Prevention of Depression and Anxiety in Subclinical Adolescents: Effects of a Transdiagnostic Internet-Delivered CBT Program
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.2.1. Anxiety and Depression Measures
2.2.2. Transdiagnostic Measures
2.2.3. Reported Top Problems
2.2.4. Feasibility and Acceptability
2.3. AMTE
2.4. Therapist Involvement via Phone Calls
2.5. Design and Procedure
2.6. Statistical Analysis
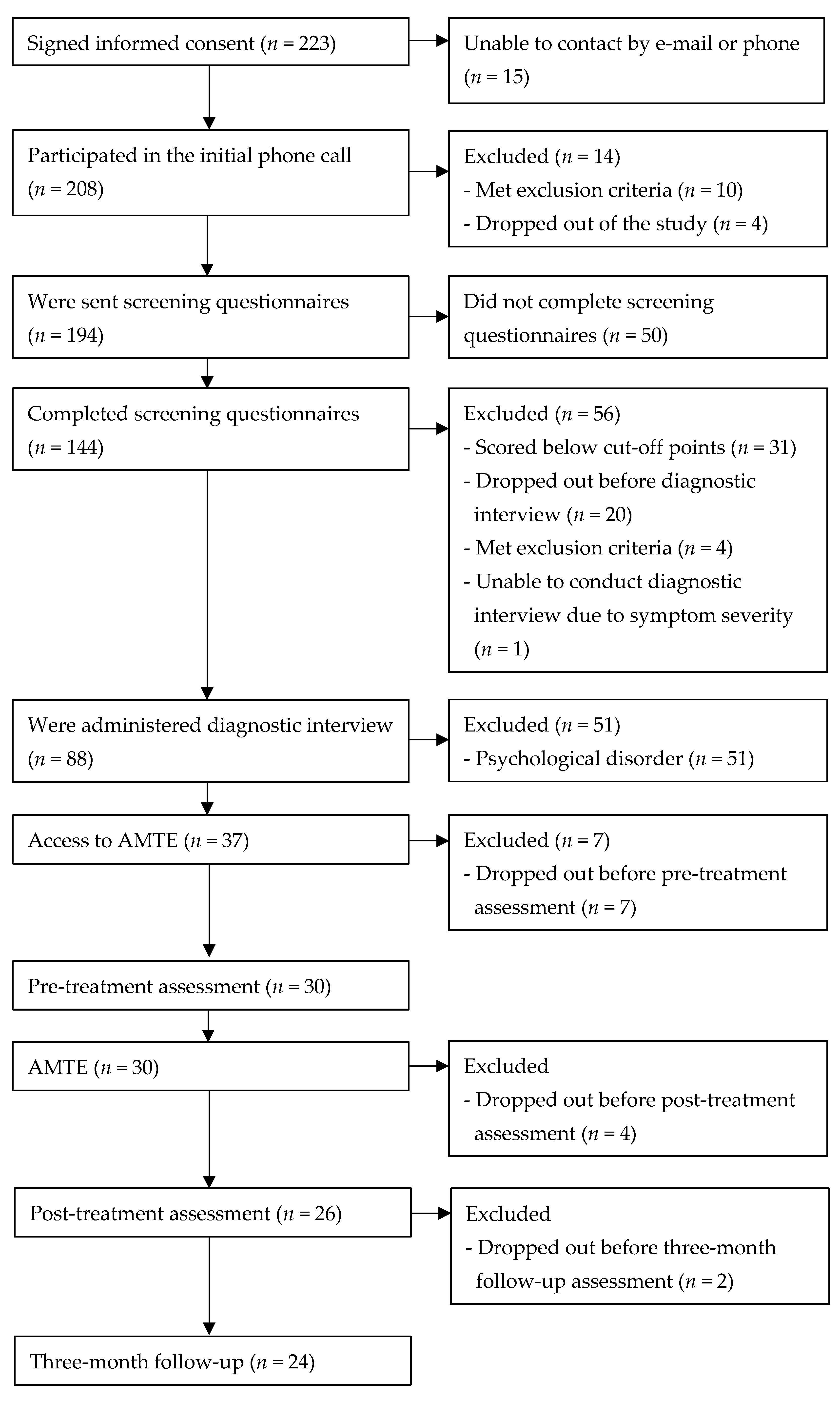
3. Results
3.1. Changes in Self-Reported and Clinician-Rated Anxiety and Depression Measures
3.2. Changes in Self-Reported Transdiagnostic Variables
3.3. Changes in Self-Reported and Parent-Reported Top Problems Assessment Ratings
3.4. Self-Reported and Parent-Reported Feasibility and Acceptability
3.4.1. Adolescent Report
3.4.2. Parent Report
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrades-Tobar, M.; García, F.E.; Concha-Ponce, P.; Valiente, C.; Lucero, C. Predictores de síntomas de ansiedad, depresión y estrés a partir del brote epidémico de COVID-19. Rev. Psicopatol. Psicol. Clin. 2021, 26, 13–22. [Google Scholar] [CrossRef]
- Sandín, B.; Valiente, R.M.; García-Escalera, J.; Campagne, D.M.; Chorot, P. Psychological impact of the COVID-19 pandemic: Negative and positive effects in Spanish population during the mandatory national quarantine. Rev. Psicopatol. Psicol. Clin. 2020, 25, 1e–21e. [Google Scholar] [CrossRef]
- Orgilés, M.; Espada, J.P.; Delvecchio, E.; Francisco, R.; Mazzeschi, C.; Pedro, M.; Morales, A. Anxiety and depressive symptoms in children and adolescents during COVID-19 pandemic: A transcultural approach. Psicothema 2021, 33, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Petukhova, M.; Sampson, N.A.; Zaslavsky, A.M.; Wittchen, H.-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 2012, 21, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Balász, J.; Miklósi, M.; Keresztény, Á.; Hoven, C.W.; Carli, V.; Wasserman, C.; Apter, A.; Bobes, J.; Brunner, R.; Cosman, D.; et al. Adolescent subthreshold-depression and anxiety: Psychopathology, functional impairment and increased suicide risk. J. Child Psychol. Psychiatry 2013, 54, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Birmaher, B.; Ryan, N.D.; Williamson, D.E.; Brent, D.A.; Kaufman, J.; Dahl, R.E.; Perel, J.; Nelson, B. Childhood and adolescent depression: A review of the past 10 years. Part I. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 1427–1439. [Google Scholar] [CrossRef]
- Axelson, D.A.; Birmaher, B. Relation between anxiety and depressive disorders in childhood and adolescence. Depress. Anxiety 2001, 14, 67–78. [Google Scholar] [CrossRef]
- Mrazek, P.J.; Haggerty, R.J. (Eds.) Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research; National Academies Press: Washington, DC, USA, 1994; pp. 24–25. [Google Scholar] [CrossRef]
- Stockings, E.A.; Degenhardt, L.; Dobbins, T.; Lee, Y.Y.; Erskine, H.E.; Whiteford, H.A.; Patton, G. Preventing depression and anxiety in young people: A review of the joint efficacy of universal, selective and indicated prevention. Psychol. Med. 2016, 46, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Werner-Seidler, A.; Spanos, S.; Calear, A.L.; Perry, Y.; Torok, M.; O’Dea, B.; Christensen, H.; Newby, J.M. School-based depression and anxiety prevention programs: An updated systematic review and meta-analysis. Clin. Psychol. Rev. 2021, 89, 102079. [Google Scholar] [CrossRef]
- Sandín, B.; Chorot, P.; Valiente, R.M. Transdiagnóstico: Nueva frontera en psicología clínica. Rev. Psicopatol. Psicol. Clin. 2012, 17, 185–203. [Google Scholar] [CrossRef] [Green Version]
- García-Escalera, J.; Chorot, P.; Valiente, R.M.; Reales, J.M.; Sandín, B. Efficacy of transdiagnostic cognitive-behavioral therapy for anxiety and depression in adults, children and adolescents: A meta-analysis. Rev. Psicopatol. Psicol. Clin. 2016, 21, 147–175. [Google Scholar] [CrossRef] [Green Version]
- Ehrenreich-May, J.; Kennedy, S.M.; Sherman, J.A.; Bilek, E.L.; Buzzella, B.A.; Bennett, S.M.; Barlow, D.H. Unified Protocols for Transdiagnostic Treatment of Emotional Disorders in Children and Adolescents: Therapist Guide; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Ehrenreich-May, J.; Kennedy, S.M.; Sherman, J.A.; Bilek, E.L.; Buzzella, B.A.; Shannon, M.B.; Barlow, D.H. Protocolo Unificado para el Tratamiento Transdiagnóstico de los Trastornos Emocionales en Niños y Adolescentes: Manual del Terapeuta; (Spanish translation and adaptation by Bonifacio Sandín and Julia García-Escalera); Pirámid: Madrid, Spain, 2022. [Google Scholar]
- Ehrenreich-May, J.; Bilek, E.L. Universal prevention of anxiety and depression in a recreational camp setting: An initial open trial. Child Youth Care Forum 2011, 40, 435–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Escalera, J.; Valiente, R.M.; Chorot, P.; Ehrenreich-May, J.; Kennedy, S.M.; Sandín, B. The Spanish version of the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders in Adolescents (UP-A) adapted as a school-based anxiety and depression prevention program: Study protocol for a cluster randomized controlled trial. JMIR Res. Protoc. 2017, 6, e149. [Google Scholar] [CrossRef] [PubMed]
- García-Escalera, J.; Chorot, P.; Sandín, B.; Ehrenreich-May, J.; Prieto, A.; Valiente, R.M. An open trial applying the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders in Adolescents (UP-A) adapted as a school-based prevention program. Child Youth Care Forum 2019, 48, 29–53. [Google Scholar] [CrossRef]
- García-Escalera, J.; Valiente, R.M.; Sandín, B.; Ehrenreich-May, J.; Prieto, A.; Chorot, P. The Unified Protocol for Transdiagnostic Treatment of Emotional Disorders in Adolescents (UP-A) adapted as a school-based anxiety and depression prevention program: An initial cluster randomized wait-list-controlled trial. Behav. Ther. 2020, 51, 461–473. [Google Scholar] [CrossRef]
- Ramdhonee-Dowlot, K.; Balloo, K.; Essau, C.A. Effectiveness of the Super Skills for Life programme in enhancing the emotional wellbeing of children and adolescents in residential care institutions in a low- and middle-income country: A randomised waitlist-controlled trial. J. Affect. Disord. 2021, 278, 327–338. [Google Scholar] [CrossRef]
- Essau, C.A.; Ollendick, T.H. The Super Skills for Life Programme; University of Roehampton: London, UK, 2013. [Google Scholar]
- Essau, C.A.; Ollendick, T.M. Super Skills for Life–Adolescent Version; University of Roehampton: London, UK, 2016. [Google Scholar]
- Ehrenreich-May, J.; Rosenfield, D.; Queen, A.H.; Kennedy, S.M.; Remmes, C.S.; Barlow, D.H. An initial waitlist-controlled trial of the unified protocol for the treatment of emotional disorders in adolescents. J. Anxiety Disord. 2017, 46, 46–55. [Google Scholar] [CrossRef]
- Kennedy, S.M.; Halliday, E.; Ehrenreich-May, J. Trajectories of change and intermediate indicators of non-response to transdiagnostic treatment for children and adolescents. J. Clin. Child. Adolesc. Psychol. 2021, 50, 904–918. [Google Scholar] [CrossRef]
- Queen, A.H.; Barlow, D.H.; Ehrenreich-May, J. The trajectories of adolescent anxiety and depressive symptoms over the course of a transdiagnostic treatment. J. Anxiety Disord. 2014, 28, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.A.; Ehrenreich-May, J. Changes in risk factors during the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders in Adolescents. Behav. Ther. 2020, 51, 869–881. [Google Scholar] [CrossRef]
- Sloan, E.; Hall, K.; Moulding, R.; Bryce, S.; Mildred, H.; Staiger, P.K. Emotion regulation as a transdiagnostic treatment construct across anxiety, depression, substance, eating and borderline personality disorders: A systematic review. Clin. Psychol. Rev. 2017, 57, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G.; Titov, N. Advantages and limitations of internet-based interventions for common mental disorders. World Psychiatry 2014, 13, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Carlbring, P.; Andersson, G.; Cuijpers, P.; Riper, H.; Hedman-Lagerlöf, E. Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: An updated systematic review and meta-analysis. Cogn. Behav. Ther. 2018, 47, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etzelmueller, A.; Vis, C.; Karyotaki, E.; Baumeister, H.; Titov, N.; Berking, M.; Cuijpers, P.; Riper, H.; Ebert, D.D. Effects of internet-based cognitive behavioral therapy in routine care for adults in treatment for depression and anxiety: Systematic review and meta-analysis. J. Med. Internet Res. 2020, 22, e18100. [Google Scholar] [CrossRef] [PubMed]
- Păsărelu, C.R.; Andersson, G.; Bergman Nordgren, L.; Dobrean, A. Internet-delivered transdiagnostic and tailored cognitive behavioral therapy for anxiety and depression: A systematic review and meta-analysis of randomized controlled trials. Cogn. Behav. Ther. 2017, 46, 1–28. [Google Scholar] [CrossRef]
- Vigerland, S.; Lenhard, F.; Bonnert, M.; Lalouni, M.; Hedman, E.; Ahlen, J.; Olén, O.; Serlachius, E.; Ljótsson, B. Internet-delivered cognitive behavior therapy for children and adolescents: A systematic review and meta-analysis. Clin. Psychol. Rev. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Sandín, B.; Valiente, R.M.; García-Escalera, J.; Pineda, D.; Espinosa, V.; Magaz, A.M.; Chorot, P. Protocolo unificado para el tratamiento transdiagnóstico de los trastornos emocionales en adolescentes a través de internet (iUP-A): Aplicación web y protocolo de un ensayo controlado aleatorizado. Rev. Psicopatol. Psicol. Clin. 2019, 24, 197–215. [Google Scholar] [CrossRef] [Green Version]
- Sandín, B.; García-Escalera, J.; Valiente, R.M.; Espinosa, V.; Chorot, P. Clinical utility of an internet-delivered version of the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders in Adolescents (iUP-A): A pilot open trial. Int. J. Environ. Res. Public Health 2020, 17, 8306. [Google Scholar] [CrossRef]
- Păsărelu, C.R.; Dobrean, A.; Andersson, G.; Zaharie, G.C. Feasibility and clinical utility of a transdiagnostic internet-delivered rational emotive and behavioral intervention for adolescents with anxiety and depressive disorders. Internet Interv. 2021, 26, 100479. [Google Scholar] [CrossRef]
- Sandín, B.; Chorot, P.; Valiente, R.M.; Chorpita, B.F. Development of a 30-item version of the Revised Child Anxiety and Depression Scale. Rev. Psicopatol. Psicol. Clin. 2010, 15, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Piqueras, J.A.; Pineda, D.; Martin-Vivar, M.; Sandín, B. Confirmatory factor analysis and psychometric properties of the Revised Child Anxiety and Depression Scale−30 (RCADS-30) in clinical and non-clinical samples. Rev. Psicopatol. Psicol. Clin. 2017, 22, 183–196. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, D.V.; Lecrubier, Y.; Shytle, D.; Milo, K.; Hergueta, T.; Colón-Soto, M.; Díaz, V.; Soto, O. Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). Version 1.1; Medical Outcome Systems: Tampa, FL, USA, 2000. [Google Scholar]
- Colón-Soto, M.; Díaz, V.; Soto, O.; Santana, C. Mini International Neuropsychiatric Interview para Niños y Adolescentes (MINI-KID). Versión en Español; Medical Outcome Systems: Tampa, FL, USA, 2005. [Google Scholar]
- Sheehan, D.V.; Sheehan, K.H.; Shytle, R.D.; Janavs, J.; Bannon, Y.; Rogers, J.E.; Milo, K.M.; Stock, S.L.; Wilkinson, B. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J. Clin. Psychiatry 2010, 71, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Piqueras, J.A.; Martín-Vivar, M.; Sandin, B.; San Luis, C.; Pineda, D. The Revised Child Anxiety and Depression Scale: A systematic review and reliability generalization meta-analysis. J. Affect. Disord. 2017, 218, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Sandín, B.; Chorot, P.; Valiente, R.M. TCC de los Trastornos de Ansiedad: Innovaciones en Niños y Adolescentes; Klinik: Madrid, Spain, 2016. [Google Scholar]
- Sandín, B.; Chorot, P.; Valiente, R.M. Cuestionario PSWQ-11 para Niños y Adolescentes (PSWQN-11); Universidad Nacional de Educación a Distancia: Madrid, Spain, 2010; Unpublished. [Google Scholar]
- Sandín, B.; Chorot, P.; Valiente, R.M.; Lostao, L. Validación española del cuestionario de preocupación PSWQ: Estructura factorial y propiedades psicométricas. Rev. Psicopatol. Psicol. Clin. 2009, 14, 107–122. [Google Scholar] [CrossRef] [Green Version]
- Guy, W. Clinical Global Impressions, ECDEU Assessment Manual for Psychopharmacology, Revised (DHEW Publ. No. ADM 76-338); U.S. Department of Health, Education, and Welfare: Rockville, MD, USA, 1976; pp. 218–222. [Google Scholar]
- Berk, M.; Ng, F.; Dodd, S.; Callaly, T.; Campbell, S.; Bernardo, M.; Trauer, T. The validity of the CGI severity and improvement scales as measures of clinical effectiveness suitable for routine clinical use. J. Eval. Clin. Pract. 2008, 14, 979–983. [Google Scholar] [CrossRef]
- Sandín, B. Escalas Panas de afecto positivo y negativo para niños y adolescentes (PANASN). Rev. Psicopatol. Psicol. Clin. 2003, 8, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Molina, J.; Sandín, B.; Chorot, P. Sensibilidad a la ansiedad y presión psicológica: Efectos sobre el rendimiento deportivo en adolescentes. Cuad. Psicol. Deporte 2014, 14, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Silverman, W.K.; Fleisig, W.; Rabian, B.; Peterson, R.A. Childhood Anxiety Sensitivity Index. J. Clin. Child Psychol. 1991, 20, 162–168. [Google Scholar] [CrossRef]
- Sandín, B. Ansiedad, Miedos y Fobias en Niños y Adolescentes; Dykinson: Madrid, Spain, 1997. [Google Scholar]
- Sandín, B.; Chorot, P.; Santed, M.A.; Valiente, R.M. Análisis factorial confirmatorio del Índice de Sensibilidad a la Ansiedad para Niños. Psicothema 2002, 14, 333–339. [Google Scholar]
- Kennedy, S.M.; Ehrenreich-May, J. Assessment of emotional avoidance in adolescents: Psychometric properties of a new multidimensional measure. J. Psychopathol. Behav. Assess. 2017, 39, 279–290. [Google Scholar] [CrossRef]
- García-Escalera, J.; Chorot, P.; Valiente, R.M.; Sandín, B.; Tonarely, N.; Ehrenreich-May, J. Versión Española de la Emotional Avoidance Strategy Inventory for Adolescents (EASI-A); Universidad Nacional de Educación a Distancia: Madrid, Spain, 2016; Unpublished. [Google Scholar]
- Weisz, J.R.; Chorpita, B.F.; Frye, A.; Ng, M.Y.; Lau, N.; Bearman, S.K.; Ugueto, A.M.; Langer, D.A.; Hoagwood, K.E. The Research Network on Youth Mental Health. Youth top problems: Using idiographic, consumer-guided assessment to identify treatment needs and to track change during psychotherapy. J. Consult. Clin. Psychol. 2011, 79, 369–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandín, B.; Valiente, R.M.; García-Escalera, J.; Chorot, P. Feasibility and Acceptability Questionnaire (FAQ); Universidad Nacional de Educación a Distancia: Madrid, Spain, 2020; Unpublished. [Google Scholar]
- Rapee, R.M.; Wignall, A.; Sheffield, J.; Kowalenko, N.; Davis, A.; McLoone, J.; Spence, S.H. Adolescents’ reactions to universal and indicated prevention programs for depression: Perceived stigma and consumer satisfaction. Prev. Sci. 2006, 7, 167–177. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows, Version 25.0; IBM Corp.: Armonk, NY, USA, 2017. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.P.; Chango, J.; Szwedo, D.; Schad, M. Long-term sequelae of sub-clinical depressive symptoms in early adolescence. Dev. Psychopathol. 2014, 26, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beesdo, K.; Knappe, S.; Pine, D.S. Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. Psychiatr. Clin. N. Am. 2009, 32, 483–524. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, J.L.; Hamlat, E.J.; Stange, J.P.; Abramson, L.Y.; Alloy, L.B. Pubertal timing and vulnerabilities to depression in early adolescence: Differential pathways to depressive symptoms by sex. J. Adolesc. 2014, 37, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Katon, W.; Richardson, L.; Russo, J.; McCarty, C.A.; Rockhill, C.; McCauley, E.; Richards, J.; Grossman, D.C. Depressive symptoms in adolescence: The association with multiple health risk behaviors. Gen. Hosp. Psychiatry 2010, 32, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Pine, D.S.; Cohen, E.; Cohen, P.; Brook, J. Adolescent depressive symptoms as predictors of adult depression: Moodiness or mood disorder? Am. J. Psychiatry 1999, 156, 133–135. [Google Scholar] [CrossRef]
- Rivas-Vazquez, R.A.; Saffa-Biller, D.; Ruiz, I.; Blais, M.A.; Rivas-Vazquez, A. Current issues in anxiety and depression: Comorbid, mixed, and subthreshold disorders. Prof. Psychol. Res. Pract. 2004, 35, 74–83. [Google Scholar] [CrossRef]
- Sandín, B.; Chorot, P.; Valiente, R.M. Psicopatología de la ansiedad y trastornos de ansiedad: Hacia un enfoque transdiagnóstico. In Manual de Psicopatología, 3rd ed.; Belloch, A., Sandín, B., Ramos, F., Eds.; McGraw-Hill: Madrid, Spain, 2020; Volume 1, pp. 3–34. [Google Scholar]
- Lenhard, F.; Vigerland, S.; Andersson, E.; Rück, C.; Mataix-Cols, D.; Thulin, U.; Ljótsson, B.; Serlachius, E. Internet-delivered cognitive behavior therapy for adolescents with obsessive-compulsive disorder: An open trial. PLoS ONE 2014, 9, e100773. [Google Scholar] [CrossRef]
- Melville, K.M.; Casey, L.M.; Kavanagh, D.J. Dropout from internet-based treatment for psychological disorders. Br. J. Clin. Psychol. 2010, 49, 455–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trosper, S.E.; Buzzella, B.A.; Bennett, S.M.; Ehrenreich, J.T. Emotion regulation in youth with emotional disorders: Implications for a unified treatment approach. Clin. Child Fam. Psychol. Rev. 2009, 12, 234–254. [Google Scholar] [CrossRef] [PubMed]
- Osma, J.; Martínez-García, L.; Quilez-Orden, A.; Peris-Baquero, Ó. Unified Protocol for the Transdiagnostic Treatment of Emotional Disorders in medical conditions: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 5077. [Google Scholar] [CrossRef] [PubMed]
- Sakiris, N.; Berle, D.A. Systematic review and meta-analysis of the Unified Protocol as a transdiagnostic emotion regulation based intervention. Clin. Psychol. Rev. 2019, 72, 101751. [Google Scholar] [CrossRef] [PubMed]
| Completer Sample Analyses n = 24 | Intention-to-Treat Analyses n = 30 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Pre- Treatment | Post- Treatment | Follow- Up | Pre- Treatment | Post- Treatment | Follow- Up | ||||||||||
| M | SD | M | SD | M | SD | χ² | p | M | SD | M | SD | M | SD | χ² | p | |
| EAN | 7.63 | 4.50 | 6.50 | 4.34 | 5.75 | 5.10 | 4.42 | 0.110 | 7.43 | 4.55 | 6.63 | 4.45 | 6.03 | 5.08 | 4.15 | 0.126 |
| CDN | 11.04 | 7.31 | 11.58 | 8.59 | 8.00 | 8.54 | 10.57 | 0.005 | 11.10 | 6.65 | 11.80 | 7.91 | 8.93 | 8.07 | 9.92 | 0.007 |
| RCADS-30 | ||||||||||||||||
| Total | 19.08 | 10.30 | 16.71 | 9.74 | 13.13 | 10.29 | 12.51 | 0.002 | 18.50 | 9.50 | 17.03 | 10.06 | 14.17 | 10.68 | 11.76 | 0.003 |
| MDD | 3.38 | 2.30 | 2.79 | 2.19 | 1.96 | 2.84 | 8.61 | 0.014 | 3.43 | 2.14 | 3.03 | 2.11 | 2.37 | 2.74 | 7.98 | 0.019 |
| PD | 1.00 | 1.38 | 1.79 | 2.50 | 0.71 | 1.60 | 11.89 | 0.003 | 0.93 | 1.41 | 1.77 | 2.69 | 0.90 | 2.11 | 11.51 | 0.003 |
| SP | 5.63 | 3.35 | 4.25 | 3.19 | 3.88 | 3.42 | 4.52 | 0.104 | 5.40 | 3.14 | 4.40 | 3.36 | 4.10 | 3.55 | 4.23 | 0.120 |
| SAD | 0.67 | 1.13 | 0.79 | 1.61 | 0.58 | 1.41 | 3.75 | 0.153 | 0.73 | 1.11 | 0.90 | 1.56 | 0.73 | 1.41 | 2.93 | 0.231 |
| GAD | 5.58 | 3.49 | 4.58 | 2.99 | 4.29 | 2.69 | 3.41 | 0.182 | 5.30 | 3.32 | 4.50 | 2.84 | 4.27 | 2.59 | 3.17 | 0.205 |
| OCD | 2.83 | 2.41 | 2.50 | 2.02 | 1.71 | 1.99 | 14.00 | 0.001 | 2.70 | 2.25 | 2.43 | 1.98 | 1.80 | 1.95 | 12.80 | 0.002 |
| ANX | 15.71 | 8.98 | 13.92 | 8.41 | 11.17 | 8.13 | 8.96 | 0.011 | 15.07 | 8.37 | 14.00 | 8.75 | 11.80 | 8.63 | 8.40 | 0.015 |
| PSWQN-11 | 23.71 | 7.87 | 20.75 | 6.49 | 17.42 | 6.30 | 11.38 | 0.003 | 23.23 | 8.27 | 20.93 | 7.37 | 18.27 | 7.43 | 10.69 | 0.005 |
| CGI-S | 2.42 | 0.72 | 1.50 | 0.78 | 1.50 | 0.72 | 25.39 | 0.000 | 2.40 | 0.72 | 1.60 | 0.81 | 1.60 | 0.77 | 27.56 | 0.000 |
| Completer Sample Analyses n = 24 | Intention-to-Treat Analyses n = 30 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Pre- Treatment | Post- Treatment | Follow- Up | Pre- Treatment | Post- Treatment | Follow- Up | ||||||||||
| M | SD | M | SD | M | SD | χ² | p | M | SD | M | SD | M | SD | χ² | p | |
| PANASN | ||||||||||||||||
| PA | 22.38 | 3.59 | 23.67 | 3.13 | 23.04 | 3.98 | 4.52 | 0.104 | 22.20 | 3.45 | 23.37 | 3.30 | 22.87 | 3.93 | 5.30 | 0.071 |
| NA | 16.88 | 2.64 | 16.42 | 2.86 | 15.63 | 3.45 | 2.93 | 0.231 | 16.87 | 2.73 | 16.60 | 3.02 | 15.97 | 3.53 | 2.36 | 0.307 |
| EASI-A | 24.92 | 11.42 | 22.50 | 11.22 | 19.75 | 12.25 | 7.68 | 0.021 | 26.07 | 12.34 | 24.47 | 12.40 | 22.27 | 13.53 | 7.22 | 0.027 |
| CASI | 25.63 | 5.99 | 25.17 | 5.35 | 23.33 | 5.27 | 11.03 | 0.004 | 25.90 | 5.59 | 25.80 | 5.68 | 24.33 | 5.84 | 10.26 | 0.006 |
| Completer Sample Analyses n = 24 | Intention-to-Treat Analyses n = 30 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Pre- Treatment | Post- Treatment | Follow- Up | Pre- Treatment | Post- Treatment | Follow- Up | ||||||||||||
| n | M | SD | M | SD | M | SD | χ² | p | n | M | SD | M | SD | M | SD | χ² | p | |
| Problem 1 (A) | 22 | 5.68 | 1.62 | 2.64 | 2.15 | 2.23 | 1.69 | 23.46 | 0.000 | 26 | 5.50 | 1.61 | 2.62 | 2.08 | 2.27 | 1.69 | 30.54 | 0.000 |
| Problem 2 (A) | 22 | 6.00 | 1.93 | 2.77 | 2.22 | 2.45 | 2.36 | 25.33 | 0.000 | 26 | 5.96 | 1.93 | 2.85 | 2.13 | 2.58 | 2.27 | 27.02 | 0.000 |
| Problem 3 (A) | 22 | 6.05 | 1.96 | 2.68 | 2.06 | 2.27 | 2.76 | 19.75 | 0.000 | 26 | 6.23 | 2.12 | 3.04 | 2.34 | 2.69 | 2.94 | 19.85 | 0.000 |
| Problem 1 (P) | 20 | 6.15 | 2.21 | 2.85 | 1.81 | 2.25 | 2.10 | 25.49 | 0.000 | 24 | 6.21 | 2.19 | 2.67 | 1.74 | 2.17 | 1.95 | 31.01 | 0.000 |
| Problem 2 (P) | 19 | 6.53 | 2.20 | 2.89 | 2.05 | 2.37 | 2.41 | 23.06 | 0.000 | 23 | 6.65 | 2.04 | 3.04 | 2.31 | 2.61 | 2.61 | 24.70 | 0.000 |
| Problem 3 (P) | 18 | 6.11 | 2.22 | 2.61 | 2.00 | 2.56 | 2.81 | 20.13 | 0.000 | 22 | 6.32 | 2.08 | 2.82 | 2.30 | 2.77 | 2.91 | 21.78 | 0.000 |
| Experience with the Online Platform (Range: 0–10) | M | SD |
| How easy has it been for you to use the AMTE online platform? | 9.04 | 1.00 |
| How easy has it been for you to understand what the videos and Dr. AMTE were telling you? | 8.88 | 1.04 |
| How useful has what you have been taught in the AMTE program (through the videos, the avatar, the PDFs, the homework assignments, etc.) been for you? | 8.83 | 1.17 |
| How easy has it been for you to include the AMTE program in your daily routine? | 8.25 | 1.23 |
| To what degree have you been able to do the exercises and homework assignments without technical or computer problems? | 8.63 | 1.38 |
| To what extent have you applied what you have learned from AMTE to your real life? | 7.71 | 1.37 |
| Satisfaction with the Program (Range: 0–10) | M | SD |
| How much have you learned from the program? | 8.79 | 1.06 |
| How effective has the program been in helping you cope with your problems? | 8.50 | 0.98 |
| How much have you enjoyed doing the program? | 7.83 | 1.27 |
| To what extent would you recommend the program to other adolescents? | 8.92 | 1.10 |
| How many skills to cope with emotions did you have before the program? | 4.88 | 2.09 |
| How many skills to cope with emotions do you have now? | 8.46 | 1.10 |
| Therapeutic Alliance (Range: 0–10) | M | SD |
| How much has your therapist helped you deal with your top problems? | 9.04 | 0.91 |
| How appreciated by your therapist have you felt? | 9.50 | 0.78 |
| To what extent have you felt that you and your therapist respected each other? | 9.67 | 0.57 |
| To what extent have you agreed with your therapist on what things were important for you to work on or overcome? | 9.33 | 0.87 |
| To what extent have you felt that your therapist cared about you? | 9.63 | 0.65 |
| How correct do you think the way you and your therapist have worked to solve your problems has been? | 9.42 | 0.83 |
| Experience with the Online Platform (Range: 0–10) | M | SD |
| How easy has it been for your son/daughter to use the AMTE online platform? | 8.46 | 1.79 |
| How easy has it been for your son/daughter to understand what the videos and Dr. AMTE were telling him/her? | 8.37 | 2.10 |
| How useful has what your son/daughter has been taught in the AMTE program (through the videos, the avatar, the PDFs, the homework assignments, etc.) been for him/her? | 8.71 | 1.33 |
| How easy has it been for your son/daughter to include the AMTE program in his/her daily routine? | 7.87 | 1.75 |
| To what degree has your son/daughter been able to do the exercises and homework assignments without technical or computer problems? | 8.25 | 2.13 |
| To what extent has your son/daughter applied what he/she has learned from AMTE to his/her real life? | 8.25 | 1.57 |
| Experience with the parent’s section of the online platform | n (%) (yes) | |
| Have you ever logged in to the parent’s section? | 14 (58.3%) | |
| To what extent have you found the parent’s section useful to help your son/daughter during treatment? (range: 0–10) | 8.08 | 3.37 |
| Satisfaction with the program (range: 0–10) | M | SD |
| How much has your son/daughter learned from the program? | 8.79 | 1.14 |
| How effective has the program been in helping your son/daughter cope with his/her problems? | 8.71 | 1.40 |
| How much has your son/daughter enjoyed doing the program? | 7.75 | 1.80 |
| To what extent would you recommend the program to other adolescents? | 9.58 | 0.83 |
| How many skills to cope with emotions did your son/daughter have before the program? | 4.50 | 2.72 |
| How many skills to cope with emotions does he/she have now? | 8.50 | 1.14 |
| Therapeutic alliance (range: 0–10) | M | SD |
| How much has the therapist helped your son/daughter deal with his/her top problems? | 9.42 | 0.72 |
| How appreciated by the therapist have you felt? | 9.75 | 0.61 |
| To what extent have you felt that you and the therapist respected each other? | 9.79 | 0.59 |
| To what extent have you agreed with the therapist on what things were important for your son/daughter to work on or to overcome? | 9.83 | 0.48 |
| To what extent have you felt that the therapist cared about your son/daughter? | 9.75 | 0.53 |
| How correct do you think the way they have worked to solve your son’s/daughter’s problems has been? | 9.75 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, J.C.; Valiente, R.M.; García-Escalera, J.; Arnáez, S.; Espinosa, V.; Sandín, B.; Chorot, P. Prevention of Depression and Anxiety in Subclinical Adolescents: Effects of a Transdiagnostic Internet-Delivered CBT Program. Int. J. Environ. Res. Public Health 2022, 19, 5365. https://doi.org/10.3390/ijerph19095365
Schmitt JC, Valiente RM, García-Escalera J, Arnáez S, Espinosa V, Sandín B, Chorot P. Prevention of Depression and Anxiety in Subclinical Adolescents: Effects of a Transdiagnostic Internet-Delivered CBT Program. International Journal of Environmental Research and Public Health. 2022; 19(9):5365. https://doi.org/10.3390/ijerph19095365
Chicago/Turabian StyleSchmitt, Julia C., Rosa M. Valiente, Julia García-Escalera, Sandra Arnáez, Victoria Espinosa, Bonifacio Sandín, and Paloma Chorot. 2022. "Prevention of Depression and Anxiety in Subclinical Adolescents: Effects of a Transdiagnostic Internet-Delivered CBT Program" International Journal of Environmental Research and Public Health 19, no. 9: 5365. https://doi.org/10.3390/ijerph19095365
APA StyleSchmitt, J. C., Valiente, R. M., García-Escalera, J., Arnáez, S., Espinosa, V., Sandín, B., & Chorot, P. (2022). Prevention of Depression and Anxiety in Subclinical Adolescents: Effects of a Transdiagnostic Internet-Delivered CBT Program. International Journal of Environmental Research and Public Health, 19(9), 5365. https://doi.org/10.3390/ijerph19095365









