Oral Health Status in Marfan Syndrome: A Systematic Review and Meta-Analysis of 353 Cases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
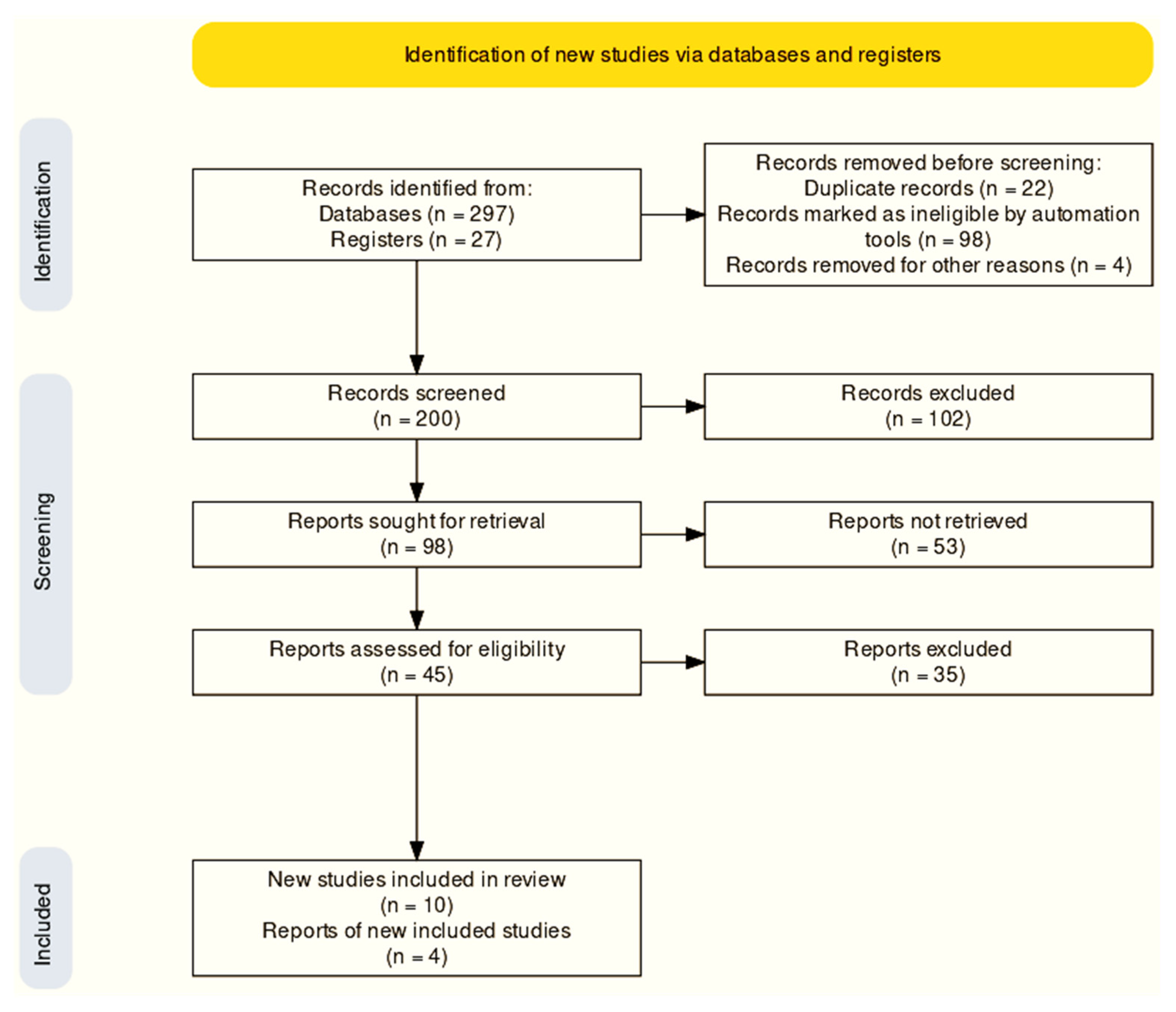
3.1. Selection of Studies
3.2. Study Characteristics
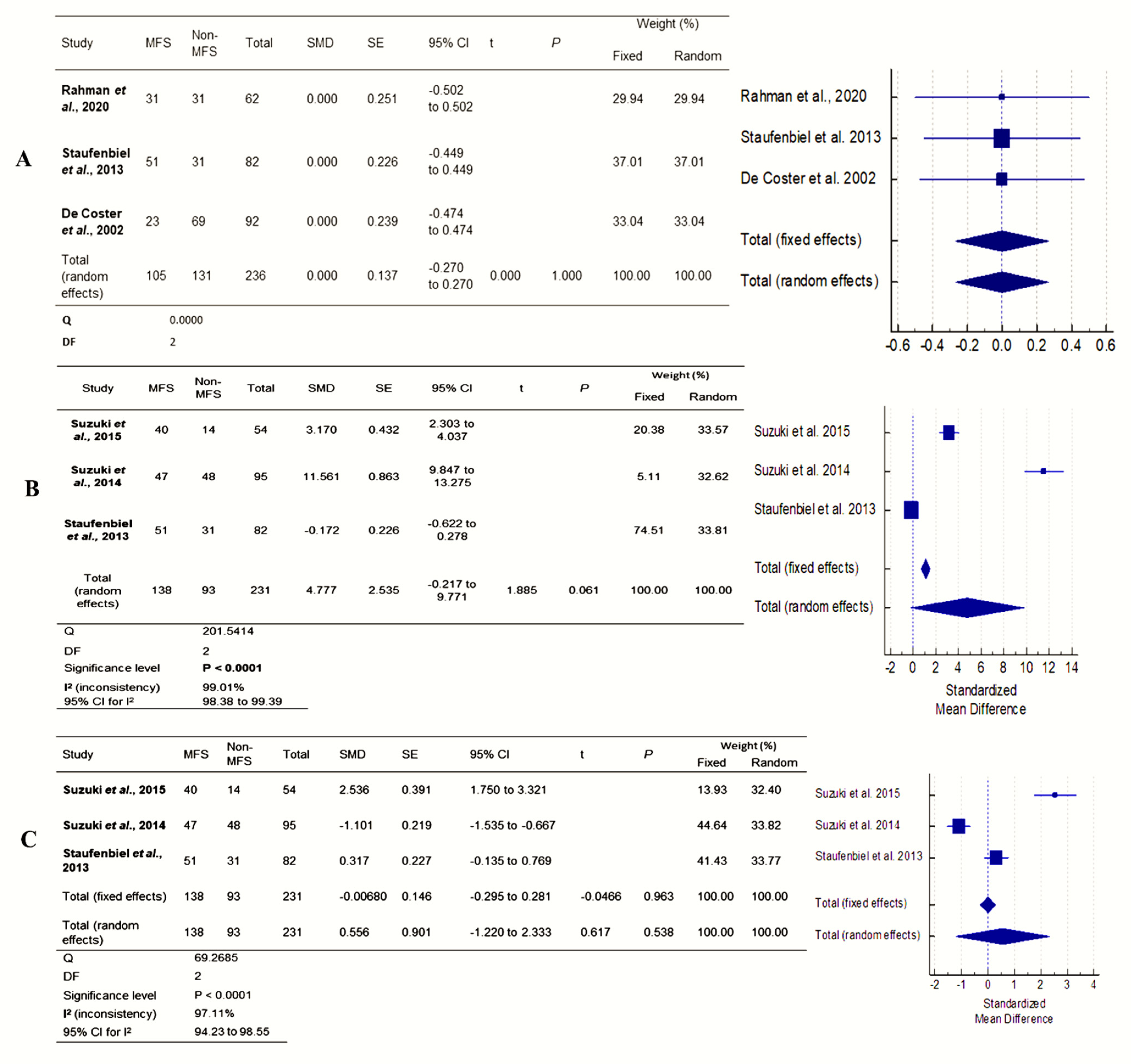
3.3. Meta-Analysis
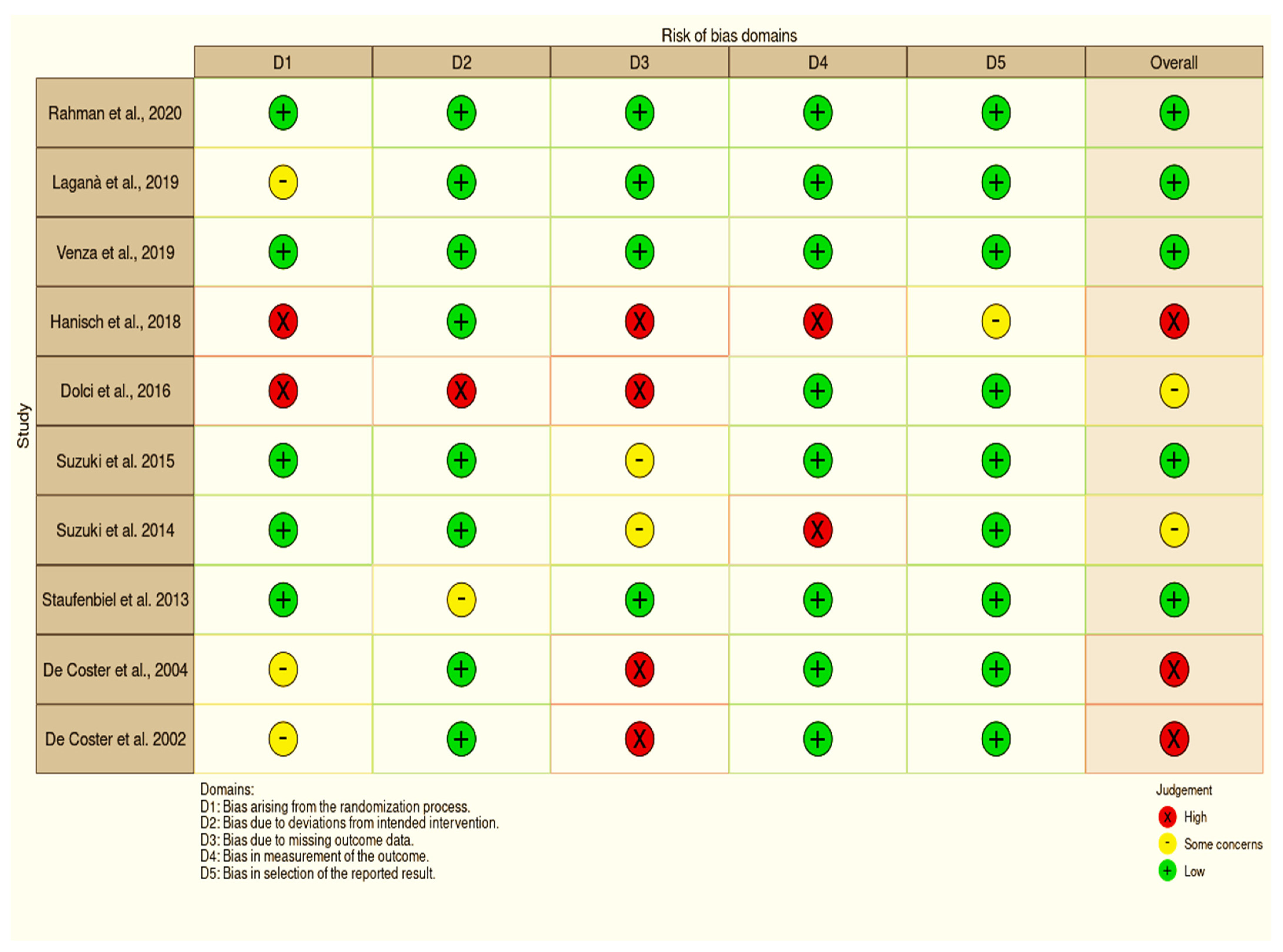
3.4. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Judge, D.P.; Rouf, R.; Habashi, J.; Dietz, H.C. Mitral valve disease in Marfan syndrome and related disorders. J. Cardiovasc. Transl. Res. 2011, 4, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Šípek, A., Jr.; Grodecká, L.; Baxová, A.; Cibulková, P.; Dvořáková, M.; Mazurová, S.; Magner, M.; Zeman, J.; Honzík, T.; Freiberger, T. Novel FBN1 gene mutation and maternal germinal mosaicism as the cause of neonatal form of Marfan syndrome. Am. J. Med. Genet. Part A 2014, 164, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Pyeritz, R.E. Marfan syndrome: Improved clinical history results in expanded natural history. Genet. Med. 2019, 21, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; De Backer, J.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammash, N.M.; Sundt, T.M.; Connolly, H.M. Marfan syndrome—Diagnosis and management. Curr. Probl. Cardiol. 2008, 33, 7–39. [Google Scholar] [CrossRef]
- Judge, D.P.; Dietz, H.C. Marfan’s syndrome. Lancet 2005, 366, 1965–1976. [Google Scholar] [CrossRef]
- Merk, D.R.; Chin, J.T.; Dake, B.A.; Maegdefessel, L.; Miller, M.O.; Kimura, N.; Tsao, P.S.; Iosef, C.; Berry, G.J.; Mohr, F.W.; et al. miR-29b participates in early aneurysm development in Marfan syndrome. Circ. Res. 2012, 110, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Staufenbiel, I.; Hauschild, C.; Kahl-Nieke, B.; Vahle-Hinz, E.; von Kodolitsch, Y.; Berner, M.; Bauss, O.; Geurtsen, W.; Rahman, A. Periodontal conditions in patients with Marfan syndrome—A multicenter case control study. BMC Oral Health 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Coster, P.J.; Martens, L.C.; De Paepe, A. Oral manifestations of patients with Marfan syndrome: A case-control study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 93, 564–572. [Google Scholar] [CrossRef]
- Nualart Grollmus, Z.C.; Morales Chávez, M.C.; Silvestre Donat, F.J. Periodontal disease associated to systemic genetic disorders. Med. Oral Patol. Oral Y Cirugía Bucal 2007, 12, 211–215. [Google Scholar]
- Straub, A.M.; Grahame, R.; Scully, C.; Tonetti, M.S. Severe periodontitis in Marfan’s syndrome: A case report. J. Periodontol. 2002, 73, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Nishi, S.E.; Alam, M.K. Effectiveness of Various Methods of Tooth Brush on Reduction of Plaque and Gingivitis in Orthodontic Patient: Meta-Analysis. Int. Med. J. 2017, 24, 150–153. [Google Scholar]
- Altman, D.G.; Egger, M.; Smith, G.D. Systematic reviews in health care: Meta-analysis in context. BMJ 2001. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Appl. Eng. Agric. 2014, 18, 727–734. [Google Scholar]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Huedo-Medina, T.B.; Sánchez-Meca, J.; Marin-Martinez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I² index? Psychol. Methods 2006, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Adam, K.; Winkler, N.; Schulz-Weidner, N.; Staufenbiel, I. Caries experience in children with Marfan syndrome—A non-interventional case-control study. DZZ Int. 2021, 5, 200. [Google Scholar]
- Laganà, G.; Fasciglione, G.F.; Biondi, A.; Coletta, M.; Ruvolo, G.; Cozza, P. Gelatinolytic activity in gingival crevicular fluid and saliva of growing patients with Marfan syndrome: A case-control study. BMC Oral Health 2019, 19, 161. [Google Scholar] [CrossRef] [Green Version]
- Venza, N.; Danesi, C.; Contò, D.; Fabi, F.; Mampieri, G.; Sangiuolo, F.; Laganà, G. Periodontal condition in growing subjects with Marfan Syndrome: A case-control study. PeerJ 2019, 7, e6606. [Google Scholar] [CrossRef]
- Hanisch, M.; Wiemann, S.; Jung, S.; Kleinheinz, J.; Bohner, L. Oral health-related quality of life in people with rare hereditary connective tissue disorders: Marfan syndrome. Int. J. Environ. Res. Public Health 2018, 15, 2382. [Google Scholar] [CrossRef] [Green Version]
- Dolci, C.; Pucciarelli, V.; Codari, M.; Marelli, S.; Trifiro, G.; Pini, A.; Sforza, C. 3D morphometric evaluation of craniofacial features in adult subjects with Marfan syndrome. In Proceedings of the 7th International Conference on 3D Body Scanning Technologies, Hometrica Consulting, Lugano, Switzerland, 30 November 2016; pp. 98–104. [Google Scholar]
- Suzuki, J.I.; Imai, Y.; Aoki, M.; Fujita, D.; Aoyama, N.; Tada, Y.; Akazawa, H.; Izumi, Y.; Isobe, M.; Komuro, I.; et al. High incidence and severity of periodontitis in patients with Marfan syndrome in Japan. Heart Vessel. 2015, 30, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.I.; Imai, Y.; Aoki, M.; Fujita, D.; Aoyama, N.; Tada, Y.; Wakayama, K.; Akazawa, H.; Izumi, Y.; Isobe, M.; et al. Periodontitis in cardiovascular disease patients with or without Marfan syndrome-a possible role of Prevotella intermedia. PLoS ONE 2014, 9, e95521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Coster, P.; Pauw, G.D.; Martens, L.; De Paepe, A. Craniofacial structure in Marfan syndrome: A cephalometric study. Am. J. Med. Genet. Part A 2004, 131, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Utreja, A.; Evans, C.A. Marfan syndrome—An orthodontic perspective. Angle Orthod. 2009, 79, 394–400. [Google Scholar] [CrossRef] [PubMed]
- von Kodolitsch, Y.; Rybczynski, M.; Vogler, M.; Mir, T.S.; Schüler, H.; Kutsche, K.; Rosenberger, G.; Detter, C.; Bernhardt, A.M.; Larena-Avellaneda, A.; et al. The role of the multidisciplinary health care team in the management of patients with Marfan syndrome. J. Multidiscip. Healthc. 2016, 9, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauss, O.; Neter, D.; Rahman, A. Prevalence of pulp calcifications in patients with Marfan syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, e56–e61. [Google Scholar] [CrossRef]
- Tsang, A.K.; Taverne, A.; Holcombe, T. Marfan syndrome: A review of the literature and case report. Spec. Care Dent. 2013, 33, 248–254. [Google Scholar] [CrossRef]
- Mallineni, S.K.; Jayaraman, J.; Yiu, C.K.; King, N.M. Concomitant occurrence of hypohyperdontia in a patient with M arfan syndrome: A review of the literature and report of a case. J. Investig. Clin. Dent. 2012, 3, 253–257. [Google Scholar] [CrossRef]
- Suda, N.; Shiga, M.; Ganburged, G.; Moriyama, K. Marfan syndrome and its disorder in periodontal tissues. J. Exp. Zool. Part B Mol. Dev. Evol. 2009, 312, 503–509. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontology 2000 2013, 62, 59–94. [Google Scholar] [CrossRef]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontology 2000 2016, 70, 142–163. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, K.; Kajiwara, T.; Ishii, K.; Hasegawa, T.; Suzuki, S.; Nakano, M.; Sawaguchi, M.; Venkataiah, V.S.; Yahata, Y.; Ito, K.; et al. Periodontal Disease Risk in Marfan Syndrome Patients Harboring a Possible Aortic Aneurysm or Dissection. Available online: https://www.researchsquare.com/article/rs-1368972/v1 (accessed on 2 March 2022).
- Snyder, H. Literature review as a research methodology: An overview and guidelines. J. Bus. Res. 2019, 104, 333–339. [Google Scholar] [CrossRef]
- Alam, M.K.; Alfawzan, A.A.; Srivastava, K.C.; Shrivastava, D.; Ganji, K.K.; Manay, S.M. Craniofacial morphology in Apert syndrome: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 5708. [Google Scholar] [CrossRef] [PubMed]
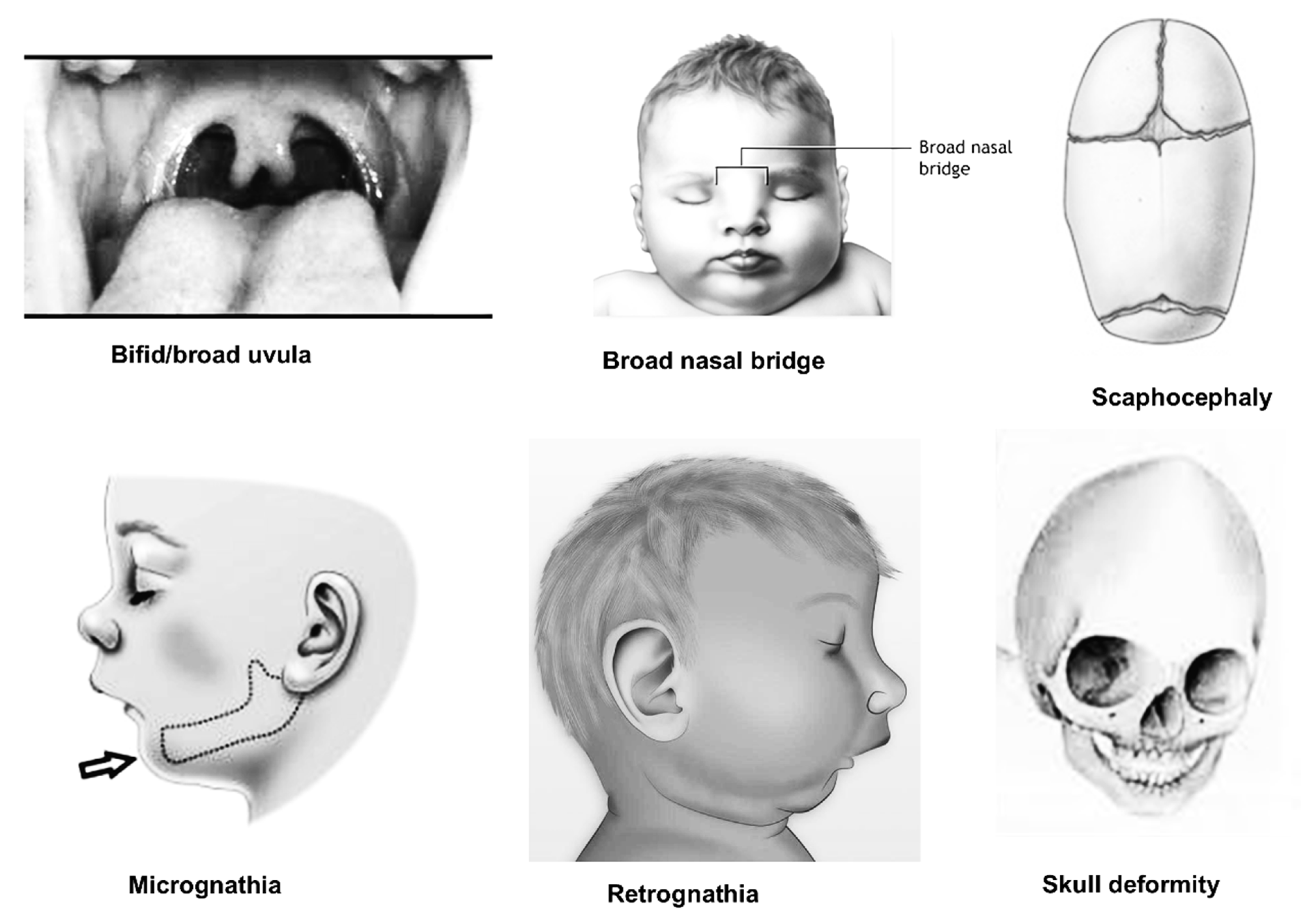

| No | Author (Year) | Country | Study Design | Participants | Age Range (Years) | Sex (M/F) | Method | Findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Rahman et al., 2020 [17] | Germany | Case-control | MFS: 31 Non-MFS: 31 | MFS: 8.77 ± 3.72 Non-MFS: 9.77 ± 3.72 | MFS = M:13; F:18 Non-MFS = M:13; F:18 | DMFT Caries restoration index Hygiene index | • Children and adolescents with MFS did not show a higher caries experience compared to a systemically healthy control group. |
| 2 | Laganà et al., 2019 [18] | Italy | Case-control | MFS: 28 Non-MFS: 23 | MFS: 8.4 ± 2.3 Non-MFS: 8.9 ± 2.9 | MFS = M:17; F:11 Non-MFS = M:12; F:11 | Zymography Western immunoblot | • Indicators of MMP activity included saliva and gingival crevicular fluid (GCF). • Periodontal matrix and inflammatory response can be significantly altered by even small variations in MMP-13 activity. |
| 3 | Venza et al., 2019 [19] | Italy | Case-control | MFS: 16 Non-MFS: 20 | MFS: 9.4 ± 2.3 Non-MFS: 10.0 ± 2.6 | MFS = M:9; F:7 Non-MFS = M:8; F:12 | Plaque index Bleeding on probing (BOP) Modified periodontal screening and recording | • Patients with MFS revealed a higher presence of plaque and consequently a generalized inflammation in the oral cavity. |
| 4 | Hanisch et al., 2018 [20] | Germany | Cross-sectional survey | MFS: 51 | MFS: 42.73 ± 14.50 | MFS = M:17; F:11 | OHIP-14 (Oral Health Impact Profile) questionnaire | • People with Marfan syndrome had a higher OHIP score than the German general public, and the vast majority of responders reported oral symptoms as a result of the disorder. Female individuals had lower OHIP-14 scores than male participants. |
| 5 | Dolci et al., 2016 [21] | Italy | Case-control | MFS: 49 Non-MFS: 661 | MFS: 18–60 Non-MFS: matched | MFS = M:18; F:31 Non-MFS = M:332; F:329 | 50 soft-tissue facial anthropometric landmarks Three-dimensional facial image using a stereophotogrammetric system | • The mandibular ramus was shorter in 96% of MFS participants compared to non-MFS subjects, and facial divergence was larger in 100% of MFS subjects. |
| 6 | Suzuki et al., 2015 [22] | Japan | Case-control | MFS: 40 Non-MFS: 14 | MFS: 34.9 ± 2.0 Non-MFS: 32.4 ± 2.2 | MFS = M:23; F:17 Non-MFS = M:10; F:4 | Periodontal status, BOP, Pocket depth | • The MFS patients and the control group had comparable pocket depths and bleeding on probing. MFS patients had a high rate of periodontitis and cardiovascular problems. |
| 7 | Suzuki et al., 2014 [23] | Japan | Case-control | MFS: 47 Non-MFS: 48 | MFS: 35.2 ± 1.8 Non-MFS: 33.5 ± 0.9 | MFS = M:29; F:18 Non-MFS = M:29; F:19 | Periodontal status, BOP, Pocket depth | • Periodontitis influenced the pathophysiology of cardiovascular complications in MFS patients. A specific periodontal pathogen might be a crucial therapeutic target to prevent CVD development. |
| 8 | Staufenbiel et al., 2013 [8] | Germany | Case-control | MFS: 51 Non-MFS: 31 | MFS: 40.20 ± 15.32 Non-MFS: 40.29 ± 13.94 | MFS = M:21; F:30 Non-MFS = M:14; F:17 | DMFT Periodontal status, BOP, Pocket depth | • Due to their overcrowded teeth, MFS patients had a tendency to display greater indicators of inflammation. For this reason, a six-month interval between professional dental cleanings is recommended to minimize the bacterial biofilm in the oral cavity, which in turn reduces the risk of systemic disorders, such as endocarditis. |
| 9 | De Coster et al., 2004 [24] | Belgium | Case-control | MFS: 17 Non-MFS: 32 | MFS: 31.4 ± 11.4 Non-MFS: matched | MFS = M:23%; F:77% Non-MFS = M:23%; F:77% | Lateral cephalometric radiographs Fourteen landmarks | • The cranial basis, the maxillary complex, the mandible body, and the jaws’ relationship to the cranial base and to each other showed significant disparities in the control group. • In MFS, the palatal height and palatal length were considerably bigger, and the height of the maxilla-alveolar processes was significantly associated to both. |
| 10 | De Coster et al., 2002 [9] | Belgium | Case-control | MFS: 23 Non-MFS: 69 | MFS: 9–53 Non-MFS: 9–53 | MFS = M:14; F:9 Non-MFS = M:42; F:27 | DMFT Gingival index | • MFS revealed a considerable number of enamel abnormalities, most of which were local hypoplastic spots, which may have been caused by local trauma or infection. MFS patients were more likely to have irregular pulp shape, root deformities, and pulp inclusions, especially when all three occurred together. Gingivitis was substantially worse in the MFS group than in the control group. |
| References | Selection | Comparability | Exposure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Rahman et al., 2020 [17] | * | * | * | * | * | * | * | * | - |
| Laganà et al., 2019 [18] | * | * | * | * | * | * | * | * | - |
| Venza et al., 2019 [19] | * | * | * | * | * | * | * | * | - |
| Hanisch et al., 2018 [20] | * | * | * | * | * | * | * | ||
| Dolci et al., 2016 [21] | * | * | * | - | * | * | - | * | - |
| Suzuki et al., 2015 [22] | * | * | * | - | * | * | * | * | - |
| Suzuki et al., 2014 [23] | * | * | * | - | * | * | * | * | - |
| Staufenbiel et al., 2013 [8] | * | * | * | - | * | * | * | * | - |
| De Coster et al., 2004 [24] | * | * | * | - | * | * | - | * | - |
| De Coster et al., 2002 [9] | * | * | * | - | * | * | - | * | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.K.; Alfawzan, A.A.; Shrivastava, D.; Srivastava, K.C.; Alswairki, H.J.; Mussallam, S.; Abutayyem, H.; Ahmed, N. Oral Health Status in Marfan Syndrome: A Systematic Review and Meta-Analysis of 353 Cases. Int. J. Environ. Res. Public Health 2022, 19, 5048. https://doi.org/10.3390/ijerph19095048
Alam MK, Alfawzan AA, Shrivastava D, Srivastava KC, Alswairki HJ, Mussallam S, Abutayyem H, Ahmed N. Oral Health Status in Marfan Syndrome: A Systematic Review and Meta-Analysis of 353 Cases. International Journal of Environmental Research and Public Health. 2022; 19(9):5048. https://doi.org/10.3390/ijerph19095048
Chicago/Turabian StyleAlam, Mohammad Khursheed, Ahmed Ali Alfawzan, Deepti Shrivastava, Kumar Chandan Srivastava, Haytham Jamil Alswairki, Samir Mussallam, Huda Abutayyem, and Naseer Ahmed. 2022. "Oral Health Status in Marfan Syndrome: A Systematic Review and Meta-Analysis of 353 Cases" International Journal of Environmental Research and Public Health 19, no. 9: 5048. https://doi.org/10.3390/ijerph19095048
APA StyleAlam, M. K., Alfawzan, A. A., Shrivastava, D., Srivastava, K. C., Alswairki, H. J., Mussallam, S., Abutayyem, H., & Ahmed, N. (2022). Oral Health Status in Marfan Syndrome: A Systematic Review and Meta-Analysis of 353 Cases. International Journal of Environmental Research and Public Health, 19(9), 5048. https://doi.org/10.3390/ijerph19095048