Rotaviruses and Noroviruses as Etiological Agents of Acute Intestinal Diseases of Ukrainian Children
Abstract
1. Introduction
2. Materials and Methods
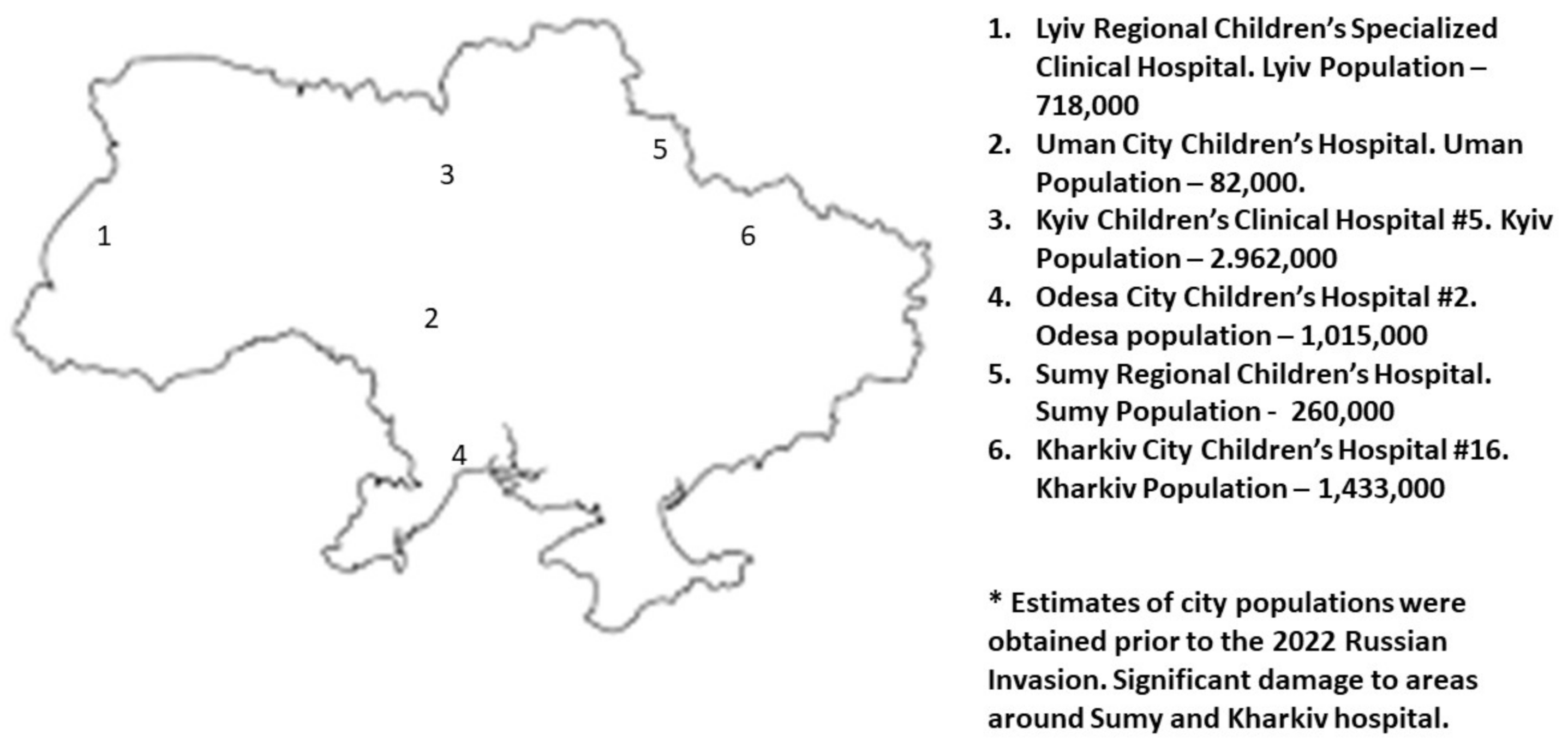
2.1. Patients and Setting
2.2. Enrollment, Consent, and Diagnosis Inclusion
2.3. Laboratory Testing
3. Results
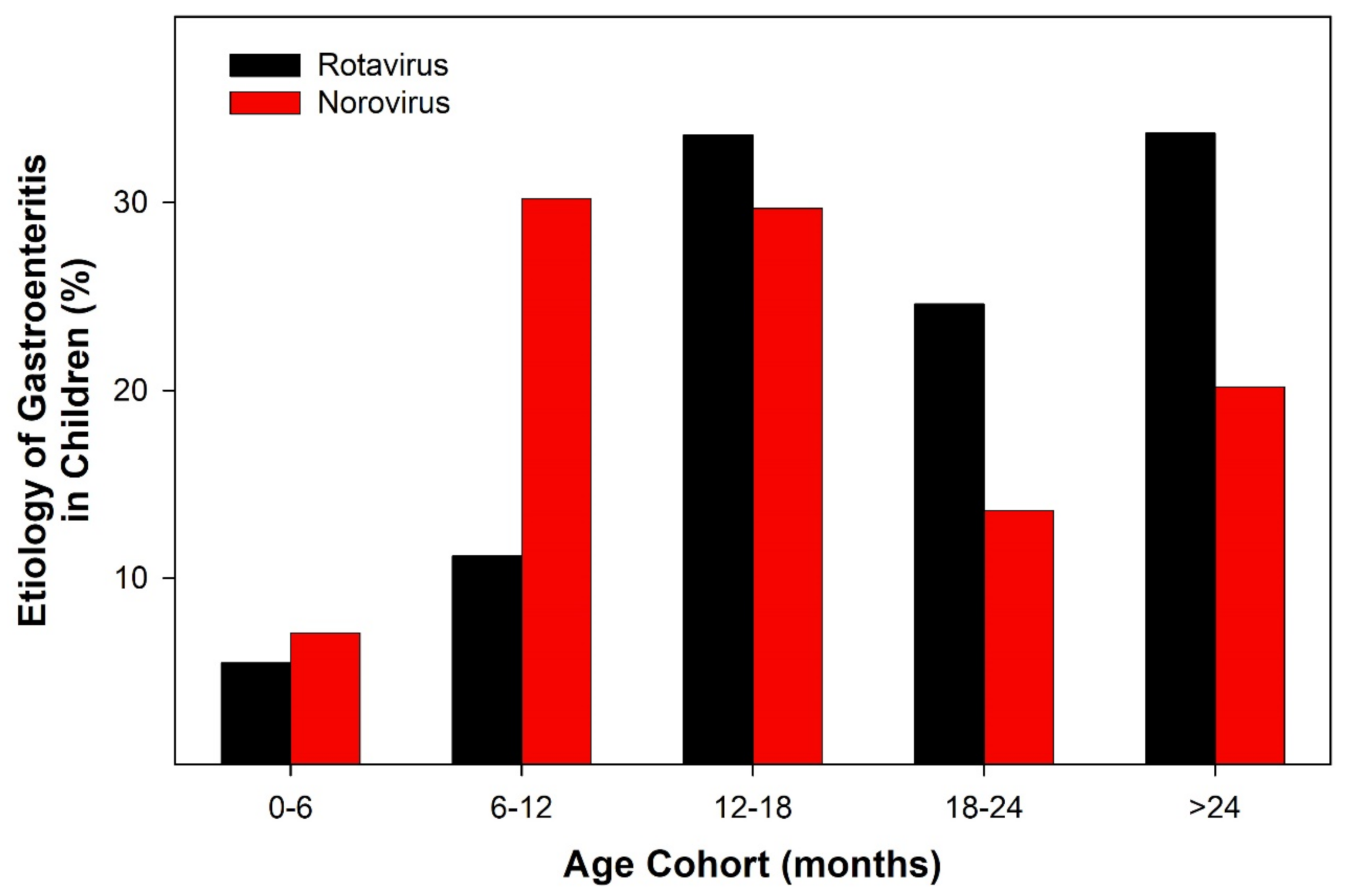
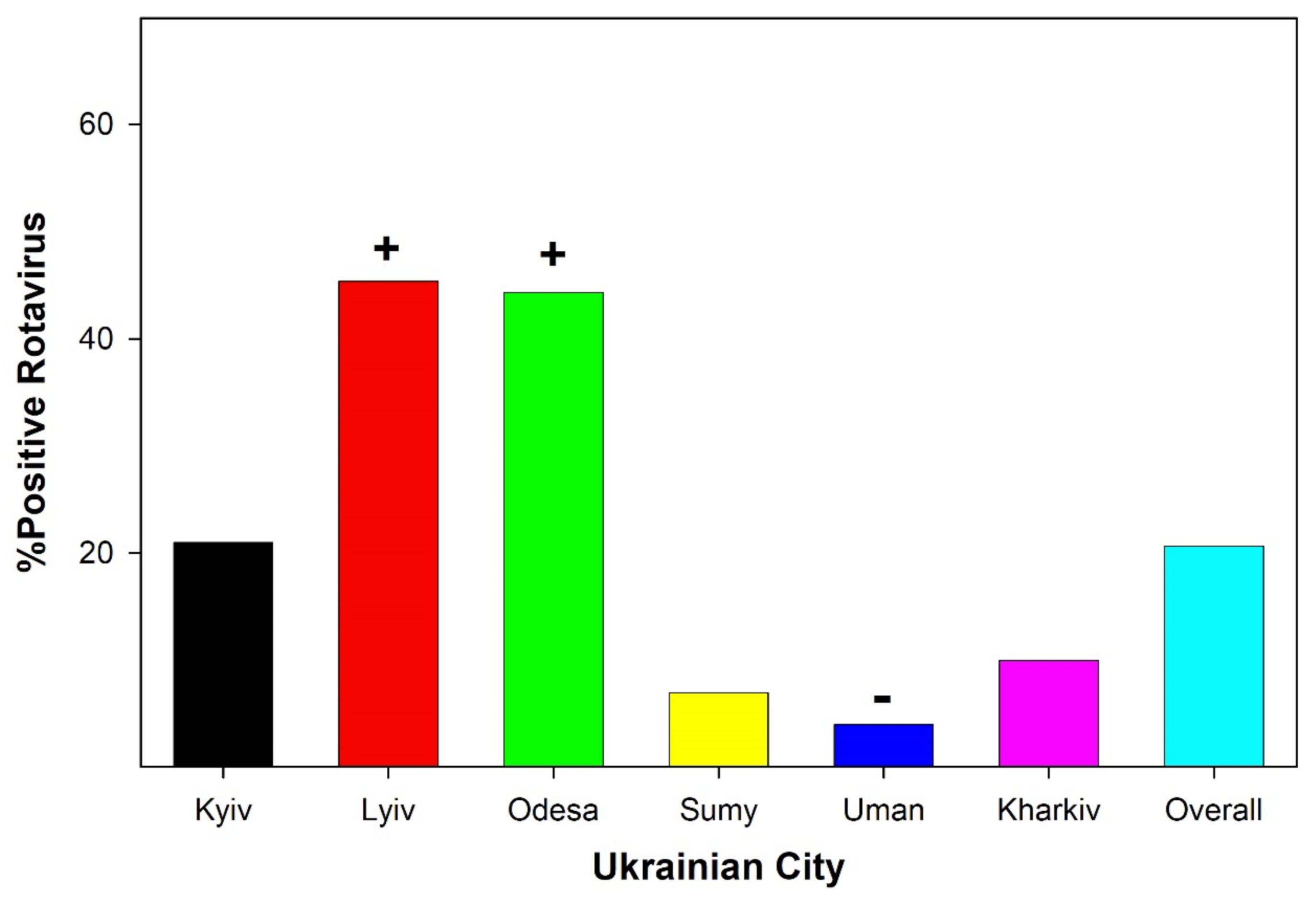
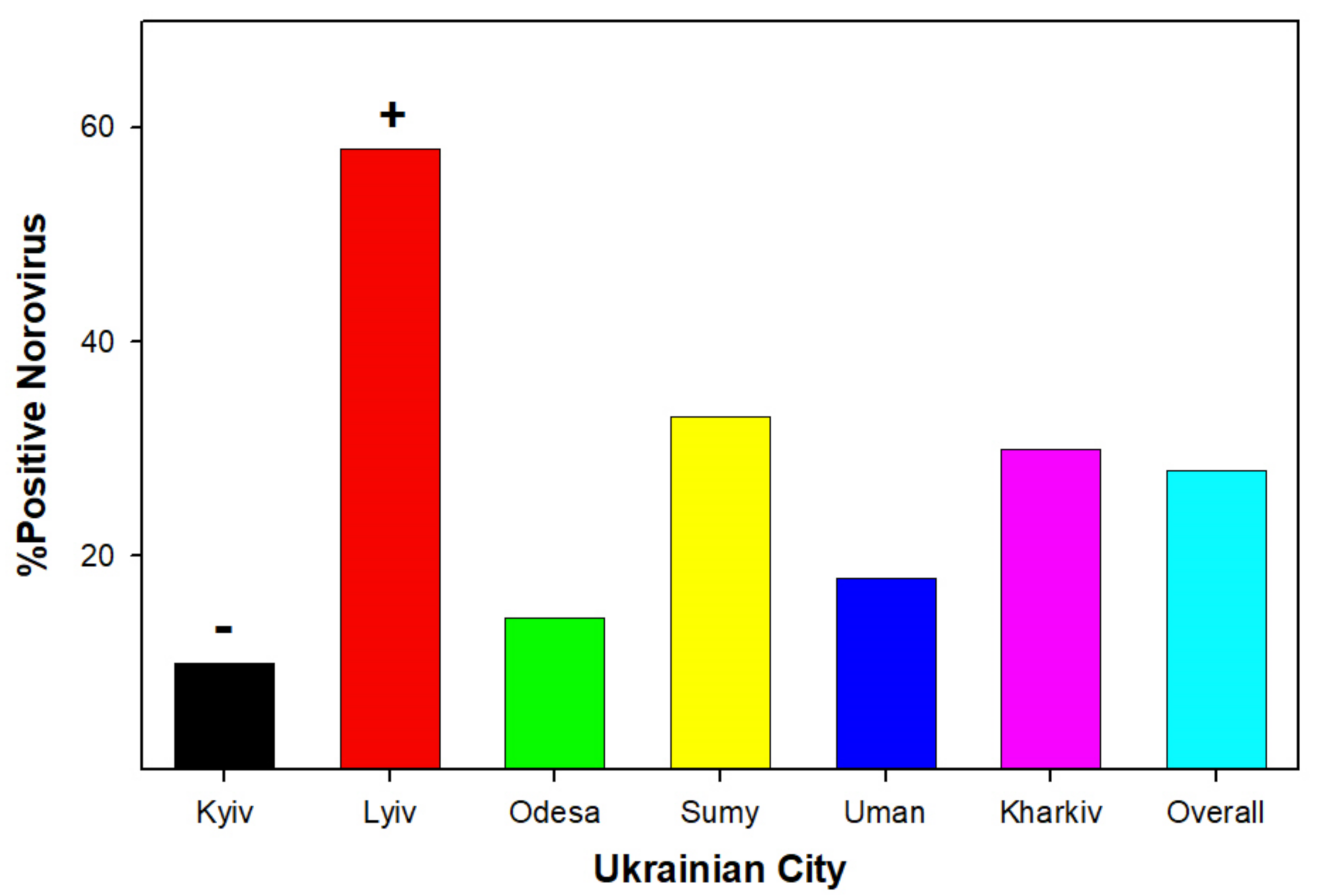
3.1. Viral Detection
3.2. Characterization of AII
3.3. Detection of Bacterial Infection in Conjunction with Viral Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, E.; Bryce, J.; Perin, J.; Newby, H. Harmful practices in the management of childhood diarrhea in low- and middle-income countries: A systematic review. BMC Public Health 2015, 15, 788. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.D.; Hummelman, E.G.; Bresee, J.S.; Miller, M.A.; Glass, R.I. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 2003, 9, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Vetter, V.; Standaert, B.; Benninghoff, B. Fifteen years of experience with the oral live-attenuated human rotavirus vaccine: Reflections on lessons learned. Expert Rev. Vaccines 2020, 19, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Rheingans, R.D.; Antil, L.; Dreibelbis, R.; Podewils, L.J.; Bresee, J.S.; Parashar, U.D. Economic Costs of Rotavirus Gastroenteritis and Cost? Effectiveness of Vaccination in Developing Countries. J. Infect. Dis. 2009, 200, S16–S27. [Google Scholar] [CrossRef]
- Ardura-Garcia, C.; Kreis, C.; Rakic, M.; Jaboyedoff, M.; Mallet, M.C.; Low, N.; Kuehni, C.E. Rotavirus disease and health care utilisation among children under 5 years of age in highly developed countries: A systematic review and meta-analysis. Vaccine 2021, 39, 2917–2928. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef]
- Sadiq, A.; Bostan, N.; Yinda, K.C.; Naseem, S.; Sattar, S. Rotavirus: Genetics, pathogenesis and vaccine advances. Rev. Med. Virol. 2018, 28, e2003. [Google Scholar] [CrossRef]
- Karst, S.M.; Tibbetts, S.A. Recent advances in understanding norovirus pathogenesis. J. Med. Virol. 2016, 88, 1837–1843. [Google Scholar] [CrossRef]
- Gheorghita, S.; Birca, L.; Donos, A.; Wasley, A.; Birca, I.; Cojocaru, R.; Melnick, A.; Ciobanu, S.; Mosina, L.; Cortese, M.M.; et al. Impact of Rotavirus Vaccine Introduction and Vaccine Effectiveness in the Republic of Moldova. Clin. Infect. Dis. 2016, 62, S140–S146. [Google Scholar] [CrossRef]
- Hovhannisyan, A.; Mkhoyan, A.; Gyulazyan, N.; Asoyan, V.; Navoyan, T.; Gevorgyan, Z.; Simonyan, M. Epidemiology and economic losses of rotavirus infection associated with hospitalization of Armenian children. J. Infect. Dev. Ctries. 2019, 13, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Khetsuriani, N.; Perehinets, I.; Nitzan, D.; Popovic, D.; Moran, T.; Allahverdiyeva, V.; Huseynov, S.; Gavrilin, E.; Slobodianyk, L.; Izhyk, O.; et al. Responding to a cVDPV1 outbreak in Ukraine: Implications, challenges and opportunities. Vaccine 2017, 35, 4769–4776. [Google Scholar] [CrossRef] [PubMed]
- Hrynash, Y.; Nadraga, A.; Dasho, M. Effectiveness of a vaccination program against mumps in Ukraine. Eur. J. Clin. Microbiol. 2008, 27, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Smiyan, O.; Popov, S.; Petrashenko, V.; Zaitsev, I.; Redko, O.; Zahorodnii, M.; Kasyan, S. Child health care system in Ukraine. Turk. Pediatr. Ars. 2020, 55, 98–104. [Google Scholar]
- Chernyshova, L.I.; Radionova, N.M.; Demchyshyna, I.V.; Kotlik, L.S.; Sadkova, O.B.; Samoilovich, E.O.; Semeiko, G.V.; Daniels, D.S.; Cohen, A.L.; Aliabadi, N. Observations on the epidemiology of rotavirus infection among hospitalized children younger than 5 years in 2 Ukrainian hospitals, 2007–2015. Vaccine 2018, 36, 7798–7804. [Google Scholar] [CrossRef]
- Mirzayeva, R.; Cortese, M.M.; Mosina, L.; Biellik, R.; Lobanov, A.; Chernyshova, L.; Lashkarashvili, M.; Turkov, S.; Iturriza-Gomara, M.; Gray, J.; et al. Rotavirus Burden among Children in the Newly Independent States of the Former Union of Soviet Socialist Republics: Literature Review and First-Year Results from the Rotavirus Surveillance Network. J. Infect. Dis. 2009, 200, S203–S214. [Google Scholar] [CrossRef][Green Version]
- Fischer, T.K.; Viboud, C.; Parashar, U.; Malek, M.; Steiner, C.; Glass, R.; Simonsen, L. Hospitalizations and Deaths from Diarrhea and Rotavirus among Children <5 Years of Age in the United States, 1993–2003. J. Infect. Dis. 2007, 195, 1117–1125. [Google Scholar] [CrossRef]
- Krawczyk, A.; Lewis, M.G.; Venkatesh, B.T.; Nair, S.N. Effect of Exclusive Breastfeeding on Rotavirus Infection among Children. Indian J. Pediatr. 2015, 83, 220–225. [Google Scholar] [CrossRef]
- Shumetie, G.; Gedefaw, M.; Kebede, A.; Derso, T. Exclusive breastfeeding and rotavirus vaccination are associated with decreased diarrheal morbidity among under-five children in Bahir Dar, northwest Ethiopia. Public Health Rev. 2018, 39, 28. [Google Scholar] [CrossRef] [PubMed]
- Zakarija-Grković, I.; Cattaneo, A.; Bettinelli, M.E.; Pilato, C.; Vassallo, C.; Buontempo, M.B.; Gray, H.; Meynell, C.; Wise, P.; Harutyunyan, S.; et al. Are our babies off to a healthy start? The state of implementation of the Global strategy for infant and young child feeding in Europe. Int. Breastfeed. J. 2020, 15, 51. [Google Scholar] [CrossRef]
- Dryha, N.O.; Stepanenko, A.V.; Rudenko, L.A.; Zhaldak, D.O.; Piven, S.M.; Plakhtiienko, I.O. Results of medical-social research on medical care quality for patients with COVID-19 of inpatient hospital departments in sumy region. Wiadomości Lek. 2021, 74, 1057–1060. [Google Scholar] [CrossRef]
- Gopchak, I.; Basiuk, T.; Bialyk, I.; Pinchuk, O.; GerAsimov, I. Dynamics of changes in surface water quality indicators of the Western Bug River basin within Ukraine using GIS technologies. PAN Wars. J. Water Land Dev. 2018, 42, 67–75. [Google Scholar] [CrossRef][Green Version]
- Koynova, I.; Rozhko, I.; Blazhko, N. Ecological threats to the valley of the Bug river (Lviv region). Natural Human Environment. Dangers, protection, education. Monograph, edited by Kazimierz H. Dygus. Warszawa 2012, 3, 55–64. [Google Scholar]
- Odnorih, Z.; Manko, R.; Malovanyy, M.; Soloviy, K. Results of surface water quality monitoring of the Western Bug River Basin in Lviv Region. J. Ecol. Eng. 2020, 21, 18–26. [Google Scholar] [CrossRef]
- Lekishvili, S.; Chayen, B.; Chayen, S. Suspected environmental and socio-economic causes of diabetes mellitus and associated ocular complications in the Sumy region, Ukraine, for the period of 2011–2016. Georgian Med. News 2018, 278, 120–126. [Google Scholar]
- Summers, A.; Bilukha, O. Suboptimal infant and young child feeding practices among internally displaced persons during conflict in eastern Ukraine. Public Health Nutr. 2017, 21, 917–926. [Google Scholar] [CrossRef]
- Tabunga, T.; Utiera, M.; Tekoaua, R.; Tibwe, T.; Tira, T.; Toatu, T.; Duituturaga, S.E.; Nilles, E.; Craig, A.T. Response to a large rotavirus outbreak on South Tarawa, Kiribati, 2013. West. Pac. Surveill. Response J. 2014, 5, 9–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chhabra, P.; Samoilovich, E.; Yermalovich, M.; Chernyshova, L.; Gheorghita, S.; Cojocaru, R.; Shugayev, N.; Sahakyan, G.; Lashkarashvili, M.; Chubinidze, M.; et al. Viral gastroenteritis in rotavirus negative hospitalized children <5 years of age from the independent states of the former Soviet Union. Infect. Genet. Evol. 2014, 28, 283–288. [Google Scholar] [CrossRef]
| Clinical Manifestations | Rotavirus Infection | Norovirus Infection |
|---|---|---|
| The Duration of Diarrhea (Days) | ||
| <1 day (0 marks) | 0 | 8.7% |
| 1–4 (1 mark) | 50.0% | 43.5% |
| 5 (2 marks) | 19.2% | 21.7% |
| >5 (3 marks) | 30.8% | 26.1% |
| The greatest number of defecations during 24 h | ||
| 1–3 (1 mark) | 26.9% | 30.4% |
| 4–5 (2 marks) | 30.8% | 34.8% |
| >5 (3 marks) | 42.3% | 34.8% |
| The duration of vomiting (days) | ||
| None (0 marks) | 11.5% | 8.7% |
| 1 (1 mark) | 50.0% | 34.8% |
| 2 (2 marks) | 23.1% | 21.7% |
| >2 (3 marks) | 15.4% | 39.1% |
| The greatest number of cases of vomiting during 24 h | ||
| None (0 marks) | 11.5% | 8.7% |
| 1 (1 mark) | 3.8% | 4.3% |
| 2–4 (2 marks) | 57.7% | 43.5% |
| >4 (3 marks) | 26.9% | 43.5% |
| Hyperthermia | ||
| <37.1 (0 marks) | 26.9% | 30.4% |
| 37.1–38.4 (1 mark) | 34.6% | 34.8% |
| 38.5–38.9 (2 mark) | 23.1% | 26.1% |
| >38.9 (3 marks) | 15.4% | 8.7% |
| Dehydration | ||
| None (0 marks) | 42.3% | 43.5% |
| 1–5% (2 marks) | 26.9% | 17.4% |
| >5% (3 marks) | 30.8% | 39.1% |
| The treatment | ||
| Not carried out (0 marks) | 0 | 0 |
| The rehydration (1 mark) | 56.5% | 39.1% |
| Hospitalization (3 marks) | 53.5% | 60.9% |
| Agent | Rotavirus | Norovirus |
|---|---|---|
| Salmonella | 0.5 | 0.14 |
| Klebsiella | 0.23 | 0.27 |
| Proteus | 0.08 | 0.09 |
| E. aeruginosa | 0.12 | 0.32 |
| Staphylococcus aureus | 0.15 | 0.05 |
| Not isolated | 0.27 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soloviov, S.O.; Todosiichuk, T.S.; Kovaliuk, O.V.; Filippelli, G.M.; Trokhymenko, O.P.; Dziublyk, I.V.; Rodd, Z.A. Rotaviruses and Noroviruses as Etiological Agents of Acute Intestinal Diseases of Ukrainian Children. Int. J. Environ. Res. Public Health 2022, 19, 4660. https://doi.org/10.3390/ijerph19084660
Soloviov SO, Todosiichuk TS, Kovaliuk OV, Filippelli GM, Trokhymenko OP, Dziublyk IV, Rodd ZA. Rotaviruses and Noroviruses as Etiological Agents of Acute Intestinal Diseases of Ukrainian Children. International Journal of Environmental Research and Public Health. 2022; 19(8):4660. https://doi.org/10.3390/ijerph19084660
Chicago/Turabian StyleSoloviov, Serhii O., Tetiana S. Todosiichuk, Olena V. Kovaliuk, Gabriel M. Filippelli, Olena P. Trokhymenko, Iryna V. Dziublyk, and Zachary A. Rodd. 2022. "Rotaviruses and Noroviruses as Etiological Agents of Acute Intestinal Diseases of Ukrainian Children" International Journal of Environmental Research and Public Health 19, no. 8: 4660. https://doi.org/10.3390/ijerph19084660
APA StyleSoloviov, S. O., Todosiichuk, T. S., Kovaliuk, O. V., Filippelli, G. M., Trokhymenko, O. P., Dziublyk, I. V., & Rodd, Z. A. (2022). Rotaviruses and Noroviruses as Etiological Agents of Acute Intestinal Diseases of Ukrainian Children. International Journal of Environmental Research and Public Health, 19(8), 4660. https://doi.org/10.3390/ijerph19084660







