosis Incidence and Its Association with Air Pollution and Meteorological Factors Using an ARIMAX Model: An Ecological Study in Ningbo of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Data Collection
2.2. Construction of the ARIMA Model
2.3. Cross-Correlation Analysis
2.4. Construction of the ARIMAX Model
2.5. Statistical Software
3. Results
3.1. Descriptive Analysis
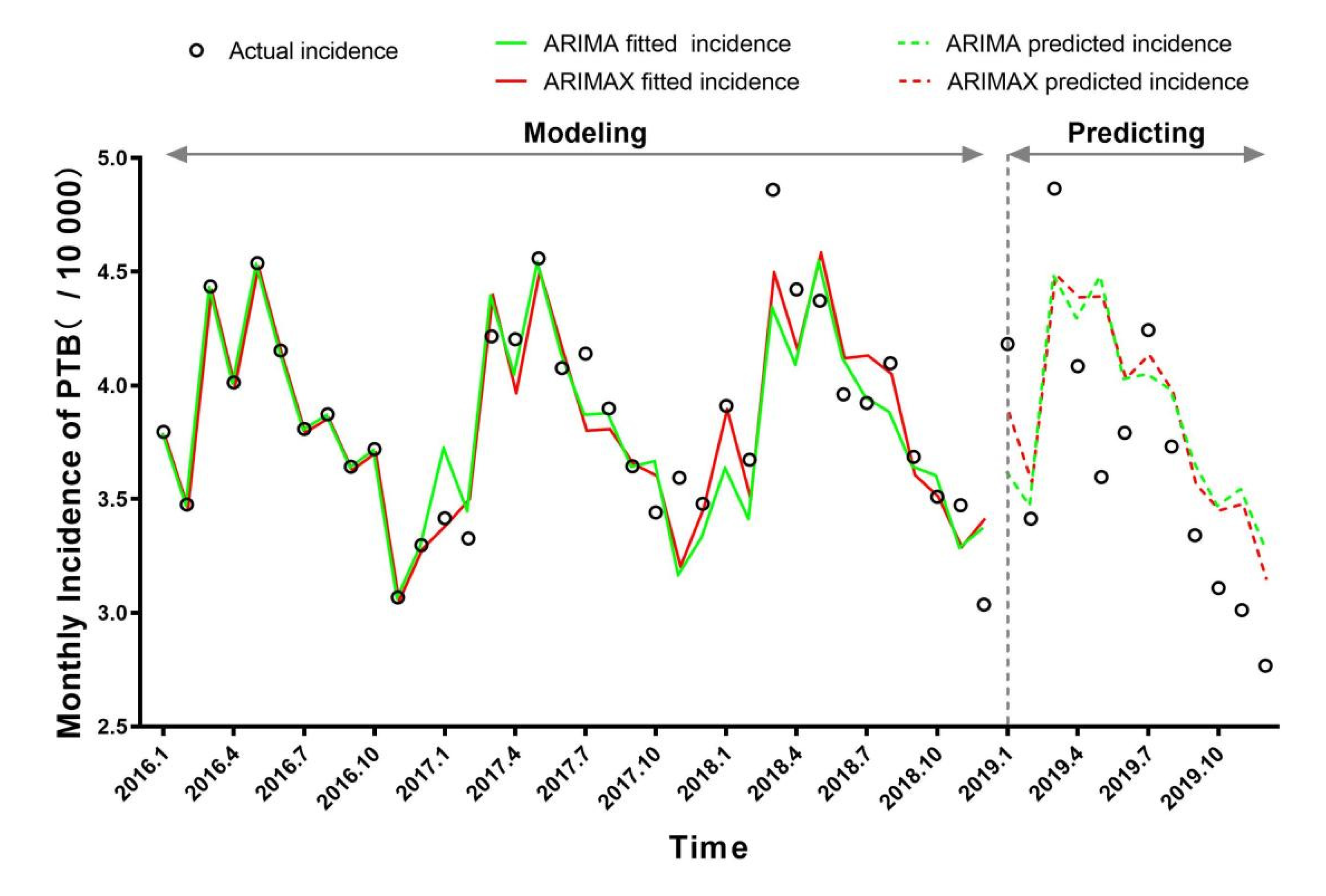
3.2. ARIMA Model
3.3. Cross-Correlation Analysis
3.3.1. CCF between the PTB and Ambient Factors
3.3.2. CCF between the Air Pollutants and Meteorological Factors
3.4. Univariate and Multivariate ARIMAX Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. The End TB Strategy. 2014. Available online: https://www.who.int/teams/global-tubercul-osis-programme/the-end-tb-strategy (accessed on 18 January 2022).
- WHO. Global Tuberculosis Report 2021. 2021. Available online: https://www.who.int/publications/digital/global-tuberculosis-report-2021 (accessed on 18 January 2022).
- Jing, Q.L.; Cheng, Q.; Marshall, J.M.; Hu, W.B.; Yang, Z.C.; Lu, J.H. Imported cases and minimum temperature drive dengue transmission in Guangzhou, China: Evidence from ARIMAX model. Epidemiol. Infect. 2018, 146, 1226–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- N’Gattia, A.K.; Coulibaly, D.; Nzussouo, N.T.; Kadjo, H.A.; Chérif, D.; Traoré, Y.; Kouakou, B.K.; Kouassi, P.D.; Ekra, K.D.; Dagnan, N.S.; et al. Effects of climatological parameters in modeling and forecasting seasonal influenza transmission in Abidjan, Cote d’Ivoire. BMC Public Health 2016, 16, 972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadsuthi, S.; Modchang, C.; Lenbury, Y.; Iamsirithaworn, S.; Triampo, W. Modeling seasonal leptospirosis transmission and its association with rainfall and temperature in Thailand using time-series and ARIMAX analyses. Asian Pac. J. Trop. Med. 2012, 5, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Chadsuthi, S.; Iamsirithaworn, S.; Triampo, W.; Modchang, C. Modeling Seasonal Influenza Transmission and Its Association with Climate Factors in Thailand Using Time-Series and ARIMAX Analyses. Comput. Math. Methods Med. 2015, 2015, 436495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharbi, M.; Quenel, P.; Gustave, J.; Cassadou, S.; La Ruche, G.; Girdary, L.; Marrama, L. Time series analysis of dengue incidence in Guadeloupe, French West Indies: Forecasting models using climate variables as predictors. BMC Infect. Dis. 2011, 11, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.Q.; Li, Y.X.; Wang, X.M.; Li, H.L.; Cai, Y.L.; Wang, B.X.; Wang, K.; Wang, W.M. The impact of air pollution on the transmission of pulmonary tuberculosis. Math. Biosci. Eng. 2020, 17, 4317–4327. [Google Scholar] [CrossRef]
- Li, Z.Q.; Pan, H.Q.; Liu, Q.; Song, H.; Wang, J.M. Comparing the performance of time series models with or without meteorological factors in predicting incident pulmonary tuberculosis in eastern China. Infect. Dis. Poverty 2020, 9, 151. [Google Scholar] [CrossRef]
- Ibironke, O.; Carranza, C.; Sarkar, S.; Torres, M.; Choi, H.T.; Nwoko, J.; Black, K.; Quintana-Belmares, R.; Osornio-Vargas, Á.; Ohman-Strickland, P.; et al. Urban Air Pollution Particulates Suppress Human T-Cell Responses to Mycobacterium Tuberculosis. Int. J. Environ. Res. Public Health 2019, 16, 4112. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cui, L.; Hou, L.; Yu, C.; Tao, N.; Liu, J.; Li, Y.; Zhou, C.; Yang, G.; Li, H. Ambient Air Pollution Exposures and Newly Diagnosed Pulmonary Tuberculosis in Jinan, China: A Time Series Study. Sci. Rep. 2018, 8, 17411. [Google Scholar] [CrossRef]
- Fares, A. Seasonality of tuberculosis. J. Glob. Infect. Dis. 2011, 3, 46–55. [Google Scholar] [CrossRef]
- Liu, L.; Wei, J.; Li, Y.; Ooi, A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air 2017, 27, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Zhang, A. Climate and air pollution alter incidence of tuberculosis in Beijing, China. Ann. Epidemiol. 2019, 37, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, S.I. Model selection and psychological theory: A discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol. Methods 2012, 17, 228–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Box, G.; Jenkins, G. Time Series Analysis: Forecasting and Control, 4th ed.; China Machine Press: Peking, China, 2011; pp. 291–292. [Google Scholar]
- Behr, M.A.; Edelstein, P.H.; Ramakrishnan, L. Revisiting the timetable of tuberculosis. BMJ 2018, 362, k2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. Available online: https://www.who.int/publications/i/item/9789240034228 (accessed on 18 January 2022).
- Wang, N.; Ma, Y.; Liu, Y.H.; Du, J.; Zhang, H.; Xie, S.H.; Zhu, K.; Lyu, X.Y.; Shu, W.; Wang, H.H.; et al. Risk of Treatment Failure in Patients with Drug-susceptible Pulmonary Tuberculosis in China. Biomed. Environ. Sci. 2016, 29, 612–617. [Google Scholar] [CrossRef]
- Shao, Y.; Yang, D.; Xu, W.; Lu, W.; Song, H.; Dai, Y.; Shen, H.; Wang, J. Epidemiology of anti-tuberculosis drug resistance in a Chinese population: Current situation and challenges ahead. BMC Public Health 2011, 11, 110. [Google Scholar] [CrossRef] [Green Version]
- Culqui-Lévano, D.R.; Rodriguez-Valín, E.; Donado-Campos, J.M. Analysis of extrapulmonary tuberculosis in Spain: 2007–2012 National Study. Enferm. Infecc. Microbiol. Clin. 2017, 35, 82–87. [Google Scholar] [CrossRef]
- Ködmön, C.; Zucs, P.; van der Werf, M.J. Migration-related tuberculosis: Epidemiology and characteristics of tuberculosis cases originating outside the European Union and European Economic Area, 2007 to 2013. Euro Surveill. 2016, 21, 30164. [Google Scholar] [CrossRef] [Green Version]
- Xiang, K.; Xu, Z.; Hu, Y.Q.; He, Y.S.; Dan, Y.L.; Wu, Q.; Fang, X.H.; Pan, H.F. Association between ambient air pollution and tuberculosis risk: A systematic review and meta-analysis. Chemosphere 2021, 277, 130342. [Google Scholar] [CrossRef]
- Huang, K.; Ding, K.; Yang, X.J.; Hu, C.Y.; Jiang, W.; Hua, X.G.; Liu, J.; Cao, J.Y.; Zhang, T.; Kan, X.H.; et al. Association between short-term exposure to ambient air pollutants and the risk of tuberculosis outpatient visits: A time-series study in Hefei, China. Environ. Res. 2020, 184, 109343. [Google Scholar] [CrossRef]
- Kim, H.; Yu, S.; Choi, H. Effects of particulate air pollution on tuberculosis development in seven major cities of Korea from 2010 to 2016: Methodological considerations involving long-term exposure and time lag. Epidemiol. Health 2020, 42, e2020012. [Google Scholar] [CrossRef] [PubMed]
- Belianin, I.I.; Nikolaeva, G.M.; Martynova, L.P. Action of dissolved ozone on mycobacterium tuberculosis and alveolar macrophages in experimental tuberculosis. Probl. Tuberk. 1997, 1997, 56–59. [Google Scholar]
- Guo, Y.; Li, S.; Tian, Z.; Pan, X.; Zhang, J.; Williams, G. The burden of air pollution on years of life lost in Beijing, China, 2004–2008: Retrospective regression analysis of daily deaths. BMJ 2013, 347, f7139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Li, G.; Qian, X.; Xu, G.; Zhao, Y.; Huang, J.; Liu, Q.; He, T.; Guo, X. The burden of ischemic heart disease related to ambient air pollution exposure in a coastal city in South China. Environ. Res. 2018, 164, 255–261. [Google Scholar] [CrossRef]
- Bie, S.; Hu, X.; Zhang, H.; Wang, K.; Dou, Z. Influential factors and spatial-temporal distribution of tuberculosis in mainland China. Sci. Rep. 2021, 11, 6274. [Google Scholar] [CrossRef]
- Maharjan, B.; Gopali, R.S.; Zhang, Y. A scoping review on climate change and tuberculosis. Int. J. Biometeorol. 2021, 65, 1579–1595. [Google Scholar] [CrossRef]
- Popovic, I.; Soares Magalhaes, R.J.; Ge, E.; Marks, G.B.; Dong, G.H.; Wei, X.; Knibbs, L.D. A systematic literature review and critical appraisal of epidemiological studies on outdoor air pollution and tuberculosis outcomes. Environ. Res. 2019, 170, 33–45. [Google Scholar] [CrossRef]
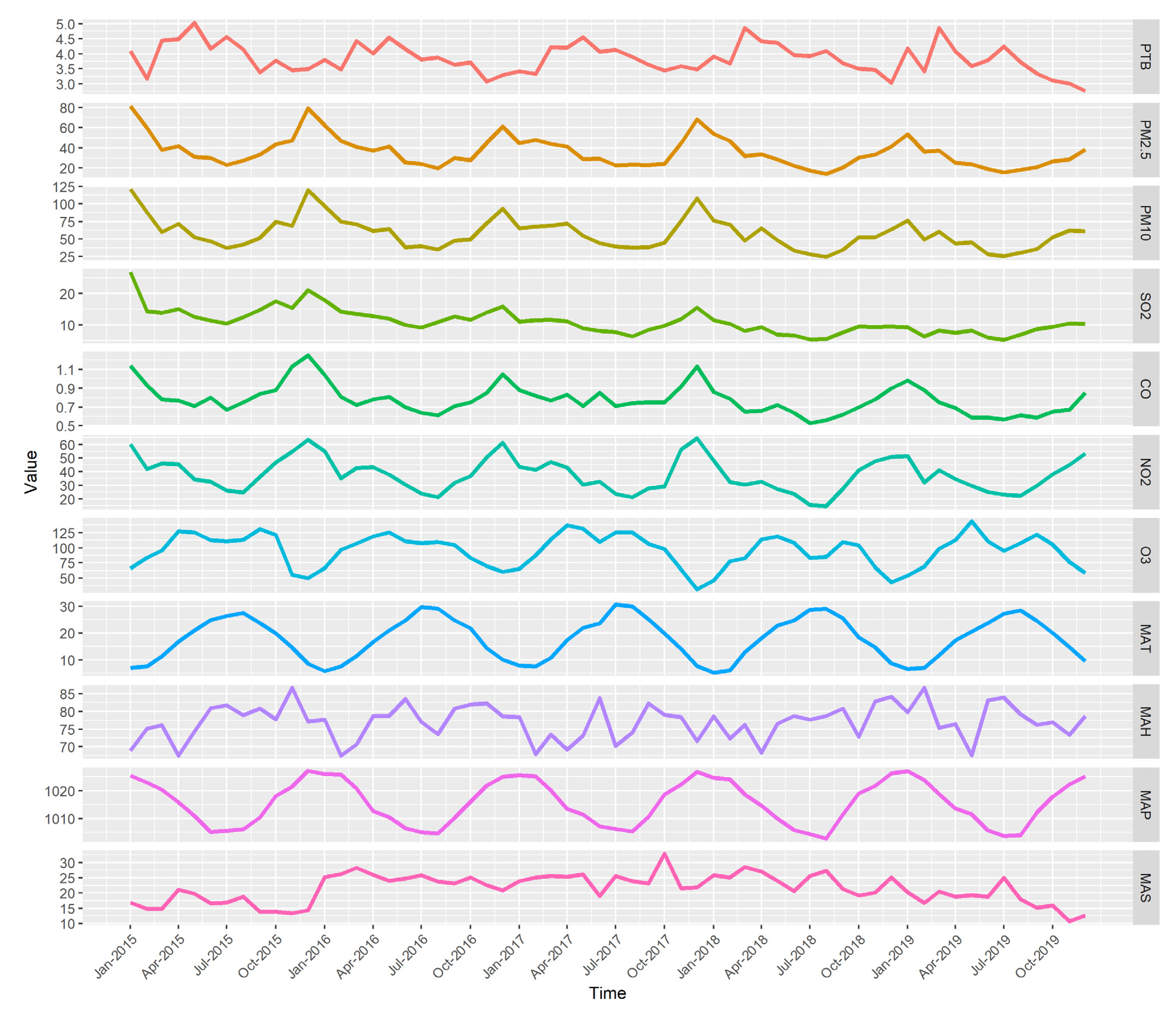
| Variables | Mean | S.D. | Min | P25 | P50 | P75 | MAX | IQR |
|---|---|---|---|---|---|---|---|---|
| Monthly incidence of PTB | 3.85 | 0.50 | 2.77 | 3.48 | 3.80 | 4.17 | 5.03 | 0.69 |
| Air pollutants concentration | ||||||||
| PM2.5 (μg/m3) | 35.79 | 14.73 | 14.35 | 24.30 | 32.64 | 43.95 | 81.42 | 19.65 |
| PM10 (μg/m3) | 57.42 | 21.51 | 24.48 | 41.62 | 52.55 | 69.57 | 121.16 | 27.95 |
| SO2 (μg/m3) | 10.90 | 3.97 | 5.130 | 8.182 | 10.36 | 12.665 | 26.900 | 4.483 |
| CO (mg/m3) | 0.78 | 0.15 | 0.53 | 0.67 | 0.75 | 0.85 | 1.25 | 0.18 |
| NO2 (μg/m3) | 37.47 | 12.25 | 14.68 | 28.76 | 35.36 | 46.19 | 64.52 | 17.43 |
| O3 (μg/m3) | 95.26 | 26.79 | 31.68 | 71.86 | 104.70 | 113.32 | 143.55 | 41.46 |
| Meteorological factors | ||||||||
| MAT (°C) | 17.79 | 7.85 | 5.32 | 10.62 | 18.21 | 24.66 | 30.57 | 14.04 |
| MAHT (°C) | 21.88 | 7.97 | 8.61 | 14.66 | 23.25 | 28.24 | 35.97 | 13.58 |
| MALT (°C) | 14.53 | 8.01 | 2.83 | 6.35 | 14.20 | 22.06 | 26.74 | 15.70 |
| MAH (%) | 76.70 | 5.23 | 63.40 | 73.49 | 77.74 | 80.78 | 86.70 | 7.29 |
| MAP (hPa) | 1015.58 | 7.77 | 1002.69 | 1009.205 | 1015.89 | 1022.41 | 1027.19 | 15.18 |
| MAS (m/s) | 21.36 | 4.72 | 10.67 | 18.64 | 21.75 | 25.18 | 32.81 | 6.54 |
| Variables | Model | BIC | Ljung–Box Test | |
|---|---|---|---|---|
| X-Squared | p-Value | |||
| Monthly incidence of PTB | ARIMA(0,0,0)(1,1,0)12 | 18.2228 | 2.1833 | 0.1395 |
| Air pollutants concentration | ||||
| PM2.5 (μg/m3) | ARIMA (1,0,0)(0,1,0)12 | 266.6336 | 0.7367 | 0.3907 |
| PM10 (μg/m3) | ARIMA (1,0,0)(0,1,0)12 | 298.6734 | 0.6880 | 0.4068 |
| SO2 (μg/m3) | ARIMA (1,0,0)(0,1,0)12 | 161.6301 | 0.5341 | 0.4649 |
| CO (mg/m3) | ARIMA (1,0,0)(1,1,0)12 | −74.6422 | 1.3417 | 0.2467 |
| NO2 (μg/m3) | ARIMA (1,0,0)(0,1,1)12 | 238.207 | 0.7107 | 0.3992 |
| O3 (μg/m3) | ARIMA (0,0,1)(1,1,0)12 | 297.4298 | 0.0011 | 0.9736 |
| Meteorological factors | ||||
| MAT (°C) | ARIMA (0,0,0)(0,1,1)12 | 126.2939 | 2.2553 | 0.1332 |
| MAHT (°C) | ARIMA (1,0,0)(0,1,1)12 | 148.7069 | 0.1210 | 0.7280 |
| MALT (°C) | ARIMA (0,0,0)(0,1,0)12 | 139.3857 | 2.5996 | 0.1069 |
| MAH (%) | ARIMA (0,0,0)(1,1,0)12 | 224.0759 | 0.0009 | 0.9757 |
| MAP (hPa) | ARIMA (0,0,0)(1,1,0)12 | 250.4339 | 0.2565 | 0.6125 |
| MAS (m/s) | ARIMA (0,1,1) | 262.4965 | 0.4851 | 0.4861 |
| Factors | Lag Periods (Months) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| PM2.5 | 0.040 | 0.145 | −0.125 | 0.353 * | 0.028 | 0.124 | −0.018 | 0.164 | −0.175 | 0.151 | 0.013 | −0.122 | 0.112 |
| PM10 | 0.068 | 0.166 | −0.114 | 0.343 * | 0.041 | 0.076 | −0.049 | 0.209 | −0.172 | 0.190 | −0.007 | −0.110 | 0.077 |
| SO2 | 0.202 | 0.069 | −0.127 | 0.176 | 0.125 | −0.161 | −0.034 | 0.151 | −0.211 | 0.031 | 0.103 | −0.270 | 0.145 |
| CO | −0.013 | 0.075 | −0.028 | −0.086 | −0.111 | 0.100 | −0.036 | 0.115 | −0.072 | 0.291 * | −0.083 | 0.165 | 0.000 |
| NO2 | 0.034 | −0.100 | −0.106 | 0.044 | 0.073 | 0.013 | −0.164 | 0.008 | −0.209 | 0.278 | −0.075 | −0.064 | 0.073 |
| O3 | −0.321 * | −0.135 | −0.279 | −0.085 | −0.014 | 0.026 | −0.064 | 0.072 | 0.111 | 0.081 | 0.113 | −0.151 | 0.216 |
| MAT | −0.206 | −0.406 * | −0.102 | −0.316 * | −0.002 | −0.081 | 0.034 | 0.093 | 0.022 | −0.126 | −0.095 | 0.146 | 0.124 |
| MAHT | 0.093 | −0.215 | −0.040 | −0.171 | 0.155 | −0.014 | 0.148 | 0.241 | 0.162 | 0.080 | −0.029 | 0.177 | 0.174 |
| MALT | −0.198 | −0.310 * | −0.040 | −0.423 * | −0.094 | −0.085 | −0.077 | −0.158 | −0.018 | −0.133 | 0.014 | 0.149 | 0.122 |
| MAH | −0.136 | −0.016 | −0.019 | −0.240 | −0.238 | 0.073 | −0.053 | −0.307 * | −0.074 | −0.273 | −0.036 | −0.085 | −0.157 |
| MAP | 0.029 | −0.136 | −0.299 * | −0.030 | 0.105 | −0.252 | −0.151 | 0.066 | 0.053 | −0.248 | 0.027 | −0.335 * | −0.066 |
| MAS | −0.091 | 0.125 | −0.112 | −0.205 | −0.169 | −0.098 | 0.068 | 0.045 | 0.091 | −0.199 | 0.038 | −0.053 | −0.240 |
| Air Pollutants | Meteorological Factors | |||||
|---|---|---|---|---|---|---|
| MAT | MAHT | MALT | MAH | MAP | MAS | |
| PM2.5 | −0.317 * | −0.154 | −0.527 * | −0.477 * | 0.022 | −0.289 * |
| PM10 | −0.319 * | −0.136 | −0.586 * | −0.605 * | 0.066 | −0.248 |
| SO2 | −0.249 | −0.059 | −0.457 * | −0.529 * | 0.428 * | −0.098 |
| CO | −0.249 | −0.097 | −0.115 | −0.059 | −0.199 | −0.150 |
| NO2 | −0.272 | −0.201 | −0.308 * | −0.400 * | −0.016 | −0.437 * |
| O3 | 0.048 | 0.122 | −0.161 | −0.403 * | 0.318 * | 0.027 |
| Model | BIC | MAPE(%) | Risk Factors | |||||
|---|---|---|---|---|---|---|---|---|
| Fitted | Test | Vars | Coef | S.E. | T | p-Value | ||
| (1) ARIMA(0,0,0)(1,1,0)12 | 9.0376 | 3.3269 | 10.6693 | sar1 | −0.5829 | 0.1907 | 3.0566 | 0.0021 * |
| (2) ARIMA(0,0,0)(1,1,0)12+PM2.5(lag3) d | 12.1682 | 3.3748 | 10.5262 d | sar1 | −0.5458 | 0.2720 | 2.0064 | 0.0264 * |
| PM2.5(lag3) | 0.0019 | 0.0087 | 0.2147 | 0.4156 | ||||
| (3) ARIMA(0,0,0)(1,1,0)12+PM10(lag3) d | 12.0662 | 3.3833 | 10.4819 d | sar1 | −0.5355 | 0.2460 | 2.1767 | 0.0183 * |
| PM10(lag3) | 0.0018 | 0.0048 | 0.3809 | 0.3528 | ||||
| (4) ARIMA(0,0,0)(1,1,0)12+CO(lag9) d | 11.1900 | 3.2306 c | 9.5569 d | sar1 | −0.5843 | 0.1903 | 3.0707 | 0.0021 * |
| PM10(lag3) | 0.0042 | 0.0041 | 1.0221 | 0.1570 | ||||
| (5) ARIMA(0,0,0)(1,1,0)12+O3(lag0) abcd | 8.2634 a | 3.0226 c | 9.7944 d | sar1 | −0.6418 | 0.1717 | 3.7374 | 0.0003 * |
| O3(lag0) | −0.0061 | 0.0029 | 2.0751 | 0.0228 * b | ||||
| (6) ARIMA(0,0,0)(1,1,0)12+MAT(lag1) abc | −1.4075 a | 2.3205c | 12.0531 | sar1 | −0.5673 | 0.1978 | 2.8679 | 0.0035 * |
| MAT(lag1) | −0.1317 | 0.0310 | 4.2421 | <0.0001 * b | ||||
| (7) ARIMA(0,0,0)(1,1,0)12+MAT(lag3) | 10.5244 | 3.3860 | 11.0464 | sar1 | −0.5146 | 0.2169 | 2.3730 | 0.0117 * |
| MAT(lag3) | −0.0595 | 0.0443 | 1.3410 | 0.0944 | ||||
| (8) ARIMA(0,0,0)(1,1,0)12+MALT(lag1) abc | 7.8580 a | 2.8932 c | 12.4087 | sar1 | −0.4583 | 0.2670 | 1.7165 | 0.0476 * |
| MALT(lag1) | −0.0697 | 0.0357 | 1.9497 | 0.0297 * b | ||||
| (9) ARIMA(0,0,0)(1,1,0)12+MALT(lag3) abc | 8.0334 a | 3.2171 c | 11.4344 | sar1 | −0.5035 | 0.2217 | 2.2709 | 0.0148 * |
| MALT(lag3) | −0.0736 | 0.0347 | 2.1198 | 0.0207 * b | ||||
| (10) ARIMA(0,0,0)(1,1,0)12+MAH(lag7) cd | 10.2365 | 3.1969 c | 10.4618 d | sar1 | −0.6337 | 0.1732 | 3.6587 | 0.0004 * |
| MAH(lag7) | −0.0157 | 0.0107 | 1.4625 | 0.0764 | ||||
| (11) ARIMA(0,0,0)(1,1,0)12+MAP(lag2) c | 9.9590 | 3.1324 c | 10.9889 | sar1 | −0.6733 | 0.1607 | 4.1897 | <0.0001 |
| MAP(lag2) | −0.0095 | 0.0059 | 1.6023 | 0.0592 | ||||
| (12) ARIMA(0,0,0)(1,1,0)12+MAP(lag11) abcd | 8.4574 a | 3.2063 c | 10.0108 d | sar1 | −0.4940 | 0.2186 | 2.2601 | 0.0152 * |
| MAP(lag11) | −0.0125 | 0.0060 | 2.0680 | 0.0232 * b | ||||
| (13) ARIMA(0,0,0)(1,1,0)12+ O3(lag0)+MAP(lag11) abcd | 8.1092 a | 2.9097 c | 9.2643 d | sar1 | −0.5608 | 0.2018 | 2.7791 | 0.0045 * |
| O3(lag0) | −0.0054 | 0.0028 | 1.9383 | 0.0306 * b | ||||
| MAP(lag11) | −0.0115 | 0.0059 | 1.9461 | 0.0301 * b | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-P.; Liu, L.-F.; Che, Y.; Huang, J.; Li, G.-X.; Sang, G.-X.; Xuan, Z.-Q.; He, T.-F. Modeling and Predicting Pulmonary Tuberculosis Incidence and Its Association with Air Pollution and Meteorological Factors Using an ARIMAX Model: An Ecological Study in Ningbo of China. Int. J. Environ. Res. Public Health 2022, 19, 5385. https://doi.org/10.3390/ijerph19095385
Chen Y-P, Liu L-F, Che Y, Huang J, Li G-X, Sang G-X, Xuan Z-Q, He T-F. Modeling and Predicting Pulmonary Tuberculosis Incidence and Its Association with Air Pollution and Meteorological Factors Using an ARIMAX Model: An Ecological Study in Ningbo of China. International Journal of Environmental Research and Public Health. 2022; 19(9):5385. https://doi.org/10.3390/ijerph19095385
Chicago/Turabian StyleChen, Yun-Peng, Le-Fan Liu, Yang Che, Jing Huang, Guo-Xing Li, Guo-Xin Sang, Zhi-Qiang Xuan, and Tian-Feng He. 2022. "Modeling and Predicting Pulmonary Tuberculosis Incidence and Its Association with Air Pollution and Meteorological Factors Using an ARIMAX Model: An Ecological Study in Ningbo of China" International Journal of Environmental Research and Public Health 19, no. 9: 5385. https://doi.org/10.3390/ijerph19095385
APA StyleChen, Y.-P., Liu, L.-F., Che, Y., Huang, J., Li, G.-X., Sang, G.-X., Xuan, Z.-Q., & He, T.-F. (2022). Modeling and Predicting Pulmonary Tuberculosis Incidence and Its Association with Air Pollution and Meteorological Factors Using an ARIMAX Model: An Ecological Study in Ningbo of China. International Journal of Environmental Research and Public Health, 19(9), 5385. https://doi.org/10.3390/ijerph19095385









