Saharan Dust and Childhood Respiratory Symptoms in Benin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Health Data
2.2. Exposure Data and Classification
2.3. Outcome Classification
2.4. Statistical Analyses
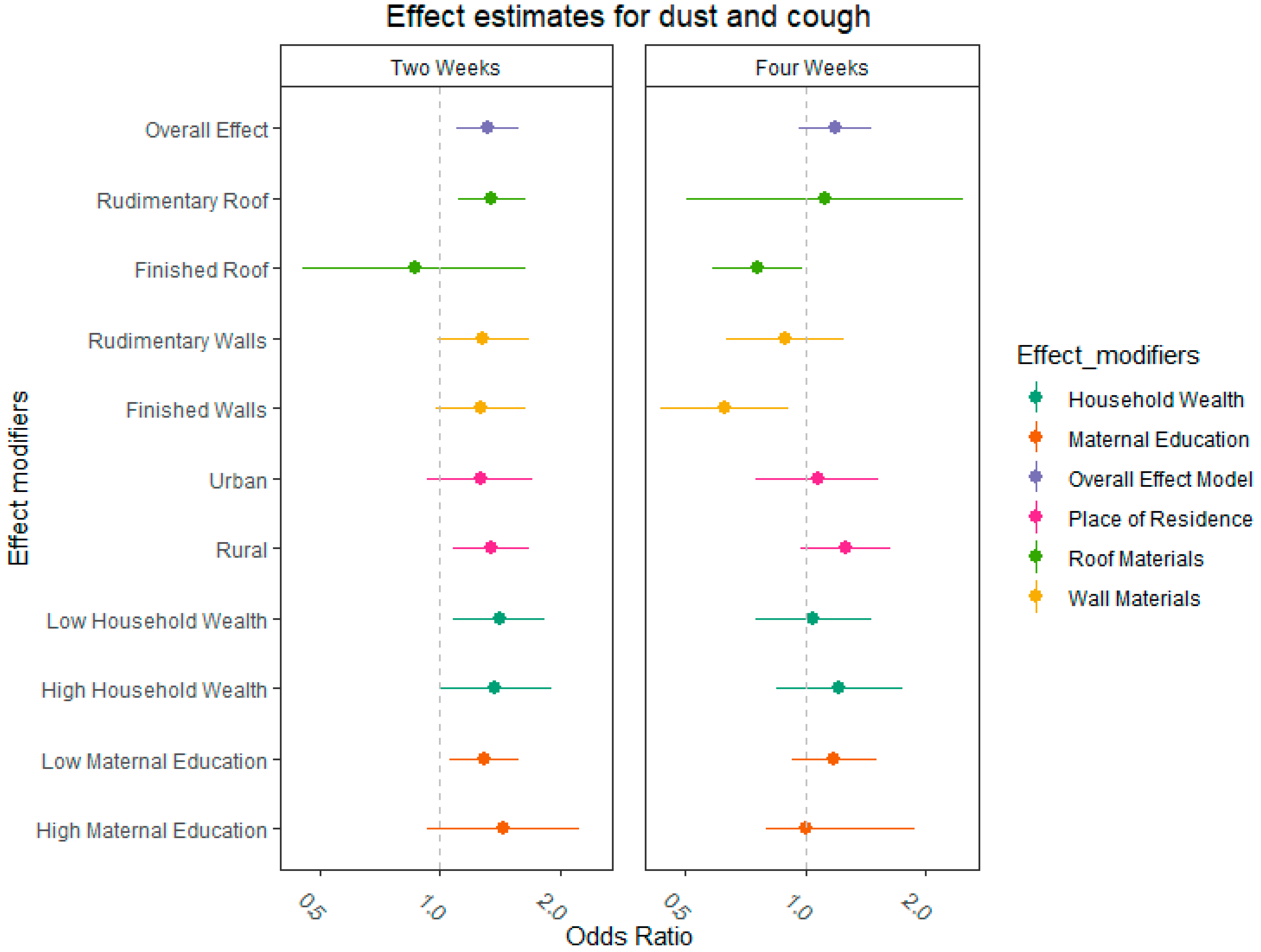
2.5. Effect Measure Modification
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Two Weeks | Four Weeks | |||
|---|---|---|---|---|
| Effect Modifier | Cochran Q Estimate | χ2 | Cochran Q Estimate | χ2 |
| Urban | 0.103 | 0.748 | 0.522 | 0.47 |
| Rural | ||||
| High education | 0.187 | 0.665 | 0.303 | 0.582 |
| Low education | ||||
| High Household wealth | 0.327 | 0.568 | 0.578 | 0.447 |
| Low Household wealth | ||||
References
- Prospero, J.M.; Lamb, P.J. African Droughts and Dust Transport to the Caribbean: Climate Change Implications. Science 2003, 302, 1024–1027. [Google Scholar] [CrossRef]
- Samoli, E.; Kougea, E.; Kassomenos, P.; Analitis, A.; Katsouyanni, K. Does the presence of desert dust modify the effect of PM10 on mortality in Athens, Greece? Sci. Total Environ. 2011, 409, 2049–2054. [Google Scholar] [CrossRef]
- Cook, A.; Weinstein, P.; Centeno, J. Health effects of natural dust. Biol. Trace Elem. Res. 2005, 103, 1–15. [Google Scholar] [CrossRef]
- De Longueville, F.; Ozer, P.; Doumbia, S.; Henry, S. Desert dust impacts on human health: An alarming worldwide reality and a need for studies in West Africa. Int. J. Biometeorol. 2013, 57, 1–19. [Google Scholar] [CrossRef]
- Kotsyfakis, M.; Zarogiannis, S.G.; Patelarou, E.; Sarogiannis, S. The health impact of Saharan dust exposure. Int. J. Occup. Med. Environ. Health 2019, 32, 749–760. [Google Scholar] [CrossRef]
- Leys, J.F.; Heidenreich, S.K.; Strong, C.L.; McTainsh, G.H.; Quigley, S. PM10 concentrations and mass transport during “Red Dawn”—Sydney 23 September 2009. Aeolian Res. 2011, 3, 327–342. [Google Scholar] [CrossRef] [Green Version]
- Evan, A.T.; Flamant, C.; Gaetani, M.; Guichard, F. The past, present and future of African dust. Nature 2016, 531, 493–495. [Google Scholar] [CrossRef]
- Evan, A.T.; Fiedler, S.; Zhao, C.; Menut, L.; Schepanski, K.; Flamant, C.; Doherty, O. Derivation of an observation-based map of North African dust emission. Aeolian Res. 2015, 16, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Francis, D.; Fonseca, R.; Nelli, N.; Cuesta, J.; Weston, M.; Evan, A.; Temimi, M. The Atmospheric Drivers of the Major Saharan Dust Storm in June 2020. Geophys. Res. Lett. 2020, 47, e2020GL090102. [Google Scholar] [CrossRef]
- Koren, I.; Kaufman, Y.J.; Washington, R.; Todd, M.C.; Rudich, Y.; Martins, J.V.; Rosenfeld, D. The Bodélé depression: A single spot in the Sahara that provides most of the mineral dust to the Amazon forest. Environ. Res. Lett. 2006, 1, 014005. [Google Scholar] [CrossRef]
- Analitis, A.; Katsouyanni, K.; Dimakopoulou, K.; Samoli, E.; Nikoloulopoulos, A.K.; Petasakis, Y.; Touloumi, G.; Schwartz, J.; Anderson, H.R.; Cambra, K.; et al. Short-Term Effects of Ambient Particles on Cardiovascular and Respiratory Mortality. Epidemiology 2006, 17, 230–233. [Google Scholar] [CrossRef]
- Anderson, H.R.; Atkinson, R.W.; Peacock, J.; Marston, L.; Konstantinou, K.; WHO Organization. Meta-Analysis of Time-Series Studies and Panel Studies of Particulate Matter (PM) and Ozone (O3): Report of a WHO Task Group; WHO Regional Office for Europe: Copenhagen, Denmark, 2004. [Google Scholar]
- Anderson, H.R.; Atkinson, R.W.; Peacock, J.L.; Sweeting, M.J.; Marston, L. Ambient particulate matter and health effects: Publication bias in studies of short-term associations. Epidemiology 2005, 16, 155–163. [Google Scholar] [CrossRef]
- Pope, C.A., III; Dockery, D.W. Health Effects of Fine Particulate Air Pollution: Lines that Connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef]
- Xing, Y.-F.; Xu, Y.-H.; Shi, M.-H.; Lian, Y.-X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef]
- Schindler, C.; Keidel, D.; Gerbase, M.W.; Zemp, E.; Bettschart, R.; Brändli, O.; Brutsche, M.; Burdet, L.; Karrer, W.; Knöpfli, B.; et al. Improvements in PM10Exposure and Reduced Rates of Respiratory Symptoms in a Cohort of Swiss Adults (SAPALDIA). Am. J. Respir. Crit. Care Med. 2009, 179, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Zemp, E.; Elsasser, S.; Schindler, C.; Künzli, N.; Perruchoud, A.P.; Domenighetti, G.; Medici, T.; Ackermann-Liebrich, U.; Leuenberger, P.; Monn, C.; et al. Long-Term Ambient Air Pollution and Respiratory Symptoms in Adults (SAPALDIA Study). Am. J. Respir. Crit. Care Med. 1999, 159, 1257–1266. [Google Scholar] [CrossRef]
- Were, F.H.; Wafula, G.A.; Lukorito, C.B.; Kamanu, T.K. Levels of PM10 and PM2.5 and Respiratory Health Impacts on School-Going Children in Kenya. J. Health Pollut. 2020, 10, 200912. [Google Scholar] [CrossRef]
- Nascimento, A.P.; Santos, J.M.; Mill, J.G.; Toledo de Almeida Albuquerque, T.; Costa Reis Júnior, N.; Reisen, V.A.; Coelho Pagel, E. Association between the incidence of acute respiratory diseases in children and ambient concentrations of SO2, PM10 and chemical elements in fine particles. Environ. Res. 2020, 188, 109619. [Google Scholar] [CrossRef]
- Fan, X.-J.; Yang, C.; Zhang, L.; Fan, Q.; Li, T.; Bai, X.; Zhao, Z.-H.; Zhang, X.; Norback, D. Asthma symptoms among Chinese children: The role of ventilation and PM10 exposure at school and home. Int. J. Tuberc. Lung Dis. 2017, 21, 1187–1193. [Google Scholar] [CrossRef]
- Gyan, K.; Henry, W.; Lacaille, S.; Laloo, A.; Lamsee-Ebanks, C.; McKay, S.; Antoine, R.M.; Monteil, M.A. African dust clouds are associated with increased paediatric asthma accident and emergency admissions on the Caribbean island of Trinidad. Int. J. Biometeorol. 2005, 49, 371–376. [Google Scholar] [CrossRef]
- Prospero, J.M. Long-term measurements of the transport of African mineral dust to the southeastern United States: Impli-cations for regional air quality. J. Geophys. Res. Atmos. 1999, 104, 15917–15927. [Google Scholar] [CrossRef] [Green Version]
- Hashizume, M.; Kim, Y.; Ng, C.F.S.; Chung, Y.; Madaniyazi, L.; Bell, M.L.; Guo, Y.L.; Kan, H.; Honda, Y.; Yi, S.-M.; et al. Health Effects of Asian Dust: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2020, 128, 066001. [Google Scholar] [CrossRef]
- Hasunuma, H.; Takeuchi, A.; Ono, R.; Amimoto, Y.; Hwang, Y.H.; Uno, I.; Shimizu, A.; Nishiwaki, Y.; Hashizume, M.; Askew, D.; et al. Effect of Asian dust on respiratory symptoms among children with and without asthma, and their sen-sitivity. Sci. Total Environ. 2021, 753, 141585. [Google Scholar] [CrossRef]
- WHO. Causes of Child Mortality. Available online: https://www.who.int/gho/child_health/mortality/causes/en/ (accessed on 1 March 2022).
- UNICEF. One is Too Many: Ending Child Deaths from Pneumonia and Diarrhoea; UNICEF: New York, NY, USA, 2016. [Google Scholar]
- UNICEF; WHO. End Preventable Deaths: Global Action Plan for Prevention and Control of Pneumonia and Diarrhoea; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Alessandrini, E.R.; Stafoggia, M.; Faustini, A.; Gobbi, G.P.; Forastiere, F. Saharan dust and the association between particulate matter and daily hospitalisations in Rome, Italy. Occup. Environ. Med. 2013, 70, 432–434. [Google Scholar] [CrossRef]
- Kanatani, K.T.; Ito, I.; Al-Delaimy, W.; Adachi, Y.; Mathews, W.C.; Ramsdell, J.W. Desert Dust Exposure Is Associated with Increased Risk of Asthma Hospitalization in Children. Am. J. Respir. Crit. Care Med. 2010, 182, 1475–1481. [Google Scholar] [CrossRef] [Green Version]
- Foreman, T. The Effect of Dust Storms on Child Health in West Africa; CDEP-CGEG Working Paper; Columbia University: New York, NY, USA, 2018. [Google Scholar]
- Adhvaryu, A.; Bharadwaj, P.; Fenske, J.; Nyshadham, A.; Stanley, R. Dust and Death: Evidence from the West African Harmattan; National Bureau of Economic Research: Cambridge, MA, USA, 2019. [Google Scholar]
- Edmond, K.; Scott, S.; Korczak, V.; Ward, C.; Sanderson, C.; Theodoratou, E.; Clark, A.; Griffiths, U.; Rudan, I.; Campbell, H. Long Term Sequelae from Childhood Pneumonia; Systematic Review and Meta-Analysis. PLoS ONE 2012, 7, e31239. [Google Scholar] [CrossRef] [Green Version]
- Grimwood, K.; Chang, A.B. Long-term effects of pneumonia in young children. Pneumonia 2015, 6, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Baek, D.; Altindag, D.; Mocan, N. Chinese Yellow Dust and Korean Infant Health. Soc. Sci. Med. 2017, 186, 78–86. [Google Scholar]
- Tulet, P.; Mallet, M.; Pont, V.; Pelon, J.; Boone, A. The 7–13 March 2006 dust storm over West Africa: Generation, transport, and vertical stratification. J. Geophys. Res. Earth Surf. 2008, 113. [Google Scholar] [CrossRef] [Green Version]
- Ouafo-Leumbe, M.-R.; Galy-Lacaux, C.; Liousse, C.; Pont, V.; Akpo, A.; Doumbia, T.; Gardrat, E.; Zouiten, C.; Sigha-Nkamdjou, L.; Ekodeck, G.E. Chemical composition and sources of atmospheric aerosols at Djougou (Benin). Arch. Meteorol. Geophys. Bioclimatol. Ser. B 2018, 130, 591–609. [Google Scholar] [CrossRef]
- ICF. Demographic and Health Surveys (Various); Funded by USAID; ICF: Rockville, MD, USA, 2020. [Google Scholar]
- Aliaga, A.; Ren, R. The Optimal Sample Sizes for Two-Stage Cluster Sampling in Demographic and Health Surveys; ORC Macro: Columbia, MD, USA, 2006. [Google Scholar]
- USAID. Geographic Displacement Procedure and Georefferenced Data Relaese Policy for the Demographic and Health Surveys. DHS. 2013. Available online: https://dhsprogram.com/pubs/pdf/SAR7/SAR7.pdf (accessed on 1 March 2022).
- Lyapustin, A. MODIS Multi-Angle Implementation of Atmospheric Correction (MAIAC) Data User’s Guide. NASA 2018, Collection 6. Available online: https://lpdaac.usgs.gov/documents/110/MCD19_User_Guide_V6.pdf (accessed on 1 March 2022).
- Kloog, I.; Zanobetti, A.; Nordio, F.; Coull, B.A.; Baccarelli, A.A.; Schwartz, J. Effects of airborne fine particles (PM 2.5) on deep vein thrombosis admissions in the northeastern United States. J. Thromb. Haemost. 2015, 13, 768–774. [Google Scholar] [CrossRef] [Green Version]
- Wellenius, G.A.; Burger, M.R.; Coull, B.A.; Schwartz, J.; Suh, H.H.; Koutrakis, P.; Schlaug, G.; Gold, D.R.; Mittleman, M.A. Ambient Air Pollution and the Risk of Acute Ischemic Stroke. Arch. Intern. Med. 2012, 172, 229–234. [Google Scholar] [CrossRef]
- Zanobetti, A.; Dominici, F.; Wang, Y.; Schwartz, J.D. A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environ. Health 2014, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Di, Q.; Dai, L.; Wang, Y.; Zanobetti, A.; Choirat, C.; Schwartz, J.D.; Dominici, F. Association of Short-term Exposure to Air Pollution with Mortality in Older Adults. JAMA J. Am. Med Assoc. 2017, 318, 2446–2456. [Google Scholar] [CrossRef]
- Nhung, N.T.T.; Amini, H.; Schindler, C.; Joss, M.K.; Dien, T.M.; Probst-Hensch, N.; Perez, L.; Künzli, N. Short-term association between ambient air pollution and pneumonia in children: A systematic review and meta-analysis of time-series and case-crossover studies. Environ. Pollut. 2017, 230, 1000–1008. [Google Scholar] [CrossRef]
- Navidi, W. Bidirectional case-crossover designs for exposures with time trends. Biometrics 1998, 54, 596–605. [Google Scholar] [CrossRef]
- Program, D. Wealth Index Construction. 2020. Available online: https://dhsprogram.com/topics/wealth-index/index.cfm (accessed on 1 March 2022).
- Tong, D.Q.; Dan, M.; Wang, T.; Lee, P. Long-term dust climatology in the western United States reconstructed from routine aerosol ground monitoring. Atmos. Chem. Phys. 2012, 12, 5189–5205. [Google Scholar] [CrossRef] [Green Version]
- Few, R. Health and climatic hazards: Framing social research on vulnerability, response and adaptation. Glob. Environ. Chang. 2007, 17, 281–295. [Google Scholar] [CrossRef]
- Haines, A.; Kovats, R.S.; Campbell-Lendrum, D.; Corvalán, C. Climate change and human health: Impacts, vulnerability, and mitigation. Lancet 2006, 367, 2101–2109. [Google Scholar] [CrossRef]
- Walson, J.L.; Berkley, J.A. The impact of malnutrition on childhood infections. Curr. Opin. Infect. Dis. 2018, 31, 231–236. [Google Scholar] [CrossRef]
- Levy, K.; Woster, A.P.; Goldstein, R.S.; Carlton, E.J. Untangling the impacts of climate change on waterborne diseases: A systematic review of relationships between diarrheal diseases and temperature, rainfall, flooding, and drought. Environ. Sci. Technol. 2016, 50, 4905–4922. [Google Scholar] [CrossRef] [Green Version]
- Pu, B.; Ginoux, P. Projection of American dustiness in the late 21st century due to climate change. Sci. Rep. 2017, 7, 5553. [Google Scholar] [CrossRef] [Green Version]
- Bhattachan, A.; D’Odorico, P.; Dintwe, K.; Okin, G.S.; Collins, S.L. Resilience and recovery potential of duneland vegetation in the southern Kalahari. Ecosphere 2014, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]

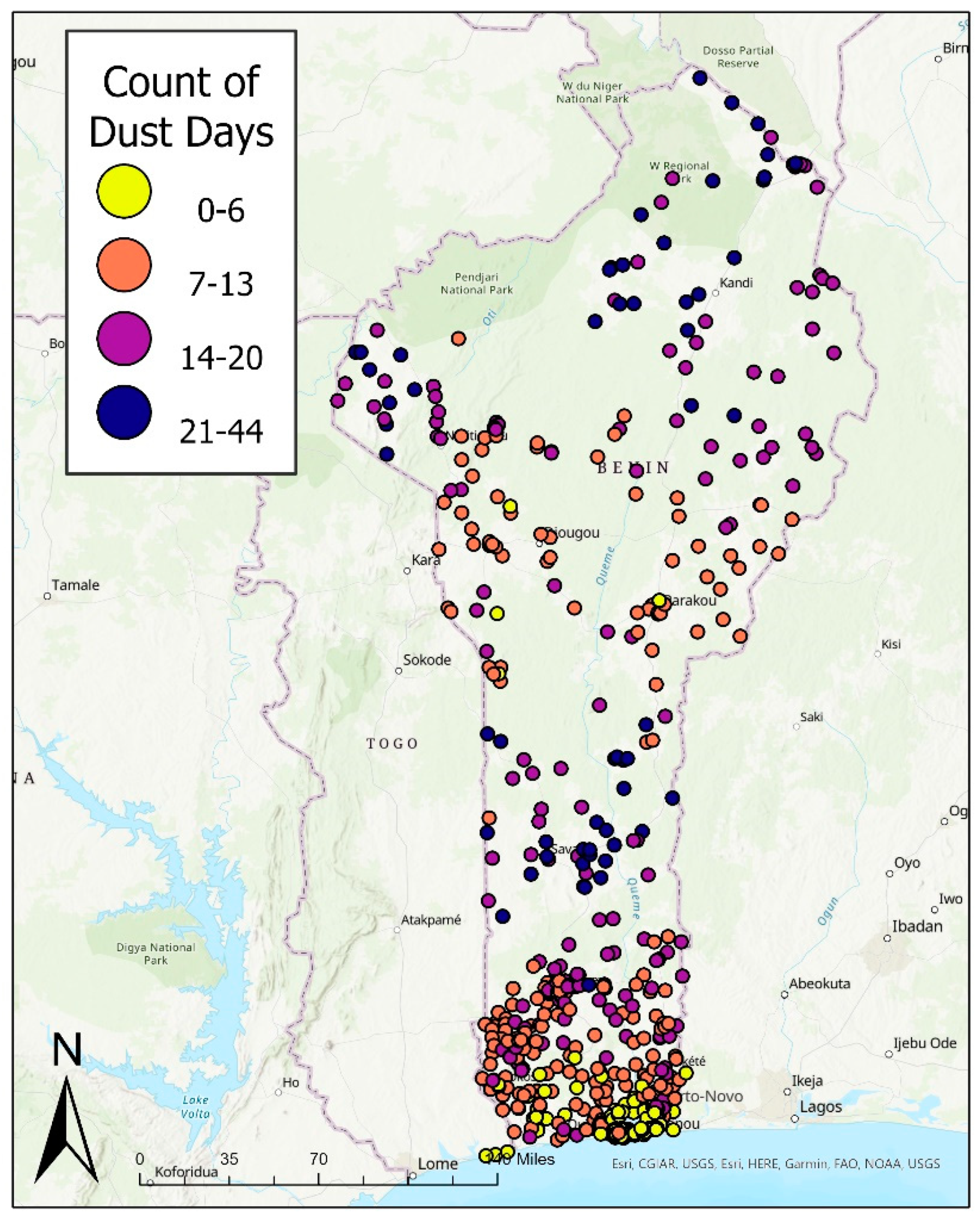
| Overall (N = 13,589) | |
|---|---|
| Outcome Status | |
| Cough | 2018 (14.9%) |
| No Cough | 10,520 (77.4%) |
| Missing | 1051 (7.7%) |
| Exposure Status | |
| Dust present | 1314 (9.7%) |
| Dust not present | 12,132 (89.3%) |
| Missing | 143 (1.1%) |
| Household Wealth | |
| 1-Low wealth | 3020 (22.2%) |
| 2 | 2776 (20.4%) |
| 3 | 2670 (19.6%) |
| 4 | 2639 (19.4%) |
| 5-High wealth | 2484 (18.3%) |
| Maternal Education | |
| Mean (SD) | 2.18 (3.65) |
| Median [Min, Max] | 0 [0, 17.0] |
| Place of Residence | |
| Urban | 5401 (39.7%) |
| Rural | 8188 (60.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McElroy, S.; Dimitrova, A.; Evan, A.; Benmarhnia, T. Saharan Dust and Childhood Respiratory Symptoms in Benin. Int. J. Environ. Res. Public Health 2022, 19, 4743. https://doi.org/10.3390/ijerph19084743
McElroy S, Dimitrova A, Evan A, Benmarhnia T. Saharan Dust and Childhood Respiratory Symptoms in Benin. International Journal of Environmental Research and Public Health. 2022; 19(8):4743. https://doi.org/10.3390/ijerph19084743
Chicago/Turabian StyleMcElroy, Sara, Anna Dimitrova, Amato Evan, and Tarik Benmarhnia. 2022. "Saharan Dust and Childhood Respiratory Symptoms in Benin" International Journal of Environmental Research and Public Health 19, no. 8: 4743. https://doi.org/10.3390/ijerph19084743
APA StyleMcElroy, S., Dimitrova, A., Evan, A., & Benmarhnia, T. (2022). Saharan Dust and Childhood Respiratory Symptoms in Benin. International Journal of Environmental Research and Public Health, 19(8), 4743. https://doi.org/10.3390/ijerph19084743






