Serotonin Receptor HTR3A Gene Polymorphisms rs1985242 and rs1062613, E-Cigarette Use and Personality
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Genetic Tests
2.3. Psychological Tests
2.4. Statistical Analysis
3. Results
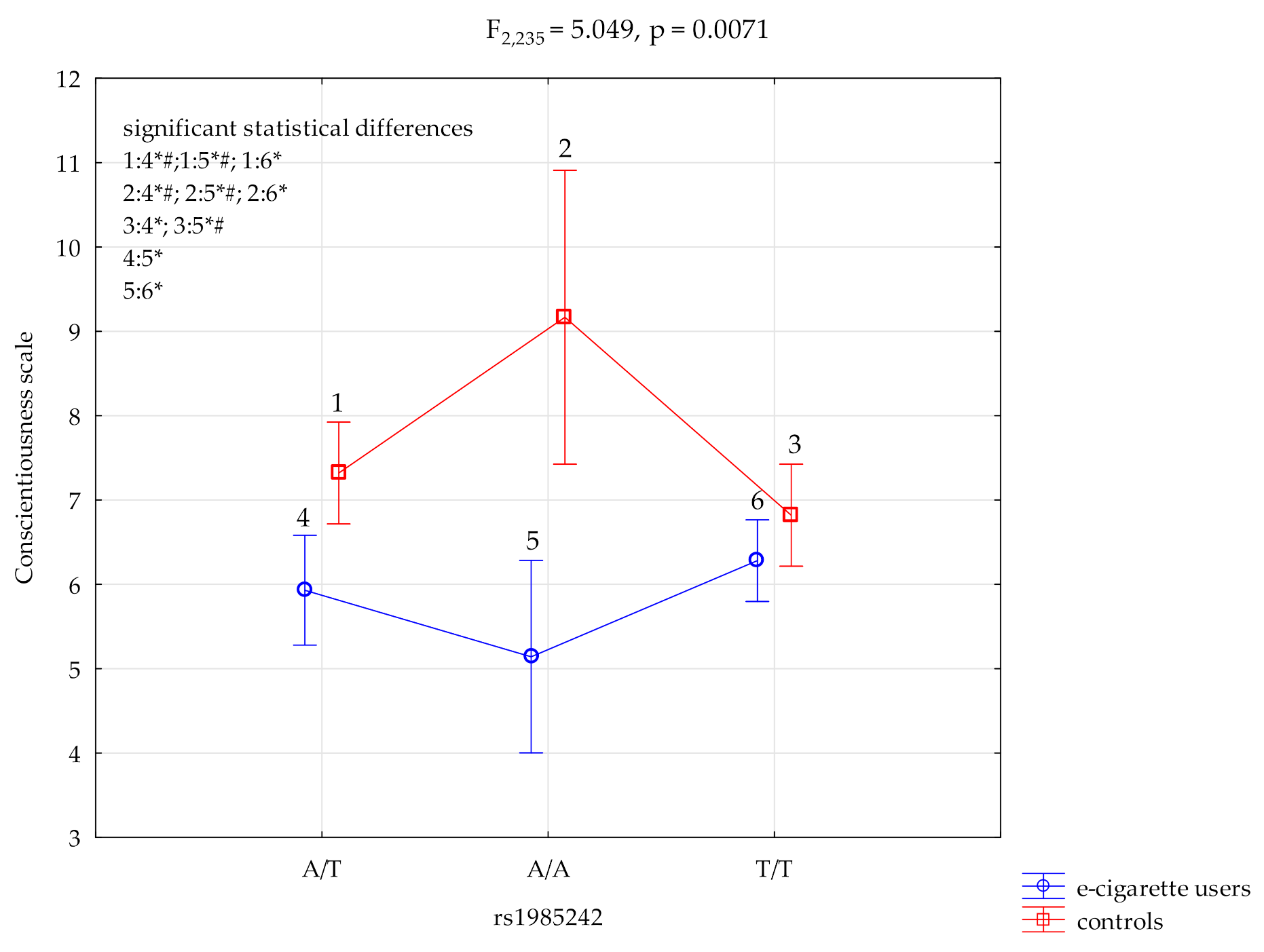
3.1. Conscientiousness Scale and HTR3A rs1985242
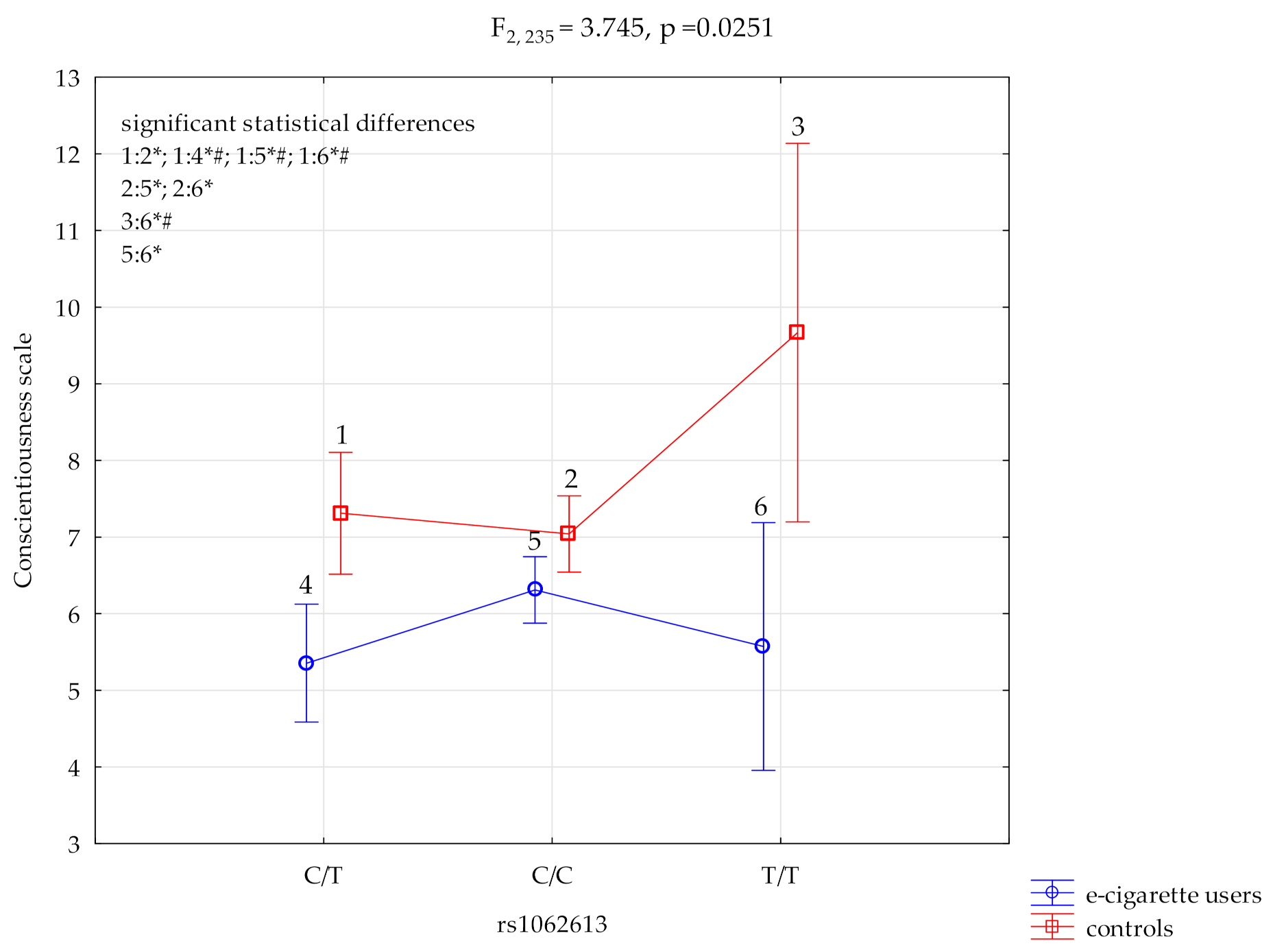
3.2. Conscientiousness Scale and HTR3A rs1062613
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bao, W.; Xu, G.; Lu, J.; Snetselaar, L.G.; Wallace, R.B. Changes in Electronic Cigarette Use Among Adults in the United States, 2014–2016. JAMA 2018, 319, 2039–2041. [Google Scholar] [CrossRef]
- Sopori, M.L. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002, 2, 372–377. [Google Scholar] [CrossRef]
- WHO Report on the Global Tobacco Epidemic 2021: Addressing New and Emerging Products; World Health Organization: Geneva, Switzerland, 2021.
- WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2025, 3rd ed.; World Health Organization: Geneva, Switzerland, 2019.
- Wipfli, H.; Avila-Tang, E.; Navas-Acien, A.; Kim, S.; Onicescu, G.; Yuan, J.; Breysse, P.; Samet, J.M. Secondhand Smoke Exposure Among Women and Children: Evidence From 31 Countries. Am. J. Public Health 2008, 98, 672–679. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults—United States. MMWR Morb. Mortal. Wkly. Rep. 2007, 56, 1157–1161. [Google Scholar]
- Hughes, J.R.; Stead, L.F.; Lancaster, T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007, 1, IDCD000031. [Google Scholar]
- Lerman, C.E.; Schnoll, R.A.; Munafò, M.R. Genetics and smoking cessation—improving outcomes in smokers at risk. Am. J. Prev. Med. 2007, 33 (Suppl. S6), S398–S405. [Google Scholar] [CrossRef][Green Version]
- Maes, H.H.; Sullivan, P.F.; Bulik, C.; Neale, M.C.; Prescott, C.A.; Eaves, L.J.; Kendler, K.S. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol. Med. 2004, 34, 1251–1261. [Google Scholar] [CrossRef]
- Liand, M.D.; Burmeister, M. New insights into the genetics of addiction. Nat. Rev. Genet. 2009, 10, 225–231. [Google Scholar]
- Sharp, B.M.; Chen, H. Neurogenetic determinants and mechanisms of addiction to nicotine and smoked tobacco. Eur. J. Neurosci. 2018, 50, 2164–2179. [Google Scholar] [CrossRef]
- Barnes, N.M.; Hales, T.G.; Lummis, S.C.; Peters, J.A. The 5-HT3 receptor—the relationship between structure and function. Neuropharmacology 2009, 56, 273–284. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Akundi, R.S.; Seidel, M.; Geyer, V.; Haus, U.; Müller, W.; Stratz, T.; Candelario-Jalil, E. Expression of 5-HT3A receptors in cells of the immune system. Scand. J. Rheumatol. Suppl. 2004, 119, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Nennig, S.; Schank, J. The Role of NFkB in Drug Addiction: Beyond Inflammation. Alcohol Alcohol. 2017, 52, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Engleman, E.A.; Rodd, Z.A.; Bell, R.L.; Murphy, J.M. The role of 5-HT3 receptors in drug abuse and as a target for pharmacotherapy. CNS Neurol. Disord. Drug Targets. 2008, 7, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.P.; Homberg, J.R. The role of serotonin in drug use and addiction. Behav. Brain Res. 2015, 277, 146–192. [Google Scholar] [CrossRef]
- Chu, L.F.; Liang, D.Y.; Li, X.; Sahbaie, P.; D’Arcy, N.; Liao, G.; Peltz, G.; Clark, J.D. From mouse to man: The 5-HT3 receptor modulates physical dependence on opioid narcotics. Pharm. Genom. 2009, 19, 193–205. [Google Scholar] [CrossRef]
- Erlendson, M.J.; D’Arcy, N.; Encisco, E.M.; Yu, J.J.; Rincon-Cruz, L.; Peltz, G.; Clark, J.D.; Chu, L. Palonosetron and hydroxyzine pre-treatment reduces the objective signs of experimentally-induced acute opioid withdrawal in humans: A double-blinded, randomized, placebo-controlled crossover study. Am. J. Drug Alcohol Abus. 2016, 43, 78–86. [Google Scholar] [CrossRef]
- Carboni, E.; Acquas, E.; Frau, R.; Di Chiara, G. Differential inhibitory effects of a 5-HT3 antagonist on drug-induced stimulation of dopamine release. Eur. J. Pharmacol. 1989, 164, 515–519. [Google Scholar] [CrossRef]
- Bétry, C.; Etiévant, A.; Oosterhof, C.; Ebert, B.; Sanchez, C.; Haddjeri, N. Role of 5-HT3 Receptors in the Antidepressant Response. Pharmaceuticals 2011, 4, 603–629. [Google Scholar] [CrossRef]
- Seneviratne, C.; Franklin, J.; Beckett, K.; Ma, J.Z.; Ait-Daoud, N.; Payne, T.J.; Johnson, B.A.; Li, M.D. Association, interaction, and replication analysis of genes encoding serotonin transporter and 5-HT3 receptor subunits A and B in alcohol dependence. Hum. Genet. 2013, 132, 1165–1176. [Google Scholar] [CrossRef]
- Enoch, M.-A.; Gorodetsky, E.; Hodgkinson, C.; Roy, A.; Goldman, D. Functional genetic variants that increase synaptic serotonin and 5-HT3 receptor sensitivity predict alcohol and drug dependence. Mol. Psychiatry 2010, 16, 1139–1146. [Google Scholar] [CrossRef]
- Yang, Z.; Seneviratne, C.; Wang, S.; Ma, J.Z.; Payne, T.J.; Wang, J.; Li, M.D. Serotonin transporter and receptor genes significantly impact nicotine dependence through genetic interactions in both European American and African American smokers. Drug Alcohol Depend. 2013, 129, 217–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, J.; Li, M.D. Association and interaction analyses of 5-HT3 receptor and serotonin transporter genes with alcohol, cocaine, and nicotine dependence using the SAGE data. Hum Genet. 2014, 133, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Niesler, B.; Flohr, T.; Nöthen, M.M.; Fischer, C.; Rietschel, M.; Franzek, E.; Albus, M.; Propping, P.; Rappold, G.A. Association between the 5′ UTR variant C178T of the serotonin receptor gene HTR3A and bipolar affective disorder. Pharmacogenetics 2001, 11, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Knisely, M.R.; Conley, Y.P.; Smoot, B.; Paul, S.M.; Levine, J.D.; Miaskowski, C. Associations Between Catecholaminergic and Serotonergic Genes and Persistent Arm Pain Severity Following Breast Cancer Surgery. J. Pain 2019, 20, 1100–1111. [Google Scholar] [CrossRef]
- Davies, P.A.; Pistis, M.; Hanna, M.C.; Peters, J.A.; Lambert, J.J.; Hales, T.G.; Kirkness, E.F. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 1999, 397, 359–363. [Google Scholar] [CrossRef]
- Dubin, A.E.; Huvar, R.; D’Andrea, M.R.; Pyati, J.; Zhu, J.Y.; Joy, K.C.; Wilson, S.J.; Galindo, J.E.; Glass, C.A.; Luo, L.; et al. The Pharmacological and Functional Characteristics of the Serotonin 5-HT3A Receptor Are Specifically Modified by a 5-HT3B Receptor Subunit. J. Biol. Chem. 1999, 274, 30799–30810. [Google Scholar] [CrossRef]
- Breitinger, H.A.; Geetha, N.; Hess, G.P. Inhibition of the serotonin 5-HT3 receptor by nicotine, cocaine, and fluoxetine investigated by rapid chemical kinetic techniques. Biochemistry 2001, 40, 8419–8429. [Google Scholar] [CrossRef]
- Schreiner, B.S.P.; Lehmann, R.; Thiel, U.; Ziemba, P.M.; Beltrán, L.R.; Sherkheli, M.A.; Jeanbourquin, P.; Hugi, A.; Werner, M.; Gisselmann, G.; et al. Direct action and modulating effect of (+)- and (−)-nicotine on ion channels expressed in trigeminal sensory neurons. Eur. J. Pharmacol. 2014, 728, 48–58. [Google Scholar] [CrossRef]
- Gurley, D.A.; Lanthorn, T.H. Nicotinic agonists competitively antagonize serotonin at mouse 5-HT3 receptors expressed in Xenopus oocytes. Neurosci. Lett. 1998, 247, 107–110. [Google Scholar] [CrossRef]
- Shen, J.; Yakel, J.L. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol. Sin. 2009, 30, 673–680. [Google Scholar] [CrossRef]
- Dani, J.A.; Bertrand, D. Nicotinic Acetylcholine Receptors and Nicotinic Cholinergic Mechanisms of the Central Nervous System. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 699–729. [Google Scholar] [CrossRef] [PubMed]
- Drisdel, R.C.; Sharp, D.; Henderson, T.; Hales, T.G.; Green, W.N. High Affinity Binding of Epibatidine to Serotonin Type 3 Receptors. J. Biol. Chem. 2008, 283, 9659–9665. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.J.; Nichols, R.A. Cross-regulation between colocalized nicotinic acetylcholine and 5-HT3 serotonin receptors on presynaptic nerve terminals. Acta Pharmacol. Sin. 2009, 30, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.V.; Rondé, P.; Spier, A.D.; Lummis, S.C.; Nichols, R.A. Nicotinic receptors co-localize with 5-HT3 serotonin receptors on striatal nerve terminals. Neuropharmacology 2000, 39, 2681–2690. [Google Scholar] [CrossRef]
- Yamauchi, J.G.; Nemecz, Á.K.; Nguyen, Q.T.; Muller, A.; Schroeder, L.F.; Talley, T.T.; Lindstrom, J.; Kleinfeld, D.; Taylor, P. Characterizing ligand-gated ion channel receptors with genetically encoded Ca++ sensors. PLoS ONE 2011, 6, 1–8. [Google Scholar] [CrossRef]
- Costa, P.; McCrae, R.R. The SAGE Handbook of Personality Theory and Assessment. The Revised NEO Personality Inventory (NEO-PI-R); Sage Publication, Inc.: Thousand Oaks, CA, USA,, 2008; pp. 179–198. [Google Scholar]
- Sutin, A.R.; Terracciano, A.; Deiana, B.; Uda, M.; Schlessinger, D.; Lakatta, E.; Costa, P.T., Jr. Cholesterol, triglycerides, and the Five-Factor Model of personality. Biol. Psychol. 2010, 84, 186–191. [Google Scholar] [CrossRef]
- Terracciano, A.; Esko, T.; Sutin, A.R.; de Moor, M.H.M.; Meirelles, O.; Zhu, G.; Tanaka, T.; Giegling, I.; Nutile, T.; Realo, A.; et al. Meta-analysis of genome-wide association studies identifies common variants in CTNNA2 associated with excitement-seeking. Transl. Psychiatry 2011, 1, e49. [Google Scholar] [CrossRef]
- Deyoung, C.G.; Hirsh, J.B.; Shane, M.S.; Papademetris, X.; Rajeevan, N.; Gray, J.R. Testing Predictions From Personality Neuroscience. Psychol. Sci. 2010, 21, 820–828. [Google Scholar] [CrossRef]
- Thompson, E. Development and Validation of an International English Big-Five Mini-Markers. Pers. Individ. Differ. 2008, 45, 542–548. [Google Scholar] [CrossRef]
- Matthews, G.; Deary, I.J. Personality Traits; University Press: Cambridge, UK, 1998. [Google Scholar]
- Deyoung, C.G.; Peterson, J.B.; Higgins, D.M. Sources of Openness/Intellect: Cognitive and Neuropsychological Correlates of the Fifth Factor of Personality. J. Pers. 2005, 73, 825–858. [Google Scholar] [CrossRef]
- John, O.P.; Srivastava, S. The Big-Five Trait Taxonomy: History, Measurement, and Theoretical Perspectives; University of California: Berkeley, CA, USA, 1999. [Google Scholar]
- Friedman, H.S.; Schustack, M.W. Personality: Classic Theories and Modern Research; Allyn & Bacon: Boston, MA, USA, 2016. [Google Scholar]
- Pietras, T.; Witusik, A.; Panek, M.; Szemraj, J.; Górski, P. Anxiety, depression and methods of stress coping in patients with nicotine dependence syndrome. Med. Sci. Monit. 2011, 17, CR272–CR276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grzywacz, A.; Suchanecka, A.; Chmielowiec, J.; Chmielowiec, K.; Szumilas, K.; Masiak, J.; Balwicki, Ł.; Michalowska-Sawczyn, M.; Trybek, G. Chmielowiec Personality Traits or Genetic Determinants—Which Strongly Influences E-Cigarette Users? Int. J. Environ. Res. Public Health 2020, 17, 365. [Google Scholar] [CrossRef] [PubMed]
- Suchanecka, A.; Chmielowiec, J.; Chmielowiec, K.; Masiak, J.; Sipak-Szmigiel, O.; Sznabowicz, M.; Czarny, W.; Michałowska-Sawczyn, M.; Trybek, G.; Grzywacz, A. Dopamine Receptor DRD2 Gene rs1076560, Personality Traits and Anxiety in the Polysubstance Use Disorder. Brain Sci. 2020, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, A.; Chmielowiec, J.; Chmielowiec, K.; Mroczek, B.; Masiak, J.; Suchanecka, A.; Sipak-Szmigiel, O.; Szumilas, K.; Trybek, G. The Ankyrin Repeat and Kinase Domain Containing 1 Gene Polymorphism (ANKK1Taq1A) and Personality Traits in Addicted Subjects. Int. J. Environ. Res. Public Health 2019, 16, 2687. [Google Scholar] [CrossRef]
- Benowitz, N.L. Clinical Pharmacology of Inhaled Drugs of Abuse: Implications in Understanding Nicotine Dependence. NIDA Res. Monogr. 1990, 99, 12–29. [Google Scholar]
- Henningfield, J.E.; Keenan, R.M. Nicotine delivery kinetics and abuse liability. J. Consult. Clin. Psychol. 1993, 61, 743–750. [Google Scholar] [CrossRef]
- Schneider, N.G.; Olmstead, R.E.; Franzon, M.A.; Lunell, E. The Nicotine Inhaler: Clinical Pharmacokinetics and Comparison with Other Nicotine Treatments. Clin. Pharmacokinet. 2001, 40, 661–684. [Google Scholar] [CrossRef]
- Caldwell, B.; Sumner, W.; Crane, J. A Systematic Review of Nicotine by Inhalation: Is There a Role for the Inhaled Route? Nicotine Tob. Res. 2012, 14, 1127–1139. [Google Scholar] [CrossRef]
- Samaha, A.-N.; Yau, W.-Y.W.; Yang, P.; Robinson, T.E. Rapid delivery of nicotine promotes behavioral sensitization and alters its neurobiological impact. Biol. Psychiatry 2005, 57, 351–360. [Google Scholar] [CrossRef]
- Wing, V.C.; Shoaib, M. Effect of infusion rate on intravenous nicotine self-administration in rats. Behav. Pharmacol. 2013, 24, 517–522. [Google Scholar] [CrossRef]
- Allain, F.; Minogianis, E.-A.; Roberts, D.; Samaha, A.-N. How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci. Biobehav. Rev. 2015, 56, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Vansickel, A.R.; Eissenberg, T. Electronic Cigarettes: Effective Nicotine Delivery After Acute Administration. Nicotine Tob. Res. 2012, 15, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Spyrou, A.; Stefopoulos, C.; Tsimopoulou, K.; Kourkoveli, P.; Tsiapras, D.; Kyrzopoulos, S.; Poulas, K.; Voudris, V. Nicotine absorption from electronic cigarette use: Comparison between experienced consumers (vapers) and naïve users (smokers). Sci. Rep. 2015, 5, 11269. [Google Scholar] [CrossRef]
- St. Helen, G.; Havel, C.; Dempsey, D.A.; Jacob, P., 3rd; Benowitz, N.L. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 2016, 111, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Sai, K.K.S.; Zuo, Y.; Rose, J.E.; Garg, P.K.; Garg, S.; Nazih, R.; Mintz, A.; Mukhin, A.G. Rapid Brain Nicotine Uptake from Electronic Cigarettes. J. Nucl. Med. 2019, 61, 928–930. [Google Scholar] [CrossRef]
- Kotov, R.; Gamez, W.; Schmidt, F.; Watson, D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychol. Bull. 2010, 136, 768–821. [Google Scholar] [CrossRef]
- Krueger, R.F.; Caspi, A.; Moffitt, T.E. Epidemiological personology: The unifying role of personality in population-based research on problem behaviors. J. Personal. 2000, 68, 967–998. [Google Scholar] [CrossRef]
- Zilberman, N.; Yadid, G.; Efrati, Y.; Neumark, Y.; Rassovsky, Y. Personality profiles of substance and behavioral addictions. Addict. Behav. 2018, 82, 174–181. [Google Scholar] [CrossRef]
- Slutske, W.S.; Caspi, A.; Moffitt, T.E.; Poulton, R. Personality and problem gambling: A prospective study of a birth cohort of young adults. Arch. Gen. Psychiatry 2005, 62, 769–775. [Google Scholar] [CrossRef]
- Lahey, B.B. Public health significance of neuroticism. Am. Psychol. 2009, 64, 241. [Google Scholar] [CrossRef]
- Cooper, M.L. Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychol. Assess. 1994, 6, 117. [Google Scholar] [CrossRef]
- Simons, J.; Correia, C.J.; Carey, K.B.; Borsari, B.E. Validating a five-factor marijuana motives measure: Relations with use, problems, and alcohol motives. J. Couns. Psychol. 1998, 45, 265. [Google Scholar] [CrossRef]
- Raynor, D.A.; Levine, H. Associations between the five-factor model of personality and health behaviors among college students. J. Am. Coll. Health 2009, 58, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Forero, D.; Navarro, J.F. Personality Traits Related to Binge Drinking: A Systematic Review. Front. Psychiatry 2017, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Oreland, L.; Lagravinese, G.; Toffoletto, S.; Nilsson, K.W.; Harro, J.; Cloninger, C.R.; Comasco, E. Personality as an intermediate phenotype for genetic dissection of alcohol use disorder. J. Neural Transm. 2018, 125, 107–130. [Google Scholar] [CrossRef]
- Petry, N.M.; Stinson, F.S.; Grant, B.F. Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 2005, 66, 564–574. [Google Scholar] [CrossRef]
- Dash, G.F.; Slutske, W.S.; Martin, N.G.; Statham, D.J.; Agrawal, A.; Lynskey, M.T. Big Five personality traits and alcohol, nicotine, cannabis, and gambling disorder comorbidity. Psychol. Addict. Behav. 2019, 33, 420–429. [Google Scholar] [CrossRef]
- Lachowicz, M.; Chmielowiec, J.; Chmielowiec, K.; Suchanecka, A.; Michałowska-Sawczyn, M.; Mierzecki, A.; Mroczek, B.; Grzywacz, A. Psychological factors and genetic characteristics of rural cannabis users. Ann. Agric. Environ. Med. 2020, 27, 260–268. [Google Scholar] [CrossRef]
- Han, H.; Liu, Q.; Yang, Z.; Wang, M.; Ma, Y.; Cao, L.; Cui, W.; Yuan, W.; Payne, T.J.; Li, L.; et al. Association and cis-mQTL analysis of variants in serotonergic genes associated with nicotine dependence in Chinese Han smokers. Transl. Psychiatry 2018, 8, 243. [Google Scholar] [CrossRef]
- Walstab, J.; Rappold, G.; Niesler, B. 5-HT3 receptors: Role in disease and target of drugs. Pharmacol. Ther. 2010, 128, 146–169. [Google Scholar] [CrossRef]
- Gatt, J.M.; Williams, L.M.; Schofield, P.R.; Dobson-Stone, C.; Paul, R.H.; Grieve, S.M.; Clark, C.R.; Gordon, E.; Nemeroff, C.B. Impact of the HTR3A gene with early life trauma on emotional brain networks and depressed mood. Depression Anxiety 2010, 27, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.-I.; Lee, S.-H.; Huh, H.J.; Chae, J.-H. Influence of the 5-HT3A Receptor Gene Polymorphism and Childhood Sexual Trauma on Central Serotonin Activity. PLoS ONE 2015, 10, e0145269. [Google Scholar] [CrossRef] [PubMed]
- Jasinska, A.J.; Chua, H.F.; Ho, S.S.; Polk, T.A.; Rozek, L.S.; Strecher, V.J. Amygdala response to smoking-cessation messages mediates the effects of serotonin transporter gene variation on quitting. NeuroImage 2012, 60, 766–773. [Google Scholar] [CrossRef] [PubMed][Green Version]
| E-Cigarette Users n = 135 Observed (Expected) | χ2 (p Value) Allele Frequencies | Controls n = 106 Observed (Expected) | χ2 (p Value) Allele Frequencies | |
|---|---|---|---|---|
| HTR3A rs1985242 | ||||
| T/T | 78 (73.3) | 4.291 (0.038) | 50 (53.1) | 2.071 (0.151) |
| A/T | 43 (52.3) | p allele freq (T) = 0.74 | 50 (43.9) | p allele freq (T) = 0.71 |
| A/A | 14 (9.3) | q allele freq (A) = 0.26 | 6 (9.1) | q allele freq (A) = 0.29 |
| HTR3A rs1062613 | ||||
| C/C | 97 (93.8) | 4.056 (0.044) | 74 (73.9) | 0.006 (0.938) |
| C/T | 31 (37.5) | p allele freq (G) = 0.83 | 29 (29.2) | p allele freq (G) = 0.83 |
| T/T | 7 (3.8) | q allele freq (T) = 0.17 | 3 (2.9) | q allele freq (T) = 0.17 |
| E-Cigarette Users | Controls | Co-Dominant Model χ2 (p Value) | OR (95% Confidence) | Additive Model Cochran-Armitage Trend Test Z (p Value) | |
|---|---|---|---|---|---|
| HTR3A rs 1985242 | |||||
| n = 135 | n = 106 | 6.456 (0.0396) * | −0.707 (0.480) | ||
| T/T | 78 (57.78%) | 50 (47.17%) | 0.520 (0.4722) | ||
| A/T | 43 (31.85%) | 50 (47.17%) | 0.55 (0.32–0.95) * | ||
| A/A | 14 (10.37%) | 6 (5.66%) | 1.50 (0.54–4.14) * | ||
| T | 199 (73.70%) | 150 (70.75%) | |||
| A | 71 (26.30%) | 62 (29.25%) | |||
| HTR3A rs1062613 | |||||
| n = 135 | n = 106 | 1.289 (0.5248) | 0.044 (0.965) | ||
| C/C | 97 (71.85%) | 74 (69.81%) | 0.001 (0.9633) | ||
| C/T | 31 (22.96%) | 29 (27.36%) | 0.81 (0.45–1.47) | ||
| T/T | 7 (5.19%) | 3 (2.83%) | 1.77 (0.44–7.10) | ||
| C | 225 (83.33%) | 177 (83.49%) | |||
| T | 45 (16.67%) | 35 (16.51%) | |||
| NEO Five Factor Inventory | E-Cigarette Users (n = 153) M ± SD | Control (n = 152) M ± SD | U Mann–Whitney Z | p Value |
|---|---|---|---|---|
| Neuroticism scale | 5.60 ± 2.19 | 4.91 ± 2.24 | 2.522 | 0.0117 * |
| Extraversion scale | 6.05 ± 2.13 | 6.96 ± 1.93 | −3.190 | 0.0014 *,# |
| Openness scale | 5.10 ± 1.92 | 5.03 ± 1.88 | 0.202 | 0.8399 |
| Agreeableness scale | 5.60 ± 2.34 | 5.88 ± 2.26 | −0.909 | 0.3632 |
| Conscientiousness scale | 6.05 ± 2.15 | 7.19 ± 2.25 | −4.147 | 0.0000 *,# |
| NEO Five Factor Inventory | Group | HTR3A rs1985242 | ANOVA (Interaction) | ||||
|---|---|---|---|---|---|---|---|
| T/T (n = 128) M ± SD | A/T (n = 93) M ± SD | A/A (n = 20) M ± SD | E-Cigarette Users/Control x HTR3A rs1985242 F (p Value) | ɳ2 | Power (Alfa = 0.05) | ||
| Neuroticism scale | E-cigarette users; n = 135 | 5.43 ± 2.11 | 6.00 ± 2.29 | 5.290 ± 2.33 | F2,235 = 0.28 (p = 0.7561) | 0.002 | 0.094 |
| Control; n = 106 | 4.88 ± 2.50 | 5.00 ± 2.02 | 4.33 ± 1.86 | ||||
| Extraversion scale | E-cigarette users; n = 135 | 5.91 ± 2.11 | 6.42 ± 2.25 | 5.71 ± 1.82 | F2,235 = 1.24 (p = 0.2907) | 0.010 | 0.269 |
| Control; n = 106 | 6.80 ± 2.02 | 7.00 ± 1.86 | 8.00 ± 1.55 | ||||
| Openness scale | E-cigarette users; n = 135 | 5.19 ± 1.58 | 5.21 ± 1.58 | 4.21 ± 1.63 | F2,235 = 1.52 (p = 0.2217) | 0.013 | 0.321 |
| Control; n = 106 | 4.94 ± 2.23 | 5.04 ± 1.43 | 5.67 ± 2.25 | ||||
| Agreeableness scale | E-cigarette users; n = 135 | 5.88 ± 2.37 | 5.07 ± 2.24 | 5.64 ± 2.37 | F2,235 = 1.95 (p = 0.1440) | 0.016 | 0.402 |
| Control; n = 106 | 5.66 ± 2.38 | 6.10 ± 2.09 | 5.83 ± 2.86 | ||||
| Conscientiousness scale | E-cigarette users; n = 135 | 6.28 ± 2.12 | 5.93 ± 2.28 | 5.14 ± 1.70 | F2,235 = 5.05 *,# (p = 0.0071) | 0.041 | 0.814 |
| Control; n = 106 | 6.82 ± 2.08 | 7.32 ± 2.38 | 9.17 ± 1.60 | ||||
| NEO Five Factor Inventory | Group | HTR3A rs1062613 | ANOVA (Interaction) | ||||
|---|---|---|---|---|---|---|---|
| C/C (n = 171) M ± SD | C/T (n = 60) M ± SD | T/T (n = 10) M ± SD | E-Cigarette Users/Control x HTR3A rs1062613 F (p Value) | ɳ2 | Power (Alfa = 0.05) | ||
| Neuroticism scale | E-cigarette users; n = 135 | 5.40 ± 2.28 | 6.19 ± 1.97 | 5.71 ± 1.50 | F2,235 = 0.64 (p = 0.5276) | 0.005 | 0.157 |
| Control; n = 106 | 4.85 ± 2.24 | 5.17 ± 2.25 | 3.67 ± 2.08 | ||||
| Extraversion scale | E-cigarette users; n = 135 | 4.86 ± 1.57 | 6.39 ± 2.20 | 6.03 ± 2.12 | F2,235 = 2.27 (p = 0.1059) | 0.019 | 0.458 |
| Control; n = 106 | 8.67 ± 0.57 | 7.24 ± 2.10 | 6.78 ± 1.86 | ||||
| Openness scale | E-cigarette users; n = 135 | 4.14 ± 1.86 | 5.19 ± 1.42 | 5.13 ± 2.05 | F2,235 = 2.66 (p = 0.0723) | 0.022 | 0.524 |
| Control; n = 106 | 7.00 ± 1.73 | 5.13 ± 1.36 | 4.91 ± 2.03 | ||||
| Agreeableness scale | E-cigarette users; n = 135 | 6.43 ± 2.07 | 4.90 ± 2.24 | 5.76 ± 2.36 | F2,235 = 0.66 (p = 0.5183) | 0.006 | 0.160 |
| Control; n = 106 | 5.67 ± 4.16 | 5.72 ± 2.22 | 5.95 ± 2.23 | ||||
| Conscientiousness scale | E-cigarette users; n = 135 | 6.31 ± 2.14 | 5.35 ± 2.18 | 5.57 ± 1.40 | F2,235 = 3.74 * (p = 0.0251) | 0.031 | 0.681 |
| Control; n = 106 | 7.04 ± 2.21 | 7.31 ± 2.35 | 9.67 ± 0.58 | ||||
| HTR3A rs1985242 and NEO FFI Conscientiousness Scale | ||||||
|---|---|---|---|---|---|---|
| {1} M = 5.93 | {2} M = 5.14 | {3} M = 6.28 | {4} M = 7.32 | {5} M = 9.17 | {6} M = 6.82 | |
| E-cigarette users HTR3A A/T {1} | 0.2388 | 0.3935 | 0.0023 *,# | 0.0007*,# | 0.0495 * | |
| E-cigarette users HTR3A A/A {2} | 0.0714 | 0.0010 *,# | 0.0002*,# | 0.0111 * | ||
| E-cigarette users HTR3A T/T {3} | 0.0087 * | 0.0019 *,# | 0.1719 | |||
| Control HTR3A A/T {4} | 0.0497 * | 0.2498 | ||||
| Control HTR3A A/A {5} | 0.0129 * | |||||
| Control HTR3A T/T {6} | ||||||
| HTR3A rs1062613 and NEO FFI Conscientiousness Scale | ||||||
| {1} M = 5.35 | {2} M = 6.31 | {3} M = 5.57 | {4} M = 7.31 | {5} M = 7.04 | {6} M = 9.67 | |
| E-cigarette users HTR3A C/T{1} | 0.0341 * | 0.8117 | 0.0006 *,# | 0.0003 *,# | 0.0012 *,# | |
| E-cigarette users HTR3A C/C {2} | 0.386 | 0.0303 | 0.0301 * | 0.0089 * | ||
| E-cigarette users HTR3A T/T {3} | 0.0584 | 0.0883 | 0.0067 *,# | |||
| Control HTR3A C/T {4} | 0.571 | 0.0748 | ||||
| Control HTR3A C/C {5} | 0.0411 * | |||||
| Control HTR3A T/T {6} | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchanecka, A.; Chmielowiec, J.; Chmielowiec, K.; Trybek, G.; Jaroń, A.; Czarny, W.; Król, P.; Masiak, J.; Grzywacz, A. Serotonin Receptor HTR3A Gene Polymorphisms rs1985242 and rs1062613, E-Cigarette Use and Personality. Int. J. Environ. Res. Public Health 2022, 19, 4746. https://doi.org/10.3390/ijerph19084746
Suchanecka A, Chmielowiec J, Chmielowiec K, Trybek G, Jaroń A, Czarny W, Król P, Masiak J, Grzywacz A. Serotonin Receptor HTR3A Gene Polymorphisms rs1985242 and rs1062613, E-Cigarette Use and Personality. International Journal of Environmental Research and Public Health. 2022; 19(8):4746. https://doi.org/10.3390/ijerph19084746
Chicago/Turabian StyleSuchanecka, Aleksandra, Jolanta Chmielowiec, Krzysztof Chmielowiec, Grzegorz Trybek, Aleksandra Jaroń, Wojciech Czarny, Paweł Król, Jolanta Masiak, and Anna Grzywacz. 2022. "Serotonin Receptor HTR3A Gene Polymorphisms rs1985242 and rs1062613, E-Cigarette Use and Personality" International Journal of Environmental Research and Public Health 19, no. 8: 4746. https://doi.org/10.3390/ijerph19084746
APA StyleSuchanecka, A., Chmielowiec, J., Chmielowiec, K., Trybek, G., Jaroń, A., Czarny, W., Król, P., Masiak, J., & Grzywacz, A. (2022). Serotonin Receptor HTR3A Gene Polymorphisms rs1985242 and rs1062613, E-Cigarette Use and Personality. International Journal of Environmental Research and Public Health, 19(8), 4746. https://doi.org/10.3390/ijerph19084746








