SARS-CoV-2 Circulation in the School Setting: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Analysis
3. Results
3.1. SARS-CoV-2 Positivity Rates in Educational Settings (Screening Studies)
3.2. SARS-CoV-2 Seroprevalence in Educational Settings (Serosurveys)
3.3. Onward Transmission of SARS-CoV-2 in Educational Settings (Contact Tracing)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viner, R.M.; Mytton, O.T.; Bonell, C.; Melendez-Torres, G.J.; Ward, J.; Hudson, L.; Waddington, C.; Thomas, J.; Russell, S.; van der Klis, F.; et al. Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared with Adults: A Systematic Review and Meta-analysis. JAMA Pediatrics 2021, 175, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Tsankov, B.K.; Allaire, J.M.; Irvine, M.A.; Lopez, A.A.; Sauvé, L.J.; Vallance, B.A.; Jacobson, K. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 103, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, X.; Dozier, M.; He, Y.; Kirolos, A.; Lang, Z.; Mathews, C.; Siegfried, N.; Theodoratou, E. What is the evidence for transmission of COVID-19 by children in schools? A living systematic review. J. Glob. Health 2020, 10, 021104. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Dozier, M.; He, Y.; Kirolos, A.; Lang, Z.; Song, P.; Theodoratou, E. The role of children in the transmission of SARS-CoV2: Updated rapid review. J. Glob. Health 2020, 10, 021101. [Google Scholar] [CrossRef]
- Gettings, J.; Czarnik, M.; Morris, E.; Haller, E.; Thompson-Paul, A.M.; Rasberry, C.; Lanzieri, T.M.; Smith-Grant, J.; Aholou, T.M.; Thomas, E.; et al. Mask Use and Ventilation Improvements to Reduce COVID-19 Incidence in Elementary Schools—Georgia, November 16–December 11, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 779–784. [Google Scholar] [CrossRef]
- van den Berg, P.; Schechter-Perkins, E.M.; Jack, R.S.; Epshtein, I.; Nelson, R.; Oster, E.; Branch-Elliman, W. Effectiveness of 3 Versus 6 ft of Physical Distancing for Controlling Spread of Coronavirus Disease 2019 among Primary and Secondary Students and Staff: A Retrospective, Statewide Cohort Study. Clin. Infect. Dis. 2021, 73, 1871–1878. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Open Med. 2009, 3, e123–e130. [Google Scholar]
- Greenland, S. Quantitative methods in the review of epidemiologic literature. Epidemiol. Rev. 1987, 9, 1–30. [Google Scholar] [CrossRef]
- Thompson, S.G.; Higgins, J.P. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002, 21, 1559–1573. [Google Scholar] [CrossRef]
- Macaskill, P.; Walter, S.D.; Irwig, L. A comparison of methods to detect publication bias in meta-analysis. Stat. Med. 2001, 20, 641–654. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmet, S.; Ekinci, E.; Wouters, I.; Decru, B.; Beuselinck, K.; Malhotra-Kumar, S.; Theeten, H. No SARS-CoV-2 carriage observed in children attending daycare centers during the intial weeks of the epidemic in Belgium. J. Med. Virol. 2021, 93, 1828–1831. [Google Scholar] [CrossRef] [PubMed]
- Szépfalusi, Z.; Schmidthaler, K.; Sieber, J.; Kopanja, S.; Götzinger, F.; Schoof, A.; Hoz, J.; Willinger, B.; Makristathis, A.; Weseslindtner, L.; et al. Lessons from low seroprevalence of SARS-CoV-2 antibodies in schoolchildren: A cross-sectional study. Pediatr. Allergy Immunol. 2021, 32, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Mossong, J.; Mombaerts, L.; Veiber, L.; Pastore, J.; Coroller, G.L.; Schnell, M.; Masi, S.; Huiart, L.; Wilmes, P. SARS-CoV-2 transmission in educational settings during an early summer epidemic wave in Luxembourg, 2020. BMC Infect. Dis. 2021, 21, 417. [Google Scholar] [CrossRef] [PubMed]
- Kriger, O.; Lustig, Y.; Cohen, C.; Amit, S.; Biber, A.; Barkai, G.; Talmi, L.; Gefen-Halevi, S.; Mechnik, B.; Regev-Yochay, G. The Sheba Medical Center healthcare workers’ children’s school: Can we open schools safely? Clin. Microbiol. Infect. 2021, 27, 474.e1–474.e3. [Google Scholar] [CrossRef]
- Lübke, N.; Schupp, A.K.; Bredahl, R.; Kraus, U.; Hauka, S.; Andrée, M.; Ehlkes, L.; Klein, T.; Graupner, A.; Horn, J.; et al. Screening for SARS-CoV-2 infections in daycare facilities for children in a large city in Germany. medRxiv 2021. medRxiv: 2021.02.26.21252510. [Google Scholar] [CrossRef]
- Hommes, F.; van Loon, W.; Thielecke, M.; Abramovich, I.; Lieber, S.; Hammerich, R.; Gehrke-Beck, S.; Linzbach, E.; Schuster, A.; von dem Busche, K.; et al. SARS-CoV-2 Infection, Risk Perception, Behaviour and Preventive Measures at Schools in Berlin, Germany, during the Early Post-Lockdown Phase: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 2739. [Google Scholar] [CrossRef]
- Gillespie, D.L.; Meyers, L.A.; Lachmann, M.; Redd, S.C.; Zenilman, J.M. The Experience of 2 Independent Schools with In-Person Learning During the COVID-19 Pandemic. J. Sch. Health 2021, 91, 347–355. [Google Scholar] [CrossRef]
- Volpp, K.G.; Kraut, B.H.; Ghosh, S.; Neatherlin, J. Minimal SARS-CoV-2 Transmission After Implementation of a Comprehensive Mitigation Strategy at a School—New Jersey, 20 August–27 November 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 377–381. [Google Scholar] [CrossRef]
- Cooper, D.M.; Zulu, M.Z.; Jankeel, A.; Ibraim, I.C.; Ardo, J.; Kasper, K.; Stephens, D.; Meyer, A.; Stehli, A.; Condon, C.; et al. SARS-CoV-2 acquisition and immune pathogenesis among school-aged learners in four diverse schools. Pediatr. Res. 2021, 90, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Hoch, M.; Vogel, S.; Eberle, U.; Kolberg, L.; Gruenthaler, V.; Fingerle, V.; Ackermann, N.; Sing, A.; Liebl, B.; Huebner, J.; et al. Feasibility and Diagnostic Accuracy of Saliva-Based SARS-CoV-2 Screening in Educational Settings and Children Aged <12 Years. Diagnostics 2021, 11, 1797. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Coltella, L.; Ranno, S.; Bianchi di Castelbianco, F.; Murru, P.M.; Sonnino, R.; Mazzone, T.; Piccioni, L.; Linardos, G.; Chiavelli, S.; et al. School in Italy: A safe place for children and adolescents. Ital. J. Pediatr. 2021, 47, 23. [Google Scholar] [CrossRef]
- Doron, S.; Ingalls, R.R.; Beauchamp, A.; Boehm, J.S.; Boucher, H.W.; Chow, L.H.; Corridan, L.; Goehringer, K.; Golenbock, D.; Larsen, L.; et al. Weekly SARS-CoV-2 screening of asymptomatic kindergarten to grade 12 students and staff helps inform strategies for safer in-person learning. Cell Rep. Med. 2021, 2, 100452. [Google Scholar] [CrossRef] [PubMed]
- Bordi, L.; Parisi, G.; Sberna, G.; Amendola, A.; Mariani, B.; Meoni, G.; Orazi, D.; Bartoletti, P.; Lombardozzi, L.; Barca, A.; et al. Effective screening strategy against SARS-CoV-2 on self-collected saliva samples in primary school setting: A pilot project. J. Infect. 2021, 83, e8–e10. [Google Scholar] [CrossRef]
- Azienda Sanitaria Locale Liguria (ASL3). Report Settimanale Sull’Attività di Screening Nelle Scuole—Aggiornamento al 29 Maggio 2021. Available online: https://www.asl3.liguria.it/components/com_publiccompetitions/includes/download.php?id=16954:dati-screening-scuole-covid-19.pdf (accessed on 30 May 2021).
- Judd, A. COVID-19 Schools Infection Survey, England: Round 4, Antibody Data, March 2021. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/covid19schoolsinfectionsurveyengland/round4antibodydatamarch2021 (accessed on 28 May 2021).
- Crowe, J.; Schnaubelt, A.T.; SchmidtBonne, S.; Angell, K.; Bai, J.; Eske, T.; Nicklin, M.; Pratt, C.; White, B.; Crotts-Hannibal, B.; et al. Assessment of a Program for SARS-CoV-2 Screening and Environmental Monitoring in an Urban Public School District. JAMA Netw. Open. 2021, 4, e2126447. [Google Scholar] [CrossRef]
- Kriemler, S.; Ulyte, A.; Ammann, P.; Peralta, G.P.; Berger, C.; Puhan, M.A.; Radtke, T. Surveillance of Acute SARS-CoV-2 Infections in School Children and Point-Prevalence During a Time of High Community Transmission in Switzerland. Front. Pediatr. 2021, 9, 645577. [Google Scholar] [CrossRef]
- Comune di Pesaro. Il Sindaco Scrive alla Regione, «Ora Serve Screening Periodico per Istituti Superiori e Medie. In Caso di Risposta Negativa, Pronti a Ripeterlo». Available online: http://www.comune.pesaro.pu.it/novita-in-comune/dettaglio/news/il-sindaco-scrive-alla-regione-ora-serve-screening-periodico-per-istituti-superiori-e-medie-in-ca/?tx_news_pi1%5Bcontroller%5D=News&tx_news_pi1%5Baction%5D=detail&cHash=4251e47288443964cf5db0ffc41e2303 (accessed on 9 February 2021).
- Comune dell’Aquila. Screening: Oggi 1800 Tamponi, 2 Dubbi. Available online: https://www.comune.laquila.it/index.php?id_oggetto=3&id_doc=7725&id_sez_ori=&template_ori=2 (accessed on 21 February 2021).
- Comune di Pesaro. Screening “Scuole sicure”. Della Dora e Ceccarelli: «Operazione Necessaria. Tamponi Unica Arma per Evitare altri Contagi». Available online: http://www.comune.pesaro.pu.it/novita-in-comune/dettaglio/news/screening-scuole-sicure-della-dora-e-ceccarelli-operazione-necessaria-tamponi-unica-arma-per-e/?tx_news_pi1%5Bcontroller%5D=News&tx_news_pi1%5Baction%5D=detail&cHash=7d1d874e167ff90dd12b31b58ba7702c (accessed on 9 March 2021).
- Comune dell’Aquila. Coronavirus, screening della popolazione scolastica a Sassa: 299 tamponi tutti negativi. Available online: https://www.comune.laquila.it/index.php?id_oggetto=3&id_cat=0&id_doc=7908&id_sez_ori=17&template_ori=3&>p=1 (accessed on 19 May 2021).
- Tönshoff, B.; Müller, B.; Elling, R.; Renk, H.; Meissner, P.; Hengel, H.; Garbade, S.F.; Kieser, M.; Jeltsch, K.; Grulich-Henn, J.; et al. Prevalence of SARS-CoV-2 Infection in Children and Their Parents in Southwest Germany. JAMA Pediatr. 2021, 175, 586–593. [Google Scholar] [CrossRef]
- Kirsten, C.; Unrath, M.; Lück, C.; Dalpke, A.H.; Berner, R.; Armann, J. SARS-CoV-2 seroprevalence in students and teachers: A longitudinal study from May to October 2020 in German secondary schools. BMJ Open 2021, 11, e049876. [Google Scholar] [CrossRef]
- Lachassinne, E.; de Pontual, L.; Caseris, M.; Lorrot, M.; Guilluy, C.; Naud, A.; Dommergues, M.A.; Pinquier, D.; Wannepain, E.; Hausherr, E.; et al. SARS-CoV-2 transmission among children and staff in daycare centres during a nationwide lockdown in France: A cross-sectional, multicentre, seroprevalence study. Lancet Child Adolesc. Health 2021, 5, 256–264. [Google Scholar] [CrossRef]
- Ulyte, A.; Radtke, T.; Abela, I.A.; Haile, S.R.; Berger, C.; Huber, M.; Schanz, M.; Schwarzmueller, M.; Trkola, A.; Fehr, J.; et al. Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school children in the canton of Zurich, Switzerland: Prospective cohort study of 55 schools. BMJ 2021, 372, n616. [Google Scholar] [CrossRef] [PubMed]
- Armann, J.P.; Kirsten, C.; Galow, L.; Kahre, E.; Haag, L.; Dalpke, A.; Lück, C.; Berner, R. SARS-CoV-2 transmissions in students and teachers: Seroprevalence follow-up study in a German secondary school in November and December 2020. BMJ Paediatr. Open 2021, 5, e001036. [Google Scholar] [CrossRef] [PubMed]
- Fontanet, A.; Tondeur, L.; Grant, R.; Temmam, S.; Madec, Y.; Bigot, T.; Grzelak, L.; Cailleau, I.; Besombes, C.; Ungeheuer, M.N.; et al. SARS-CoV-2 infection in schools in a northern French city: A retrospective serological cohort study in an area of high transmission, France, January to April 2020. Euro. Surveill. 2021, 26, 2001695. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.P.; Piñera, C.; De La Maza, V.; Lagomarcino, A.J.; Simian, D.; Torres, B.; Urquidi, C.; Valenzuela, M.T.; O’Ryan, M. Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Prevalence in Blood in a Large School Community Subject to a Coronavirus Disease 2019 Outbreak: A Cross-sectional Study. Clin. Infect. Dis. 2021, 73, e458–e465. [Google Scholar] [CrossRef] [PubMed]
- Macartney, K.; Quinn, H.E.; Pillsbury, A.J.; Koirala, A.; Deng, L.; Winkler, N.; Katelaris, A.L.; O’Sullivan, M.V.N.; Dalton, C.; Wood, N.; et al. Transmission of SARS-CoV-2 in Australian educational settings: A prospective cohort study. Lancet Child Adolesc. Health 2020, 4, 807–816. [Google Scholar] [CrossRef]
- Danis, K.; Epaulard, O.; Bénet, T.; Gaymard, A.; Campoy, S.; Botelho-Nevers, E.; Bouscambert-Duchamp, M.; Spaccaferri, G.; Ader, F.; Mailles, A.; et al. Cluster of Coronavirus Disease 2019 (COVID-19) in the French Alps, February 2020. Clin. Infect. Dis. 2020, 71, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.; Kim, K.R.; Park, H.; Kim, S.; Kim, Y.J. Stepwise School Opening and an Impact on the Epidemiology of COVID-19 in the Children. J. Korean Med. Sci. 2020, 35, e414. [Google Scholar] [CrossRef]
- Heavey, L.; Casey, G.; Kelly, C.; Kelly, D.; McDarby, G. No evidence of secondary transmission of COVID-19 from children attending school in Ireland, 2020. Eurosurveillance 2020, 25, 2000903. [Google Scholar] [CrossRef]
- Ehrhardt, J.; Ekinci, A.; Krehl, H.; Meincke, M.; Finci, I.; Klein, J.; Geisel, B.; Wagner-Wiening, C.; Eichner, M.; Brockmann, S.O. Transmission of SARS-CoV-2 in children aged 0 to 19 years in childcare facilities and schools after their reopening in May 2020, Baden-Württemberg, Germany. Euro. Surveill. 2020, 25, 2001587. [Google Scholar] [CrossRef]
- Stein-Zamir, C.; Abramson, N.; Shoob, H.; Libal, E.; Bitan, M.; Cardash, T.; Cayam, R.; Miskin, I. A large COVID-19 outbreak in a high school 10 days after schools’ reopening, Israel, May 2020. Euro. Surveill. 2020, 25, 2001352. [Google Scholar] [CrossRef]
- Jordan, I.; Fernandez de Sevilla, M.; Fumado, V.; Bassat, Q.; Bonet-Carne, E.; Fortuny, C.; Garcia-Miquel, A.; Jou, C.; Adroher, C.; Melé Casas, M.; et al. Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 Infection Among Children in Summer Schools Applying Stringent Control Measures in Barcelona, Spain. Clin. Infect. Dis. 2022, 74, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.J.; McKune, S.L.; Ryan, K.A.; Lednicky, J.A.; Crowe, S.R.; Myers, P.D.; Morris, J.G., Jr. SARS-CoV-2 Positivity on or After 9 Days Among Quarantined Student Contacts of Confirmed Cases. JAMA 2021, 325, 1561–1562. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.; Kendrick, K.; Troelstrup, T.; Gumke, M.; Edwards, J.; Chapman, S.; Propper, R.; Rivkees, S.A.; Blackmore, C. COVID-19 in Primary and Secondary School Settings During the First Semester of School Reopening—Florida, August–December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Brandal, L.T.; Ofitserova, T.S.; Meijerink, H.; Rykkvin, R.; Lund, H.M.; Hungnes, O.; Greve-Isdahl, M.; Bragstad, K.; Nygård, K.; Winje, B.A. Minimal transmission of SARS-CoV-2 from paediatric COVID-19 cases in primary schools, Norway, August to November 2020. Euro. Surveill. 2021, 26, 2002011. [Google Scholar] [CrossRef]
- Larosa, E.; Djuric, O.; Cassinadri, M.; Cilloni, S.; Bisaccia, E.; Vicentini, M.; Venturelli, F.; Giorgi Rossi, P.; Pezzotti, P.; Bedeschi, E.; et al. Secondary transmission of COVID-19 in preschool and school settings in northern Italy after their reopening in September 2020: A population-based study. Euro. Surveill. 2020, 25, 2001911. [Google Scholar] [CrossRef]
- Gettings, J.R.; Gold, J.A.W.; Kimball, A.; Forsberg, K.; Scott, C.; Uehara, A.; Tong, S.; Hast, M.; Swanson, M.R.; Morris, E.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Transmission in a Georgia School District-United States, December 2020–January 2021. Clin. Infect. Dis. 2022, 74, 319–326. [Google Scholar] [CrossRef]
- Hershow, R.B.; Wu, K.; Lewis, N.M.; Milne, A.T.; Currie, D.; Smith, A.R.; Lloyd, S.; Orleans, B.; Young, E.L.; Freeman, B.; et al. Low SARS-CoV-2 Transmission in Elementary Schools—Salt Lake County, Utah, 3 December 2020–31 January 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 442–448. [Google Scholar] [CrossRef]
- Dawson, P.; Worrell, M.C.; Malone, S.; Tinker, S.C.; Fritz, S.; Maricque, B.; Junaidi, S.; Purnell, G.; Lai, A.M.; Neidich, J.A.; et al. Pilot Investigation of SARS-CoV-2 Secondary Transmission in Kindergarten Through Grade 12 Schools Implementing Mitigation Strategies—St. Louis County and City of Springfield, Missouri, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 449–455. [Google Scholar] [CrossRef]
- Gandini, S.; Rainisio, M.; Iannuzzo, M.L.; Bellerba, F.; Cecconi, F.; Scorrano, L. A cross-sectional and prospective cohort study of the role of schools in the SARS-CoV-2 second wave in Italy. Lancet Reg. Health Eur. 2021, 5, 100092. [Google Scholar] [CrossRef]
- Barcellini, L.; Forlanini, F.; Sangiorgio, A.; Gambacorta, G.; Alberti, L.; Meta, A.; Gaia, P.; Amendola, A.; Tanzi, E.; Massa, V.; et al. Does school reopening affect SARS-CoV-2 seroprevalence among school-age children in Milan? PLoS ONE 2021, 16, e0257046. [Google Scholar] [CrossRef]
- Fukumoto, K.; McClean, C.T.; Nakagawa, K. No causal effect of school closures in Japan on the spread of COVID-19 in spring 2020. Nat. Med. 2021, 27, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Bark, D.; Dhillon, N.; St-Jean, M.; Kinniburgh, B.; McKee, G.; Choi, A. SARS-CoV-2 transmission in kindergarten to grade 12 schools in the Vancouver Coastal Health region: A descriptive epidemiologic study. CMAJ Open 2021, 9, E810–E817. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, J.; Hertegård, E.; BSvaleryd, H. The effects of school closures on SARS-CoV-2 among parents and teachers. Proc. Natl. Acad. Sci. USA 2021, 118, e2020834118. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.; Snow, K.; Danchin, M.; Mulholland, K.; Goldfeld, S.; Russell, F. SARS-CoV-2 infections and public health responses in schools and early childhood education and care centres in Victoria, Australia: An observational study. Lancet Reg. Health West Pac. 2022, 19, 100369. [Google Scholar] [CrossRef]
- Lakha, F.; King, A.; Swinkels, K.; Lee, A.C.K. Are schools drivers of COVID-19 infections-an analysis of outbreaks in Colorado, USA in 2020. J. Public Health 2022, 44, e26–e35. [Google Scholar] [CrossRef]
- Di Domenico, L.; Pullano, G.; Sabbatini, C.E.; Boëlle, P.Y.; Colizza, V. Modelling safe protocols for reopening schools during the COVID-19 pandemic in France. Nat. Commun. 2021, 12, 1073. [Google Scholar] [CrossRef]
- Li, Y.; Campbell, H.; Kulkarni, D.; Harpur, A.; Nundy, M.; Wang, X.; Nair, H. Usher Network for COVID-19 Evidence Reviews (UNCOVER) group. The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (R) of SARS-CoV-2: A modelling study across 131 countries. Lancet Infect. Dis. 2021, 21, 193–202. [Google Scholar] [CrossRef]
- Lasser, J.; Sorger, J.; Richter, L.; Thurner, S.; Schmid, D.; Klimek, P. Assessing the impact of SARS-CoV-2 prevention measures in Austrian schools using agent-based simulations and cluster tracing data. Nat. Commun. 2022, 13, 554. [Google Scholar] [CrossRef]
- Colosi, E.; Bassignana, G.; Contreras, D.A.; Poirier, C.; Boëlle, P.Y.; Cauchemez, S.; Yazdanpanah, Y.; Lina, B.; Fontanet, A.; Barrat, A.; et al. Screening and vaccination against COVID-19 to minimise school closure: A modelling study. Lancet Infect. Dis. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Engzell, P.; Frey, A.; Verhagen, M.D. Learning loss due to school closures during the COVID-19 pandemic. Proc. Natl. Acad. Sci. USA 2021, 118, e2022376118. [Google Scholar] [CrossRef]
- Lee, J. Mental health effects of school closures during COVID-19. Lancet Child Adolesc. Health 2020, 4, 421. [Google Scholar] [CrossRef]
- Monnier, M.; Moulin, F.; Thierry, X.; Vandentorren, S.; Côté, S.; Barbosa, S.; Falissard, B.; Plancoulaine, S.; Charles, M.A.; Simeon, T.; et al. Children’s mental and behavioral health, schooling, and socioeconomic characteristics during school closure in France due to COVID-19: The SAPRIS project. Sci. Rep. 2021, 11, 22373. [Google Scholar] [CrossRef] [PubMed]
- Hammerstein, S.; König, C.; Dreisörner, T.; Frey, A. Effects of COVID-19-Related School Closures on Student Achievement-A Systematic Review. Front. Psychol. 2021, 12, 746289. [Google Scholar] [CrossRef] [PubMed]
- Stuart, E.A.; Dowdy, D.W. Evidence-based COVID-19 policy-making in schools. Nat. Med. 2021, 27, 2078–2079. [Google Scholar] [CrossRef]
- Young, B.C.; Eyre, D.W.; Kendrick, S.; White, C.; Smith, S.; Beveridge, G.; Nonnenmacher, T.; Ichofu, F.; Hillier, J.; Oakley, S.; et al. Daily testing for contacts of individuals with SARS-CoV-2 infection and attendance and SARS-CoV-2 transmission in English secondary schools and colleges: An open-label, cluster-randomised trial. Lancet 2021, 398, 1217–1229. [Google Scholar] [CrossRef]
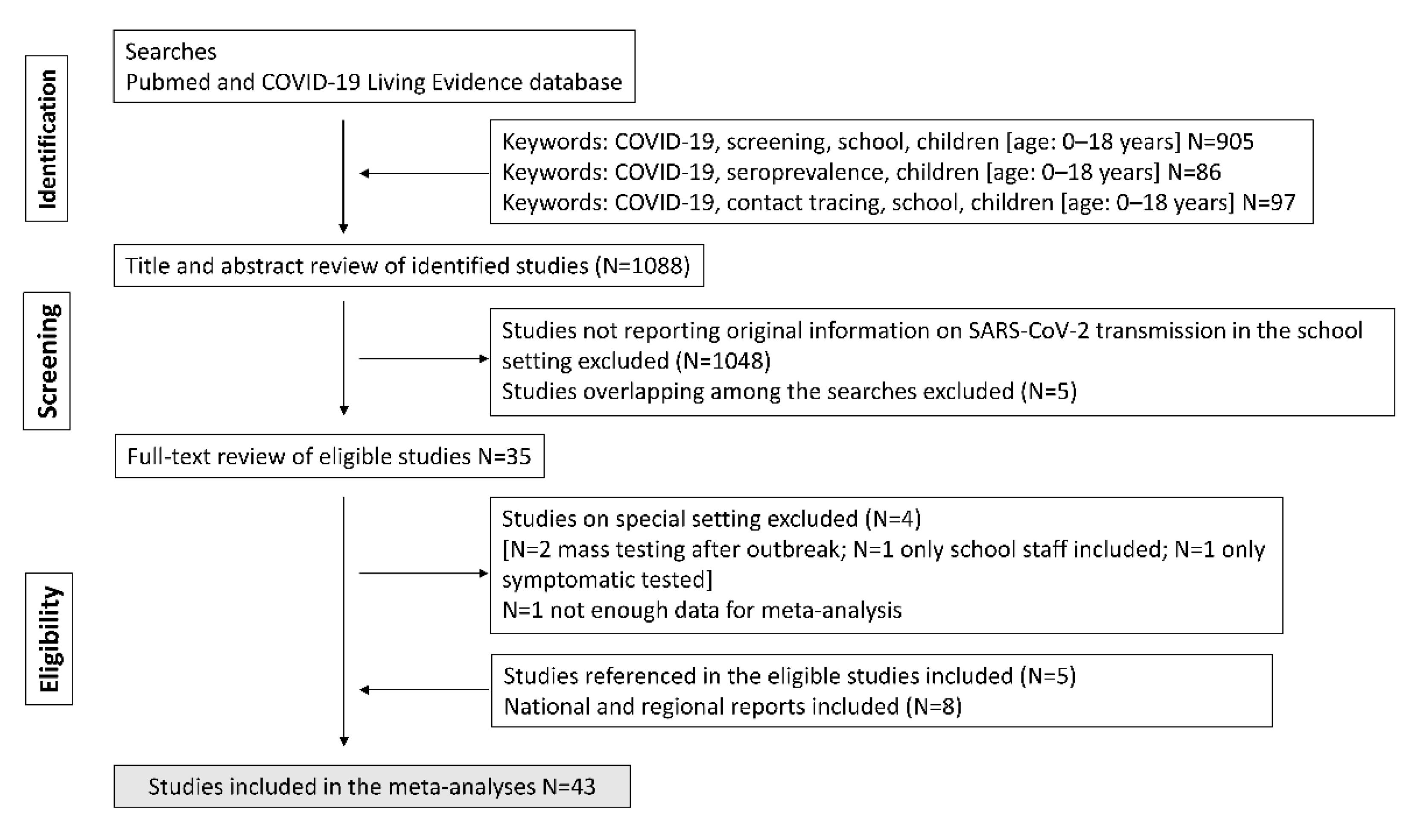
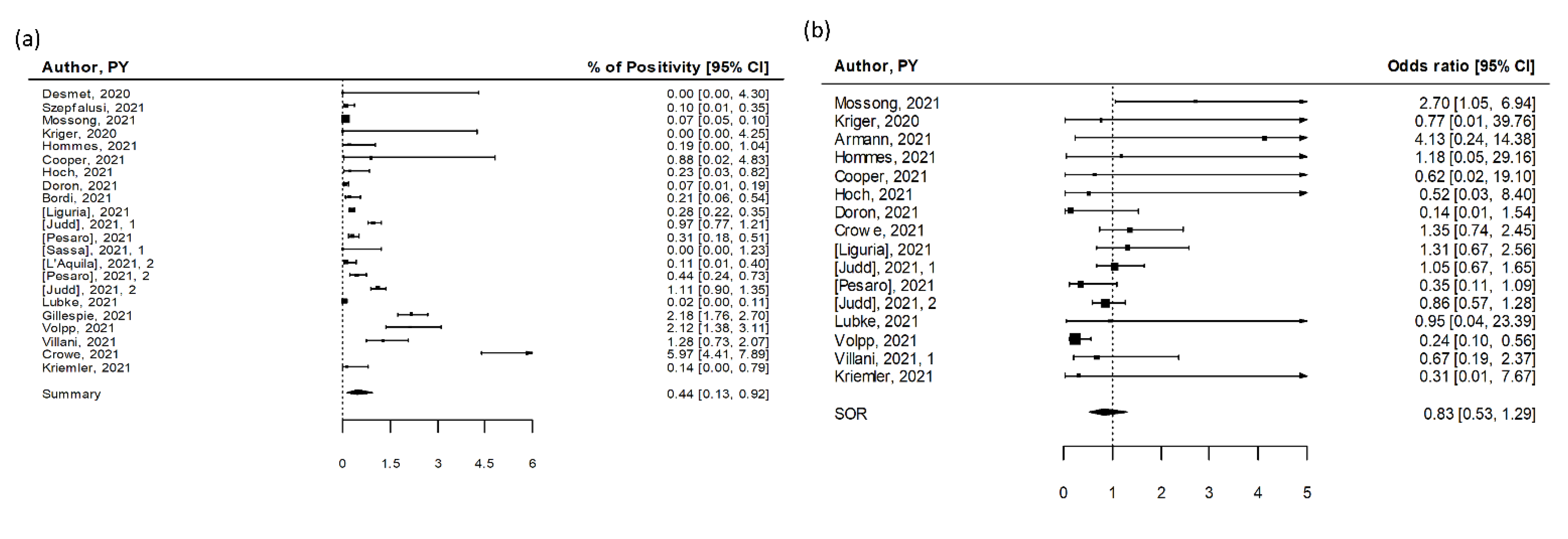
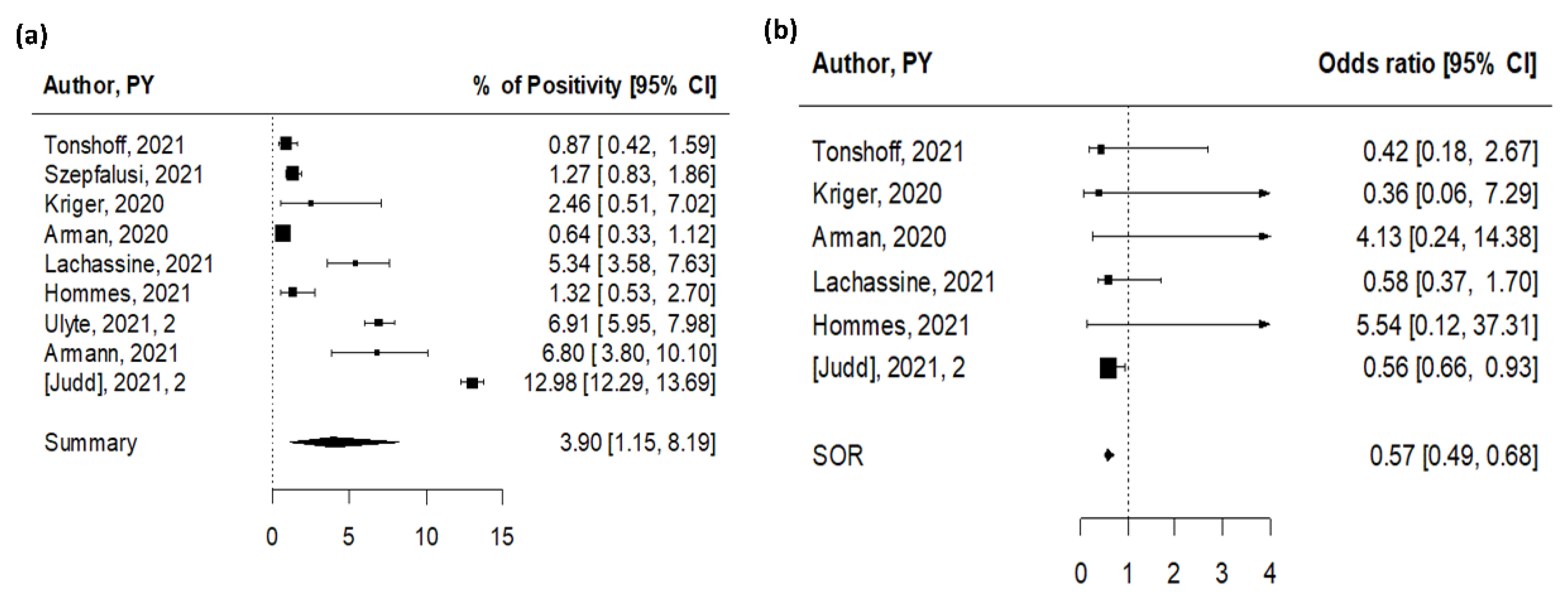
| n | Summary | Low 95% CI | Up 95% CI | I2 (%) | Study Design | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| % SARS-CoV-2 positivity | Contract tracing * | 15 | 2.54 | 0.76 | 5.31 | 100 | ||
| Screening | 22 | 0.44 | 0.13 | 0.92 | 97 | |||
| 6 | 1.14 | 0.01 | 4.19 | 98 | Cohorts | 0.03 | ||
| 16 | 0.31 | 0.05 | 0.81 | 95 | Cross-sectionals | |||
| Serosurvey | 9 | 3.90 | 1.15 | 8.19 | 100 | |||
| 3 | 10.31 | 2.44 | 22.74 | 98 | Cohorts | 0.005 | ||
| 6 | 1.49 | 0.07 | 4.69 | 88 | Cross-sectionals | |||
| OR for young vs. old | Susceptibility in contract tracing | 6 | 0.60 | 0.25 | 1.47 | 63 | ||
| Infectivity in contract tracing | 3 | 0.26 | 0.11 | 0.63 | 44 | |||
| Screening | 15 | 0.83 | 0.53 | 1.29 | 41 | |||
| 5 | 0.62 | 0.20 | 1.94 | 69 | Cohorts | 0.56 | ||
| 10 | 0.98 | 0.74 | 1.32 | 23 | Cross-sectionals | |||
| Serosurvey | 6 | 0.57 | 0.46 | 0.68 | 21 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caini, S.; Martinoli, C.; La Vecchia, C.; Raimondi, S.; Bellerba, F.; D’Ecclesiis, O.; Sasso, C.; Basso, A.; Cammarata, G.; Gandini, S. SARS-CoV-2 Circulation in the School Setting: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 5384. https://doi.org/10.3390/ijerph19095384
Caini S, Martinoli C, La Vecchia C, Raimondi S, Bellerba F, D’Ecclesiis O, Sasso C, Basso A, Cammarata G, Gandini S. SARS-CoV-2 Circulation in the School Setting: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(9):5384. https://doi.org/10.3390/ijerph19095384
Chicago/Turabian StyleCaini, Saverio, Chiara Martinoli, Carlo La Vecchia, Sara Raimondi, Federica Bellerba, Oriana D’Ecclesiis, Clementina Sasso, Alessandra Basso, Giulio Cammarata, and Sara Gandini. 2022. "SARS-CoV-2 Circulation in the School Setting: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 9: 5384. https://doi.org/10.3390/ijerph19095384
APA StyleCaini, S., Martinoli, C., La Vecchia, C., Raimondi, S., Bellerba, F., D’Ecclesiis, O., Sasso, C., Basso, A., Cammarata, G., & Gandini, S. (2022). SARS-CoV-2 Circulation in the School Setting: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(9), 5384. https://doi.org/10.3390/ijerph19095384









