Abstract
A previous pooled analysis demonstrated significant relief of breathlessness following opioid administration in patients with chronic obstructive pulmonary disease. However, in clinical practice, it is important to know the characteristics of patients responding to opioids, the best prescription methods, and the evaluation measures that can sufficiently reflect these effects. Thus, we performed a systematic review of systemic opioids for non-cancer chronic respiratory diseases. Fifteen randomized controlled studies (RCTs), four non-randomized studies, two observational studies, and five retrospective studies were included. Recent RCTs suggested that regular oral opioid use would decrease the worst breathlessness in patients with a modified Medical Research Council score ≥ 3 by a degree of 1.0 or less on a scale of 1–10. Ergometer or treadmill tests indicated mostly consistent significant acute effects of morphine or codeine. In two non-randomized studies, about 60% of patients responded to opioids and showed definite improvement in symptoms and quality of life. Furthermore, titration of opioids in these studies suggested that a major proportion of these responders had benefits after administration of approximately 10 mg/day of morphine. However, more studies are needed to clarify the prescription method to reduce withdrawal due to adverse effects, which would lead to significant improvements in overall well-being.
1. Introduction
A recent global survey found that chronic respiratory diseases were the third leading cause of death in 2017 [1]. Furthermore, advanced chronic respiratory diseases impair quality of life (QoL) as severely as cancer before death [2]. Improvement in palliative care is therefore an important issue for these diseases.
Breathlessness is one of the most frequent symptoms of advanced chronic respiratory diseases [3,4,5]. Management of the causal diseases is necessary for the relief of dyspnea, such as inhaled steroids for asthma and bronchodilators for chronic obstructive pulmonary disease (COPD). Pulmonary rehabilitation is also available to improve exercise tolerance and relieve mental distress in advanced COPD [6,7]. In addition to these measures, systemic opioids are an important alternative for intractable breathlessness [8,9]. A previous pooled analysis revealed a significant improvement in breathlessness due to opioids in patients with COPD [10].
However, recent randomized controlled trials (RCTs) did not demonstrate any significant differences in many outcomes between opioids and placebo [11,12]. The inconsistency of these results could be a barrier to prescribing opioids for breathlessness in cases of non-cancer disease [13]. In Japan, opioids for dyspnea have not been approved by the national insurance system, possibly owing to the absence of clear evidence of their efficacy. To establish the evidence of opioids as a treatment for breathlessness, the cause of these inconsistencies should be clarified systematically, and the subgroups in which many patients do respond well to opioids should be demonstrated.
In clinical practice, physicians and patients experience difficulty in palliating breathlessness when chronic respiratory diseases, such as COPD and interstitial lung disease (ILD), advance to breathlessness corresponding to a modified Medical Research Council (mMRC) score ≥ 3 or 4. The expected efficacy of opioids should be determined for these patients. Simultaneously, both physicians and patients with non-cancer diseases prioritize the prevention of adverse events that can affect daily activities or prognosis. The safest prescription methods should be pursued in order to break the barriers that prevent the use of opioids for the palliation of breathlessness in non-cancer diseases.
For these reasons, we performed a systematic review of articles that evaluated the effects of opioids in advanced chronic respiratory diseases.
2. Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [14]. The study protocol was registered in the PROSPERO database (CRD42021267919).
2.1. Data Sources
We searched and identified articles described in English in MEDLINE (from 1980 to October 2020) and the Cochrane Library (from 1980 to October 2020). The search strategy is provided in the online Supplementary Materials (Table S1).
2.2. Study Selection
We searched for studies that evaluated the effect of oral or parenteral opioids as interventions, excluding nebulized opioids. The participants had respiratory symptoms, primarily due to chronic respiratory diseases other than cancer. We included studies that partially involved participants with chronic diseases of other organs or malignant tumors. The outcomes included clinical changes in respiratory symptoms, health-related QoL, and exercise tolerance after opioid administration. The study types were RCTs, non-randomized, observational, and retrospective studies involving >5 patients.
We excluded studies that exclusively involved respiratory symptoms due to cancer or chronic diseases of other organs, such as cardiac and neurological diseases. We also excluded studies that exclusively focused on the adverse effects of opioids or opioid prescription rates. Qualitative studies were also excluded.
YY and KMSUR screened the titles and abstracts of all studies identified by the search strategy and performed full-text assessments to identify studies for inclusion. Disagreements were resolved by discussion with a third reviewer, HO, or MN. Referring to the studies selected in previous systematic reviews, YY assessed for disagreements with our study selection. Regarding the studies that were not selected in our search strategy, at least two review authors evaluated eligibility for inclusion as hand-searched studies.
2.3. Data Extraction and Analysis
We extracted data on background respiratory diseases, baseline dyspnea level, opioid classifications, opioid dosage, duration of the intervention, breathlessness after the intervention, exercise tolerance after the intervention, QoL after the intervention, and adverse effects of opioids. Data extraction was first performed by a reviewer and then checked by another reviewer.
Data are presented descriptively. As the studies had significant heterogeneity and data varied between studies, we performed a narrative review to assess the differences in response to opioids among various severities of dyspnea, evaluation measures, and opioid prescriptions.
2.4. Risk of Bias Assessment
As for RCTs, risk of bias assessment was performed according to the Cochrane Risk of Bias (ROB) tool [15]. For studies other than RCTs, the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS) was used [16]. Two review authors independently assessed the risk of bias of the included studies and any discrepancies were resolved through discussion. The domains considered at ROB were random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other bias, whereas the RoBANS tool considered selection of participants, confounding variables, measurement of exposure, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting.
3. Results
3.1. Characteristics of Selected Studies
A total of 713 publications were identified in the initial search, of which 30 were suitable for inclusion in the systematic review. We also referenced two previous systematic reviews on opioids in respiratory diseases [10,17], and two studies [18,19] that were not selected through our database search were included. Therefore, 32 articles were included in this systematic review (Figure 1).
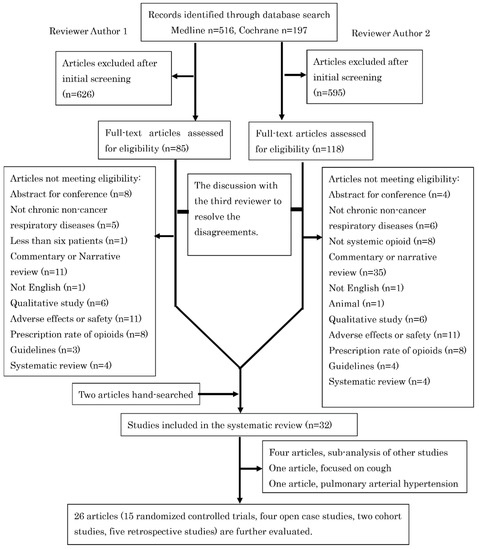
Figure 1.
The study selection process of this systematic review.
Among the five articles [20,21,22,23,24] that demonstrated sub-analysis of the previous studies [11,25,26], four [20,22,23,24] did not provide any additional information on breathlessness, QoL, or exercise tolerance and were excluded from further review. The article by Smith J. et al. [27] evaluated the effect of opioids exclusively on coughing and was excluded from further review. The article by Ferreira D.H. et al. [28] was the only study that evaluated pulmonary arterial hypertension, in which morphine did not reduce breathlessness. Because pulmonary arterial hypertension would differ in the pathogenesis of breathlessness from other chronic respiratory diseases, this study was also excluded from further review. The characteristics of the remaining 15 RCTs are shown in Table 1, Table 2 and Table 3. Further to the studies already mentioned, there were four non-randomized studies (Table 4 and Table 5), two observational studies (Table 4 and Table 5), and five retrospective studies (Table 6).

Table 1.
Characteristics of randomized controlled studies regarding regularly used systemic opioids for non-cancer chronic respiratory disease.

Table 2.
Outcomes of randomized controlled studies regarding regularly used systemic opioids for non-cancer chronic respiratory disease.

Table 3.
Randomized controlled studies regarding acute effects of systemic opioids for non-cancer chronic respiratory disease.

Table 4.
Characteristics of non-randomized or observational studies of systemic opioids for non-cancer chronic respiratory disease.

Table 5.
Outcomes of non-randomized or observational studies of systemic opioids for non-cancer chronic respiratory disease.

Table 6.
Retrospective studies of systemic opioids for non-cancer chronic respiratory diseases.
3.2. RCT Studies of Opioids to Reduce Breathlessness Due to COPD
Two relatively large-scale RCTs have recently reported no significant differences in the clinical scores of breathlessness between opioids and placebo [11,12]. The sub-analysis of patients with mMRC score ≥ 3 demonstrated that the numerical rating scale (NRS) or visual analog scale (VAS) of the worst breathlessness was significantly reduced in patients who received opioids compared to those who received placebo in both studies.
There were four other RCTs that recruited patients with dyspnea corresponding to mMRC ≥ 3. Abdallah S.J. et al. [34] showed that morphine reduced exertional breathlessness in cardiopulmonary cycle exercise testing (Table 3). Woodcock A.A. et al. [18] also demonstrated that dihydrocodeine significantly improved subjective disability by measuring the oxygen cost diagram, although the attrition bias in this study was high owing to the high percentage of withdrawal after taking dihydrocodeine due to side effects (Table 1 and Table 2). A crossover study conducted by Abernethy A.P. et al. [25] was the only RCT that recruited patients with breathlessness at rest and indicated that the VAS score of breathlessness was significantly reduced under sustained-release (SR) morphine compared to placebo (Table 1 and Table 2). Contrary to the consistent effectiveness of morphine or dihydrocodeine for dyspnea of mMRC ≥ 3, the study by Ferreira D.H. et al. [21], which is derived from the immaturely ceased portion of the same trial as that by Currow D. et al. [12], did not demonstrate any difference in breathlessness scores between oxycodone and placebo, including VAS score of the worst breathlessness in the previous 24 h. No significant improvement in breathlessness was shown by regularly used opioids in any studies which included patients with dyspnea scores corresponding to a mMRC of 2, or those which had no criteria of enrollment regarding dyspnea (Table 1 and Table 2).
Notably, the average degree of improvement in breathlessness caused by opioids was low. The difference in the worst breathlessness in the previous 24 h was −1.33 (95% confidence interval, −2.50, −0.16) on the NRS (0−10) in COPD patients with dyspnea of mMRC ≥ 3 in the study by Verberkt C.A. et al. [11]. The differences in other studies [12,21,25,29,31,33,36] were even smaller. Studies that used rougher scales, such as the studies by Poole PJ et al. [30] and Munck L.K. et al. [32], did not detect significant differences in breathlessness between opioids and placebo.
3.3. RCT Studies of Opioids to Improve Exercise Tolerance
There were no RCT studies of regularly used opioids which indicated significant differences in exercise tolerance favoring opioids (Table 1) [11,18,29,30,31,33]. Poole P.J. et al. [30] indicated that the distance in the 6MWT was significantly shorter under morphine. In contrast to these negative results, five of the seven studies on the acute effects of opioids [19,29,31,34,35,36,37] indicated significantly better results, except for the study of diamorphine using the 6MWT by Eiser N. et al. [31] and the small study by Light R.W. et al. [19] (Table 3). The study by Johnson M.A. et al. [36] also provided instructions to consume a tablet of dihydrocodeine or placebo 30 min before exercise, and the mean number of tablets used during the dihydrocodeine week was 2.8 tablets, possibly reflecting an acute effect.
The evaluation measure is another factor that can reflect the effectiveness of opioids in exercise tolerance. Six studies [11,18,29,30,31,33] evaluated exercise tolerance by using a 6 min walk test (6MWT) or 12 min walk test (12MWT). There were no significant differences in the 6MWT or 12MWT distances favoring opioids compared with the placebo groups. While the 6MWT results by Kronborg-White S. et al. [29] indicated significantly better changes on the Borg scale 1 h after the first immediate-release (IR) morphine intake, there were no other studies that indicated differences in breathlessness after 6MWT between groups that received opioids and placebo [30,31]. In contrast, four of the six studies that evaluated exercise tolerance using a cycle ergometer or treadmill [19,31,34,35,36,37] showed significantly better results in patients taking opioids [34,35,36,37].
Only one study has evaluated the effect of regularly used opioids on exercise tolerance using a cycle ergometer or treadmill, which demonstrated no significant differences in exercise tolerance or breathlessness between diamorphine and placebo [31].
3.4. RCT Studies of Opioids to Improve QOL in COPD
Verberkt C.A. et al. [11] indicated that COPD assessment test scores were significantly reduced in patients who received morphine compared to those who received placebo, favoring morphine. However, no other studies [12,21,29,30] have indicated significant differences in QoL between opioid and placebo groups (Table 5). A study by Poole P.J. et al. [30] indicated a significant worsening of chronic respiratory questionnaire (CRQ) mastery and a non-significant improvement in CRQ dyspnea in the morphine group.
3.5. The Response Rate of Opioids in RCT Studies
It is important in clinical practice to know the percentage of patients with dyspnea who prefer opioids. However, only a few studies have addressed this issue. Among the studies of patients with mMRC ≥ 3, Abdallah S.J. et al. [34] demonstrated that 75% preferred morphine over a placebo during the exercise test (Table 7). In the study by Verberkt C.A. et al. [11], 48% of the patients with mMRC ≥ 2 in the morphine group showed an improvement of ≥1 NRS, which was not significantly different from that of the placebo group (35%) (Table 2). The study by Currow D. et al. [12] demonstrated that the percentage of patients reporting less breathlessness during the past week was not different between opioid and placebo groups (48.5% vs. 49.3%). However, the participants in the placebo group also received rescue morphine in this study (Table 7).

Table 7.
Quality of life (QoL) or other comprehensive assessments in randomized controlled studies.
3.6. Non-Randomized or Observational Studies of Opioids for Chronic Respiratory Diseases
All four non-randomized studies indicated beneficial effects of opioids in patients with chronic respiratory diseases [26,38,39,40] (Table 4 and Table 5). In a study by Rocker GM et al. [38], 61% of the participants answered that opioid treatment was helpful at 4–6 months. Among these responders, nearly half answered that opioid treatment was very helpful using a 5-point Likert scale. Likewise, 63% of the patients with dyspnea of mMRC ≥ 3 showed a ≥10% reduction in the VAS score of breathlessness without unacceptable side effects in the study by Currow DC et al. [26]. An observational study by Smallwood N et al. [41] also demonstrated that 41.9% self-reported being highly compliant with morphine treatment, although the severity of baseline dyspnea was not indicated in this study (Table 4 and Table 5).
The non-randomized study by Currow DC [26] showed a small improvement in breathlessness in the enrolled patients (Table 5), which was consistent with that reported in RCTs. However, the median NRS score for dyspnea was 2.0 lower after morphine intake than before morphine intake in the patients who continued treatment, that is, the responders to opioids included in the study by Rocker GM et al. [38]. These patients also showed a significant improvement in their CRQ scores (Table 5). Likewise, 52 responders to opioids showed a larger reduction in the VAS score for breathlessness in the study by Currow DC [26] (Table 5).
3.7. Retrospective Study of Opioids for Chronic Respiratory Diseases
All five retrospective studies [5,43,44,45,46] indicated beneficial effects of opioids in patients with ILD (Table 6). The studies by Takeyasu M. et al. [43] and Matsuda Y. et al. [44] reported the effect of continuous intravenous infusion or continuous subcutaneous infusion of morphine in advanced ILD.
3.8. The Required Dosage of Opioids to Observe Benefits
Five studies determined the oral opioid dosage by titration [11,26,30,38,46]. Among them, the study by Verberkt C.A. et al. [11] started titration from 20 mg/day of morphine, and thus, this study could not determine the effect of a lower dosage. The study by Poole P.J. et al. [30] titrated morphine from 10 mg/day to 40 mg/day if there were no adverse effects or if the adverse effects were minor, and thus, this study could not determine the beneficial effect of a lower dosage (Table 1).
A non-randomized study by Rocker G.M. et al. [38] examined treatment with 2 mg of IR morphine every 4 h during the daytime, and reported that 30% and 45% of participants stated that this was very helpful or somewhat helpful, respectively. Likewise, 69.2% of the responders benefitted from 10 mg/day of SR morphine after titration from 10 mg/day in the study by Currow D. et al. [26]. These findings were consistent with those of the study by Allcroft P. [39], which revealed the beneficial effect of 10 mg/day of morphine with clonazepam (Table 4 and Table 5). However, a retrospective study by Colman R. et al. [46] showed that the median oral morphine equivalent was 30 mg/day in those who were taking SR opioids after titration from a low dose, although a precise titration protocol was not indicated (Table 6).
3.9. Adverse Events of Opioid Treatment
There have been no evident reports of severe fatal adverse events. However, the completion rate of the opioid group was approximately 10-20% lower than that of the placebo group in most RCTs regarding regularly used opioids (Table 1). Among eight RCTs, only that by Kronborg-White S. et al. [29] demonstrated a completion rate higher than 90%. Abernethy A.P. et al. [25] also showed that the completion rate of the opioid group was equal to that of the placebo group. In a non-randomized study by Currow DC [26], 18.1% withdrew owing to adverse events. Meanwhile, only 6.8% withdrew owing to adverse events in the study by Rocker G.M. et al. [38].
3.10. Risk of Bias of Included Studies
The risk of bias is shown in the online Supplement (Tables S2 and S3). The random sequence generation was mentioned in only six RCTs [11,12,21,25,29,30] and was marked as unclear risk of bias in the rest of the studies. Allocation concealment was not mentioned in most of the RCTs and only three articles were marked as low risk of bias [12,21,25]. Most of the studies mentioned the blinding of participants and personnel, but six studies did not [18,25,30,31,36,37]. Only one article [35] was rated as high risk of bias in this domain. None of the articles mentioned the blinding of outcome assessors and we rated that as unclear risk of bias. Five of the RCTs were rated as high risk of bias for attrition [18,30,31,33,34]. All the RCTs were rated as low risk of bias for selective reporting and other bias.
Similar to the RCTs, blinding of the outcome assessors was not mentioned in any of the case studies, cohort studies, or retrospective studies. All the studies were rated as low risk of bias for performance bias, incomplete outcome data reporting, and selective outcome reporting. Selection of participants was rated as low risk of bias in only three studies [26,41,42], and rated as high risk of bias in the rest of the studies. Only one article did not mention the confounding factors [39], and the rest of the articles were rated as low risk of bias.
4. Discussion
In this systematic review, we found consistent findings among heterogeneous studies on opioid use in chronic respiratory diseases. Figure 2 summarizes these findings. Although a previous meta-analysis revealed the beneficial effects of opioids in advanced COPD, it was difficult to elucidate why some studies did not show any improvement in dyspnea due to opioids. Furthermore, previous systematic reviews of opioids for dyspnea did not include recent large-scale RCTs. Additionally, we analyzed non-randomized and retrospective studies because these studies also demonstrated some important findings that could not be indicated in RCTs.
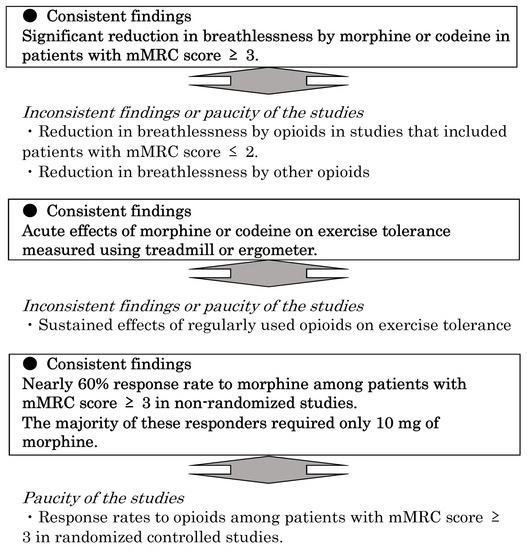
Figure 2.
Consistent findings among heterogeneous studies of opioids for breathlessness due to non-cancer chronic respiratory diseases. mMRC, modified Medical Research Council.
We revealed that the worst breathlessness could be improved by morphine treatment in COPD patients with a mMRC of ≥3 [11,12]. This is consistent with the findings of a previous study that indicated higher baseline breathlessness intensity as a predictor of beneficial responses to opioids [47]. The degree of improvement was also almost same in the two RCTs, approximately 1.0 in 0–10 NRS [11,12]. However, it should be noted that responders to opioids showed clinical improvement more clearly in the two observational studies [26,38]. These findings are important to understand how the effects of opioids should be evaluated in clinical practice.
Regarding the effect on exercise tolerance, the acute effect of morphine or codeine was generally confirmed in ergometer and treadmill studies [34,35,36,37]. However, the 6MWT and 12MWT findings were negative in patients using opioids regularly [11,18,29,30,31,33]. Regrettably, only one study [31] evaluated exercise capacity using a treadmill in patients regularly using opioids. This study used diamorphine but not morphine or codeine. Therefore, it is challenging to determine the differences between the acute and long-term effects of opioids.
Non-randomized studies are also important for determining the percentage of patients responding to opioids and their required dosage in clinical practice [26,38]. Although there is a high risk of the placebo effect, these studies would be appropriately applied to actual practice from the viewpoint of the severity of dyspnea in patients. Likewise, retrospective studies [5,43,44,45] of patients with ILD before death are also important, because no RCT involving these patients has been reported.
Although there was no serious depression of ventilation due to opioids, as indicated in previous systematic reviews [48], the low completion rate of opioid arms in RCTs could be a barrier to recommending opioids in clinical practice. In this systematic review, we could not identify factors that improved the completion rate in some studies [25,29]. Titration from a very low dose of IR morphine to SR morphine would reduce withdrawal due to adverse effects [38], while titration from SR morphine (10 mg) does not prevent adverse effects sufficiently [26,30]. However, other factors, such as the severity of baseline dyspnea, experience with prescribing opioids, and other non-pharmacological support, would also affect the continuation of opioid therapy.
Johnson M.A. et al. [36] also demonstrated relief of breathlessness and a high completion rate without any difference in the occurrence of adverse events from placebo by using dihydrocodeine before exercise. The relatively consistent findings of acute effects of opioids on exercise tolerance would also support the effectiveness of the as-needed use of opioids, although the onset of the effect of IR morphine might be too late for rescue use. No study has evaluated the long-term effects of the as-needed use of IR opioids for chronic respiratory diseases [49,50].
Opioids are currently prescribed for intractable breathlessness in non-cancer respiratory diseases without clear evidence. Although most of our findings were based on a limited number of studies, they provide suggestions for better prescription strategies for opioids in clinical practice. We clearly presented evidence of the effectiveness of morphine or codeine for COPD patients with a mMRC ≥ 3, which can be a cutoff point to consider when initiating opioids. Although adverse events are still a major obstacle to opioid use, the consistent findings regarding the acute effects of opioids on exercise tolerance support the initiation of as-needed low-dose IR morphine or codeine by setting the maximum number of uses. A gradual increment of a very low dose (<10 mg) of morphine would reduce the withdrawal of opioid usage due to adverse events. Nearly 60% responded well to morphine, and the majority of these responders required only low-dose morphine (approximately 10 mg). Although the reports of this response rate were affected by placebo effects, these findings could be directly applied in actual practice.
This systematic review has some limitations. Although some subgroups showed consistent findings in our analysis, the importance of the factors that separated the groups, such as dyspnea severity, opioid classification, acute effects of opioids, and evaluation measures of exercise tolerance, must be confirmed in future prospective studies. Because we could not determine the distribution of dyspnea severity in each study, it is uncertain whether our groupings reflected the actual dyspnea severity of the patients. Additionally, the number of the studies in each subgroup was small. In particular, the absence or small number of applicable studies prevented evaluation of some important issues, such as the effects of opioids other than morphine or codeine, usefulness of the 6MWT for measuring opioid effects, the effect of regularly used opioids on exercise tolerance, and the best prescription method to reduce adverse events.
These limitations could also be related to inconsistency regarding the improvement in QoL by opioids. Further studies are needed to clarify the prescription method to improve overall well-being by using opioids in patients with advanced chronic respiratory diseases.
5. Conclusions
Among the heterogeneous studies on opioid use in chronic respiratory diseases, patients with dyspnea corresponding to a mMRC ≥ 3 consistently showed beneficial effects of morphine or codeine. The acute effects of morphine and codeine were also consistently observed in ergometer and treadmill studies. Because a majority of the responders benefitted from low-dose morphine in non-randomized studies, as-needed or regular use of low-dose morphine would be recommended as the first option for severe dyspnea in non-cancer patients. Further studies are needed to clarify the optimal prescription methodology to reduce withdrawal due to adverse effects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19084907/s1, Table S1: Research strategy in MEDLINE and Chochrane; Table S2: Bias risk of the randomized controlled studies; Table S3: Bias risk of non-randomized studies, observational studies, five retrospective studies.
Author Contributions
Y.Y. contributed to conceptualization, data curation, formal analysis, methodology, and writing. K.M.S.-U.-R. contributed to data curation, formal analysis, and writing. M.N. and H.O. contributed to data curation. Y.H., T.Y. and S.H. contributed to conceptualization. H.M. contributed to project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number 19dk0110038h0001, 20dk0110038h0002, and 21dk0110038s0303.
Institutional Review Board Statement
This study does not involve human participants.
Informed Consent Statement
This study does not involve human participants.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
We would like to thank the International Medical Information Center (Tokyo, Japan) for their assistance in the article search.
Conflicts of Interest
All authors have declared potential conflicts of interest as follows: T.Y. belongs to an endowed chair funded by donations from Kazuteru Noguchi, JSH, Towa Pharmaceutical, Sawai Pharmaceutical, AKTIO, and Ain Pharmaciez. The other authors declare no conflict of interest associated with this manuscript.
References
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Gore, J.M.; Brophy, C.J.; Greenstone, M.A. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax 2000, 55, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Elkington, H.; White, P.; Addington-Hall, J.; Higgs, R.; Pettinari, C. The last year of life of COPD: A qualitative study of symptoms and services. Respir. Med. 2004, 98, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Rajala, K.; Lehto, J.T.; Saarinen, M.; Sutinen, E.; Saarto, T.; Myllärniemi, M. End-of-life care of patients with idiopathic pulmonary fibrosis. BMC Palliat. Care 2016, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Bajwah, S.; Higginson, I.J.; Ross, J.R.; Wells, A.U.; Birring, S.S.; Patel, A.; Riley, J. Specialist palliative care is more than drugs: A retro-spective study of ILD patients. Lung 2012, 190, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.A.; Singh, S.J.; Collier, R.; Williams, J.E.; Morgan, M.D. Pulmonary rehabilitation is successful for COPD irrespective of MRC dyspnoea grade. Respir. Med. 2009, 103, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Coventry, P.A.; Hind, D. Comprehensive pulmonary rehabilitation for anxiety and depression in adults with chronic obstruc-tive pulmonary disease: Systematic review and meta-analysis. J. Psychosom. Res. 2007, 63, 551–565. [Google Scholar] [CrossRef]
- Johnson, M.J.; Currow, D.C. Opioids for breathlessness: A narrative review. BMJ Support. Palliat. Care 2020, 10, 287–295. [Google Scholar] [CrossRef]
- Pisani, L.; Hill, N.S.; Pacilli, A.M.G.; Polastri, M.; Nava, S. Management of Dyspnea in the Terminally Ill. Chest 2018, 154, 925–934. [Google Scholar] [CrossRef]
- Ekström, M.; Nilsson, F.; Abernethy, A.A.; Currow, D.C. Effects of Opioids on Breathlessness and Exercise Capacity in Chronic Obstructive Pulmonary Disease: A Systematic Review. Ann. Am. Thorac. Soc. 2015, 12, 1079–1092. [Google Scholar] [CrossRef]
- Verberkt, C.A.; van den Beuken-van Everdingen, M.H.J.; Schols, J.M.G.A.; Hameleers, N.; Wouters, E.F.M.; Janssen, D.J.A. Effect of Sustained-Release Morphine for Refractory Breathlessness in Chronic Obstructive Pulmonary Disease on Health Status: A Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Currow, D.; Louw, S.; McCloud, P.; Fazekas, B.; Plummer, J.; McDonald, C.F.; Agar, M.; Clark, K.; McCaffrey, N.; Ekström, M.P.; et al. Regular, sustained-release morphine for chronic breathlessness: A multicentre, double-blind, randomised, placebo-controlled trial. Thorax 2020, 75, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Reedy, F.; Pearson, M.; Greenley, S.; Clark, J.; Currow, D.C.; Bajwah, S.; Fallon, M.; Johnson, M.J. Professionals’, patients’ and families’ views on the use of opioids for chronic breathlessness: A systematic review using the framework method and pillar process. Palliat. Med. 2021, 35, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.J.; Sheen, S.S.; Hahn, S.; Jang, B.H.; Son, H.J. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef]
- Kohberg, C.; Andersen, C.U.; Bendstrup, E. Opioids: An unexplored option for treatment of dyspnea in IPF. Eur. Clin. Respir. J. 2016, 3, 30629. [Google Scholar] [CrossRef][Green Version]
- Woodcock, A.A.; Johnson, M.A.; Geddes, D.M. Breathlessness, alcohol, and opiates. N. Engl. J. Med. 1982, 306, 1363–1364. [Google Scholar]
- Light, R.W.; Stansbury, D.W.; Webster, J.S. Effect of 30 mg of Morphine Alone or With Promethazine or Prochlorperazine on the Exercise Capacity of Patients With COPD. Chest 1996, 109, 975–981. [Google Scholar] [CrossRef]
- Ekström, M.; Johnson, M.J.; Huang, C.; Currow, D.C. Minimal clinically important differences in average, best, worst and current intensity and unpleasantness of chronic breathlessness. Eur. Respir. J. 2020, 56, 1902202. [Google Scholar] [CrossRef]
- Ferreira, D.H.; Louw, S.; McCloud, P.; Fazekas, B.; McDonald, C.F.; Agar, M.R.; Clark, K.; McCaffrey, N.; Ekström, M.; Currow, D.C.; et al. Controlled-Release Oxycodone vs. Placebo in the Treatment of Chronic Breathlessness—A Multisite Randomized Placebo Controlled Trial. J. Pain Symptom Manag. 2020, 59, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.T.; Currow, D.C.; Abernethy, A.P.; Johnson, M.J.; Toson, B.; Eckert, D.J. Effects of low-dose morphine on perceived sleep quality in patients with refractory breathlessness: A hypothesis generating study. Respirology 2016, 21, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Currow, D.C.; Plummer, J.; Frith, P.; Abernethy, A.P. Can we predict which patients with refractory dyspnea will respond to opioids? J. Palliat. Med. 2007, 10, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Currow, D.C.; Quinn, S.; Greene, A.; Bull, J.; Johnson, M.J.; Abernethy, A.P. The Longitudinal Pattern of Response When Morphine Is Used To Treat Chronic Refractory Dyspnea. J. Palliat. Med. 2013, 16, 881–886. [Google Scholar] [CrossRef]
- Abernethy, A.P.; Currow, D.C.; Frith, P.; Fazekas, B.S.; McHugh, A.; Bui, C. Randomised, double blind, placebo-controlled crossover trial of sustained release morphine for the management of refractory dyspnea. BMJ 2003, 327, 523–528. [Google Scholar] [CrossRef]
- Currow, D.C.; McDonald, C.; Oaten, S.; Kenny, B.; Allcroft, P.; Frith, P.; Briffa, M.; Johnson, M.J.; Abernethy, A.P. Once-Daily Opioids for Chronic Dyspnea: A Dose Increment and Pharmacovigilance Study. J. Pain Symptom Manag. 2011, 42, 388–399. [Google Scholar] [CrossRef]
- Smith, J.; Owen, E.; Earis, J.; Woodcock, A. Effect of codeine on objective measurement of cough in chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2006, 117, 831–835. [Google Scholar] [CrossRef]
- Ferreira, D.H.; Ekström, M.; Sajkov, D.; Vandersman, Z.; Eckert, D.J.; Currow, D.C. Extended-Release Morphine for Chronic Breathlessness in Pulmonary Arterial Hypertension—A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. J. Pain Symptom Manag. 2018, 56, 483–492. [Google Scholar] [CrossRef]
- Kronborg-White, S.; Andersen, C.U.; Kohberg, C.; Hilberg, O.; Bendstrup, E. Palliation of chronic breathlessness with morphine in patients with fibrotic interstitial lung disease—A randomised placebo-controlled trial. Respir. Res. 2020, 21, 195. [Google Scholar] [CrossRef]
- Poole, P.J.; Veale, A.G.; Black, P.N. The Effect of Sustained-Release Morphine on Breathlessness and Quality of Life in Severe Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1998, 157, 1877–1880. [Google Scholar] [CrossRef]
- Eiser, N.; Denman, W.T.; West, C.; Luce, P. Oral diamorphine: Lack of effect on dyspnoea and exercise tolerance in the “pink puffer” syndrome. Eur. Respir. J. 1991, 4, 926–931. [Google Scholar] [PubMed]
- Munck, L.K.; Christensen, C.B.; Pedersen, L.; Larsen, U.; Branebjerg, P.E.; Kampmann, J.P. Codeine in Analgesic Doses Does not Depress Respiration in Patients with Severe Chronic Obstructive Lung Disease. Pharmacol. Toxicol. 1990, 66, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.L.; Kronenberg, R.S.; Hedemark, L.L.; Niewoehner, D.E. Effects of chronic administration of codeine and promethazine on breathlessness and exercise tolerance in patients with chronic airflow obstruction. Br. J. Dis. Chest 1987, 81, 287–292. [Google Scholar] [CrossRef]
- Abdallah, S.J.; Wilkinson-Maitland, C.; Saad, N.; Li, P.Z.; Smith, B.M.; Bourbeau, J.; Jensen, D. Effect of morphine on breathlessness and exercise endurance in advanced COPD: A randomised crossover trial. Eur. Respir. J. 2017, 50, 1701235. [Google Scholar] [CrossRef]
- Light, R.W.; Muro, J.R.; Sato, R.I.; Stansbury, D.W.; Fischer, C.E.; Brown, S.E. Effects of oral morphine on breathlessness and exercise tolerance in patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1989, 139, 126–133. [Google Scholar] [CrossRef]
- Johnson, M.A.; Woodcock, A.A.; Geddes, D.M. Dihydrocodeine for breathlessness in “pink puffers”. Br. Med. J. 1983, 286, 675–677. [Google Scholar] [CrossRef]
- Woodcock, A.A.; Gross, E.R.; Gellert, A.; Shah, S.; Johnson, M.; Geddes, D.M. Effects of Dihydrocodeine, Alcohol, and Caffeine on Breathlessness and Exercise Tolerance in Patients with Chronic Obstructive Lung Disease and Normal Blood Gases. N. Engl. J. Med. 1981, 305, 1611–1616. [Google Scholar] [CrossRef]
- Rocker, G.M.; Simpson, A.C.; Young, J.; Horton, R.; Sinuff, T.; Demmons, J.; Donahue, M.; Hernandez, P.; Marciniuk, D. Opioid therapy for refractory dyspnea in patients with advanced chronic obstructive pulmonary disease: Patients’ experiences and outcomes. CMAJ Open 2013, 1, E27–E36. [Google Scholar] [CrossRef]
- Allcroft, P.; Margitanovic, V.; Greene, A.; Agar, M.R.; Clark, K.; Abernethy, A.P.; Currow, D.C. The Role of Benzodiazepines in Breathlessness: A Single Site, Open Label Pilot of Sustained Release Morphine Together with Clonazepam. J. Palliat. Med. 2013, 16, 741–744. [Google Scholar] [CrossRef]
- Allen, S.; Raut, S.; Woollard, J.; Vassallo, M. Low dose diamorphine reduces breathlessness without causing a fall in oxygen saturation in elderly patients with end-stage idiopathic pulmonary fibrosis. Palliat. Med 2005, 19, 128–130. [Google Scholar] [CrossRef]
- Smallwood, N.; Thompson, M.; Warrender-Sparkes, M.; Eastman, P.; Le, B.; Irving, L.; Philip, J. Integrated respiratory and palliative care may improve outcomes in advanced lung disease. ERJ Open Res. 2018, 4, 00102–02017. [Google Scholar] [CrossRef] [PubMed]
- Vicent, L.; Nunez Olarte, J.M.; Puente-Maestu, L.; Oliva, A.; López, J.C.; Postigo, A.; Martín, I.; Luna, R.; Fernández-Avilés, F.; Martínez-Sellés, M. Degree of dyspnoea at admission and discharge in patients with heart failure and respiratory diseases. BMC Palliat. Care 2017, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Takeyasu, M.; Miyamoto, A.; Kato, D.; Takahashi, Y.; Ogawa, K.; Murase, K.; Mochizuki, S.; Hanada, S.; Uruga, H.; Takaya, H.; et al. Continuous Intravenous Morphine Infusion for Severe Dyspnea in Terminally Ill Interstitial Pneumonia Patients. Intern. Med. 2016, 55, 725–729. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuda, Y.; Maeda, I.; Tachibana, K.; Nakao, K.; Sasaki, Y.; Sugimoto, C.; Arai, T.; Tokoro, A.; Akira, M.; Inoue, Y. Low-Dose Morphine for Dyspnea in Terminally Ill Patients with Idiopathic Interstitial Pneumonias. J. Palliat. Med. 2017, 20, 879–883. [Google Scholar] [CrossRef]
- Tsukuura, H.; Nishimura, K.; Taniguchi, H.; Kondoh, Y.; Kimura, T.; Kataoka, K.; Watanabe, N.; Hasegawa, Y. Opioid Use in End-of-Life Care in Patients with Interstitial Pneumonia Associated with Respiratory Worsening. J. Pain Palliat. Care Pharmacother. 2013, 27, 214–219. [Google Scholar] [CrossRef]
- Colman, R.; Singer, L.; Barua, R.; Downar, J. Outcomes of lung transplant candidates referred for co-management by palliative care: A retrospective case series. Palliat. Med. 2015, 29, 429–435. [Google Scholar] [CrossRef]
- Johnson, M.J.; Bland, J.M.; Oxberry, S.G.; Abernethy, A.P.; Currow, D.C. Opioids for chronic refractory breathlessness: Patient predic-tors of beneficial response. Eur. Respir. J. 2013, 42, 758–766. [Google Scholar] [CrossRef]
- Verberkt, C.A.; van den Beuken-van Everdingen, M.H.; Schols, J.M.G.A.; Datla, S.; Dirksen, C.D.; Johnson, M.J.; Van Kuijk, S.M.J.; Wouters, E.F.M.; Janssen, D.J.A. Respiratory adverse effects of opioids for breathlessness: A systematic review and meta-analysis. Eur. Respir. J. 2017, 50, 1701153. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. Use of short-acting opioids in the management of breathlessness: An evidence-based review. Curr. Opin. Support. Palliat. Care 2020, 14, 167–176. [Google Scholar] [CrossRef]
- Currow, D.C.; Kochovska, S.; Ferreira, D.; Johnson, M. Morphine for the symptomatic reduction of chronic breathlessness: The case for controlled release. Curr. Opin. Support. Palliat. Care 2020, 14, 177–181. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).