Effect of Cigarette and E-Cigarette Smoke Condensates on Candida albicans Biofilm Formation and Gene Expression
Abstract
1. Introduction
2. Materials and Methods
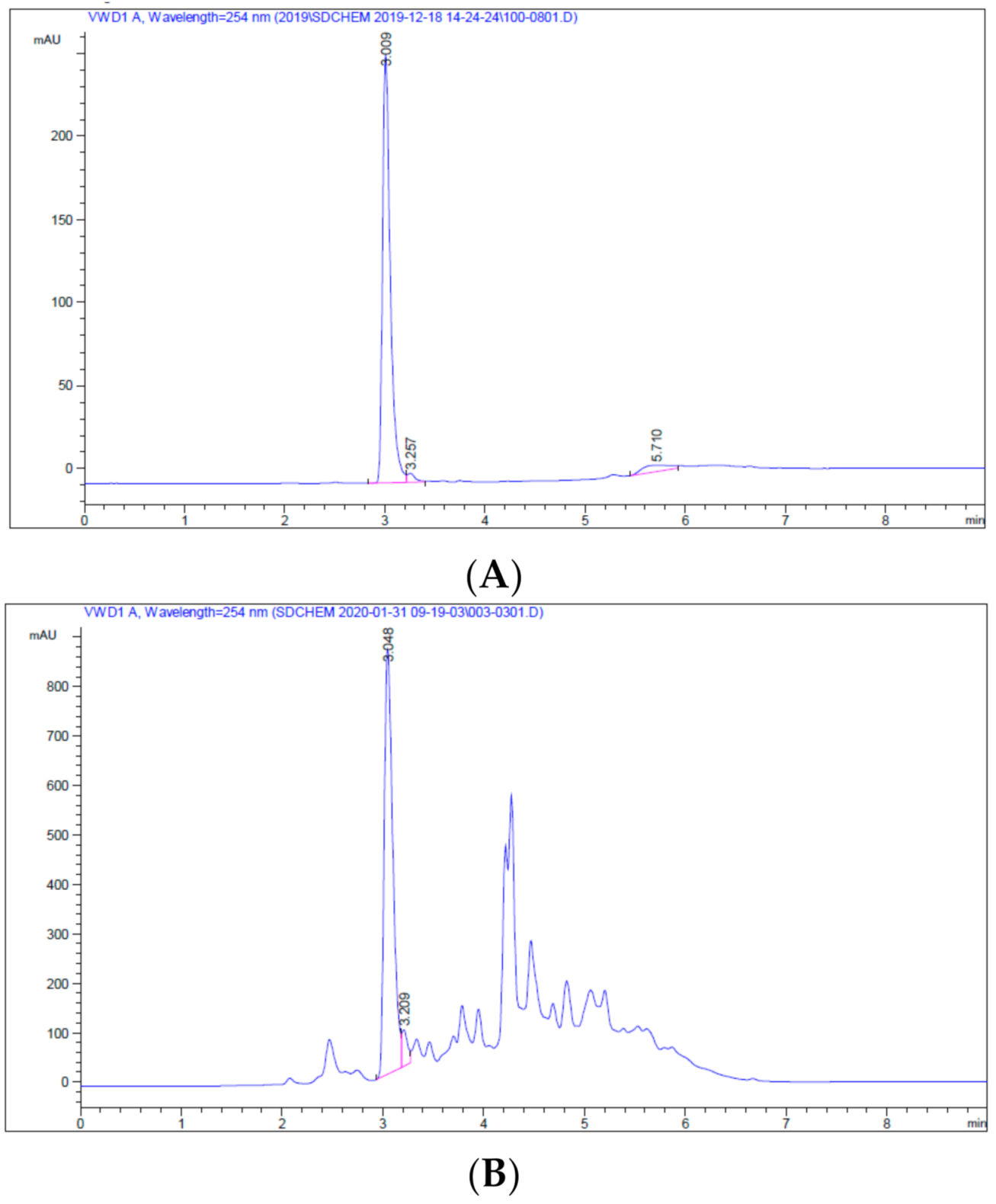
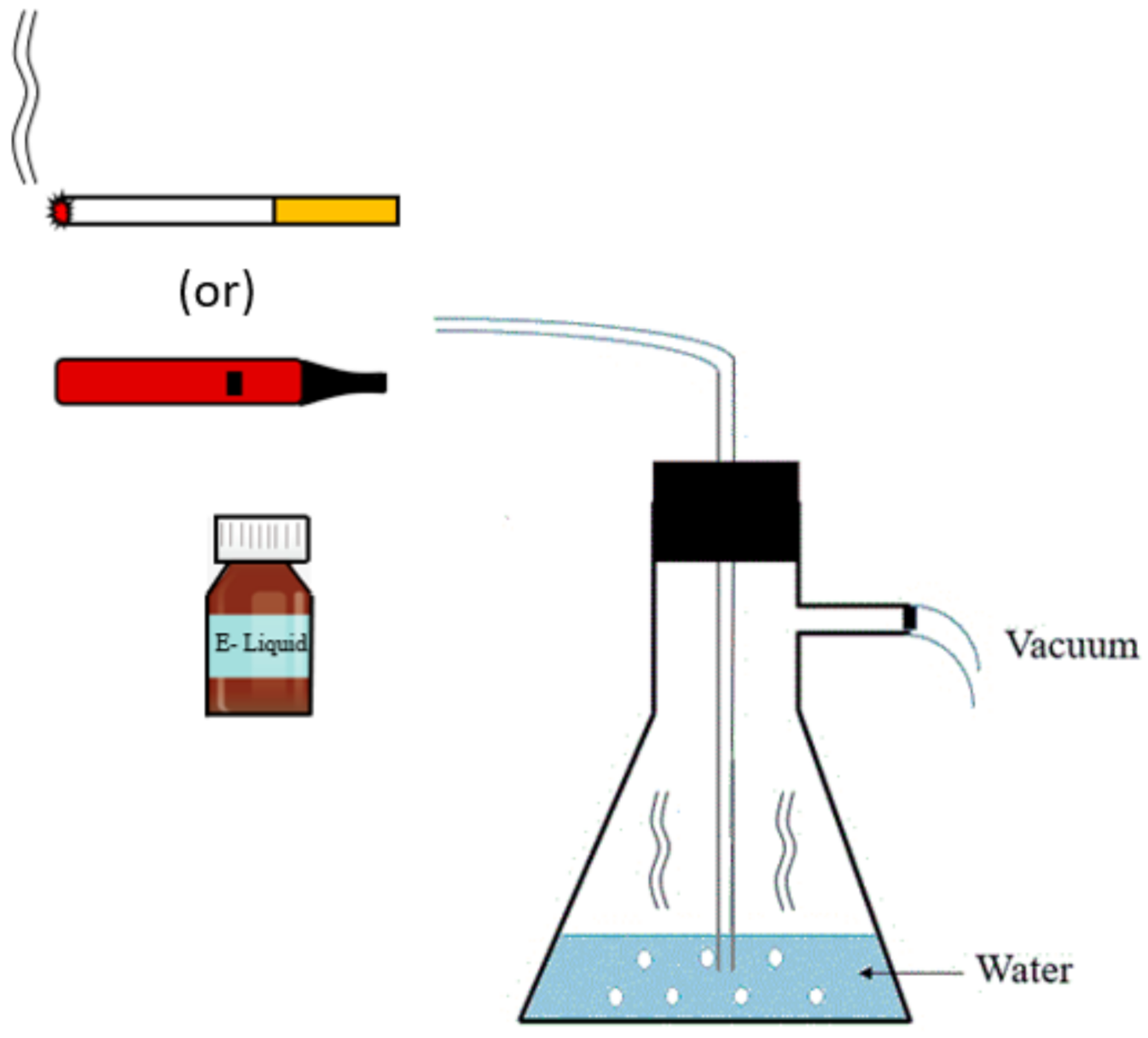
2.1. Preparation of CSC and ECSC
2.2. Candida albicans Strain, Culture Conditions, and Treatments
2.3. Measuring the Effect of N/CSC/ECSC or ECSC (−) on C. albicans Growth (MIC and MFC)
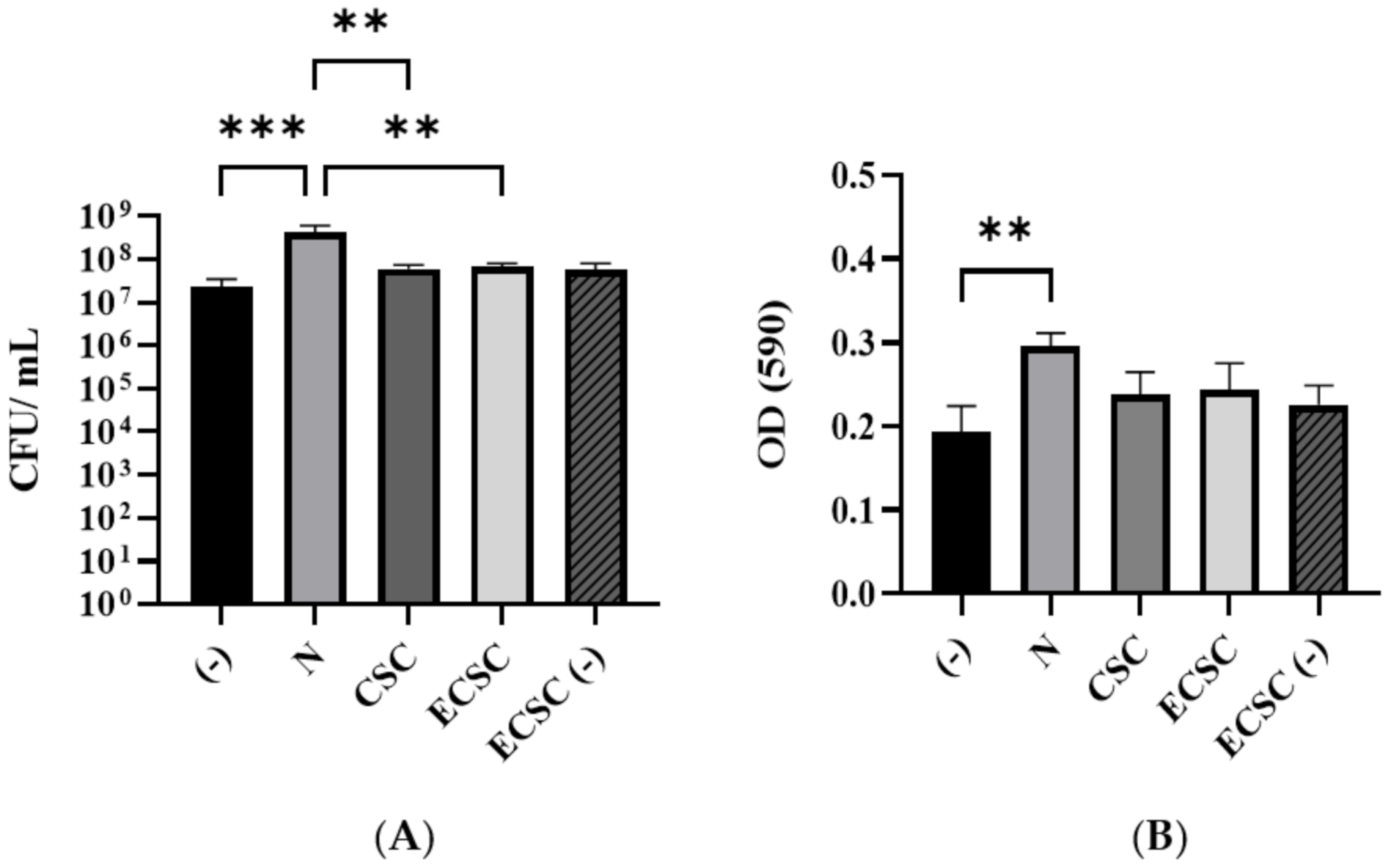
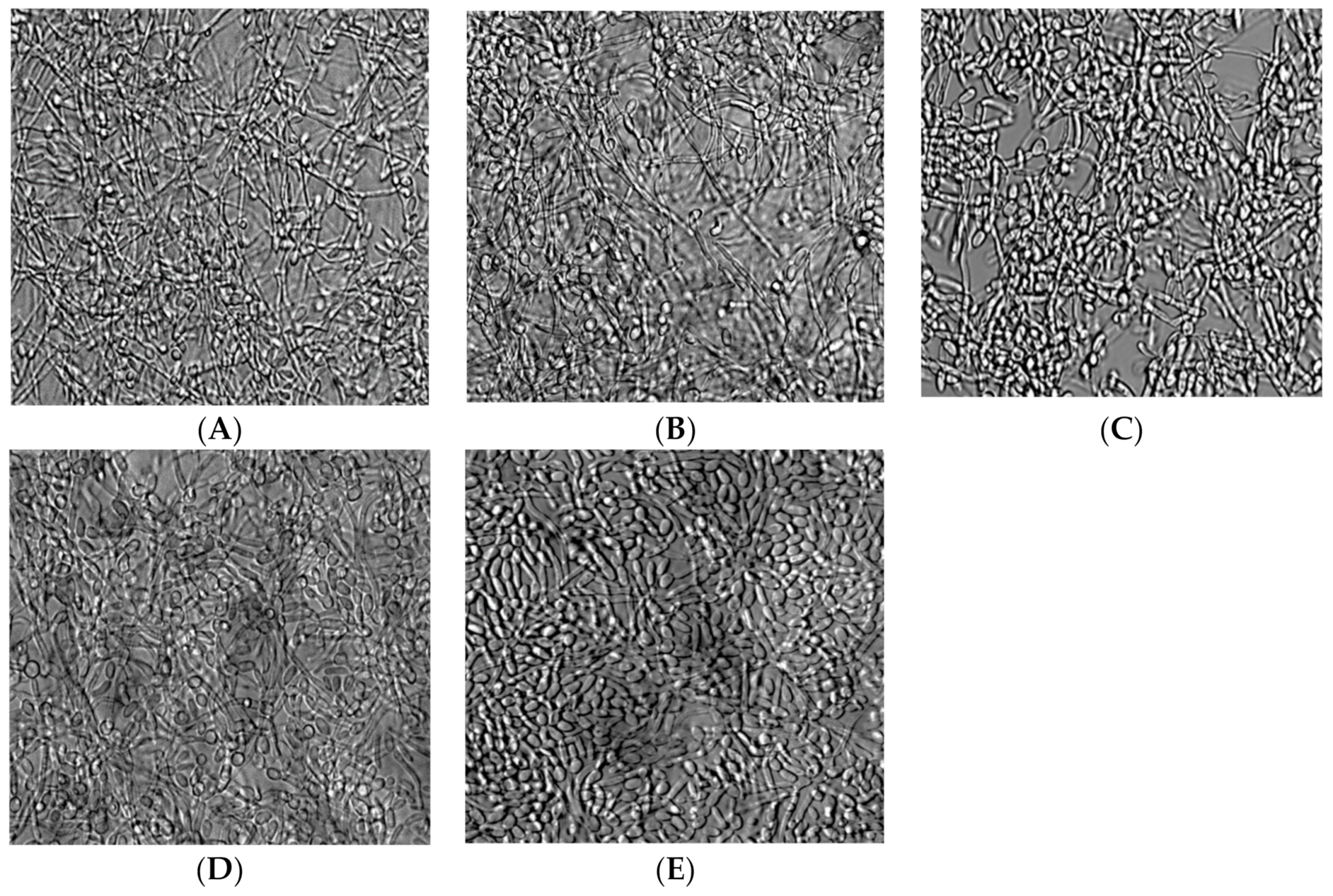
2.4. Measuring the Effect of N/CSC/ECSC+N or ECSC-N on C. albicans Biofilm Formation
2.5. Measuring the Effect of N/CSC/ECSC or ECSC (−) on C. albicans Gene Expression
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Statistical Analysis
3. Results
3.1. Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
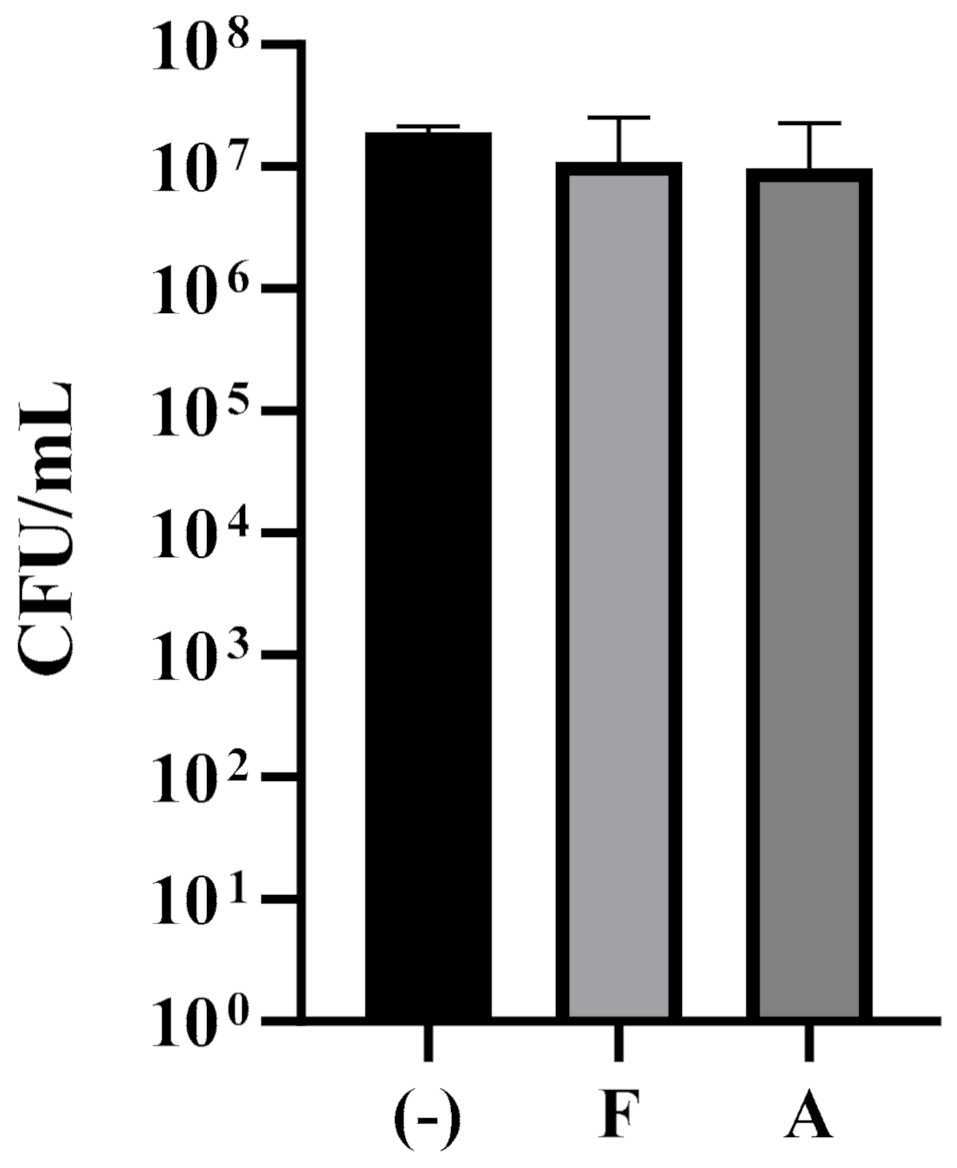
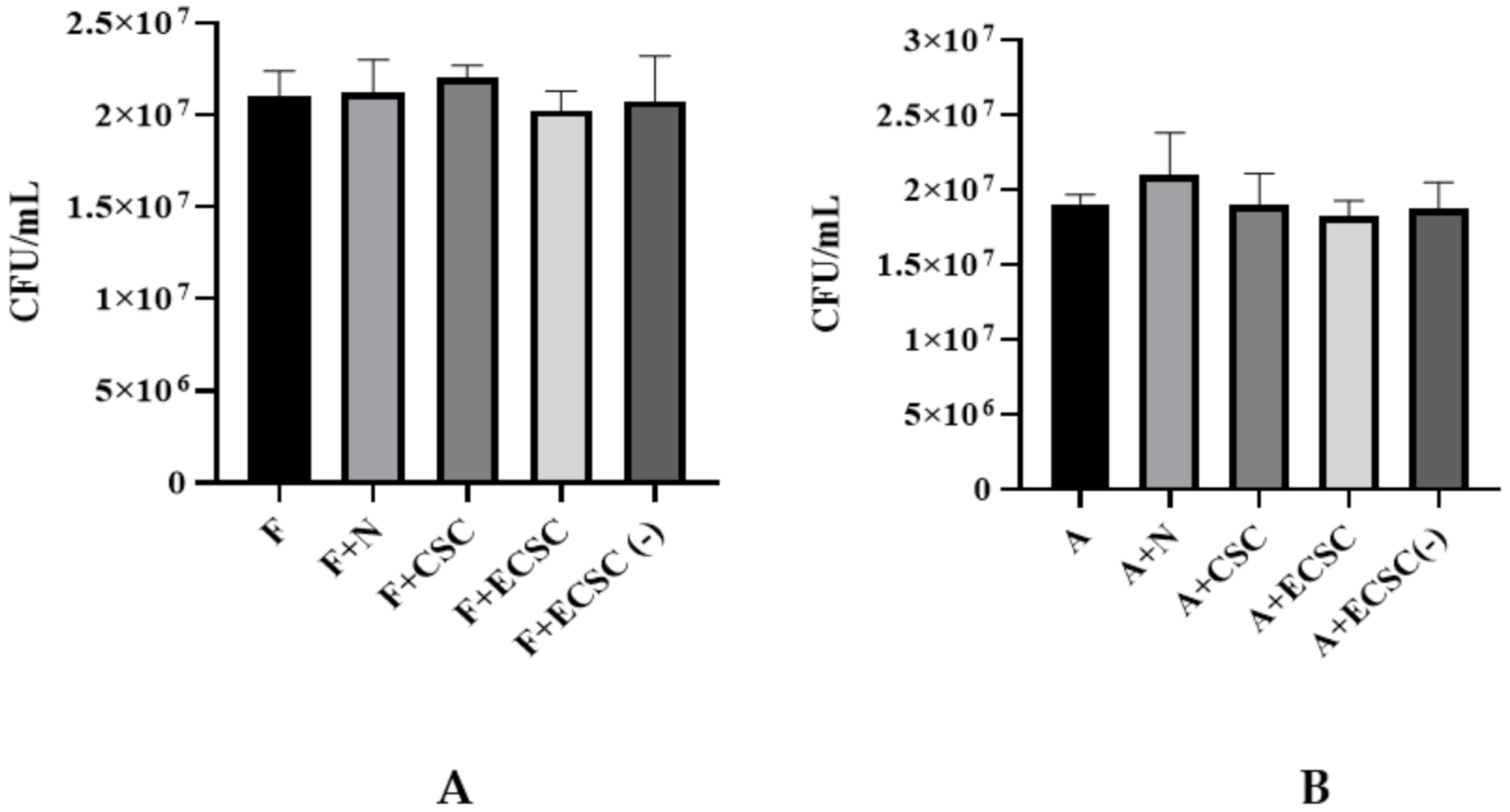
3.2. Candida albicans Biofilm Formation
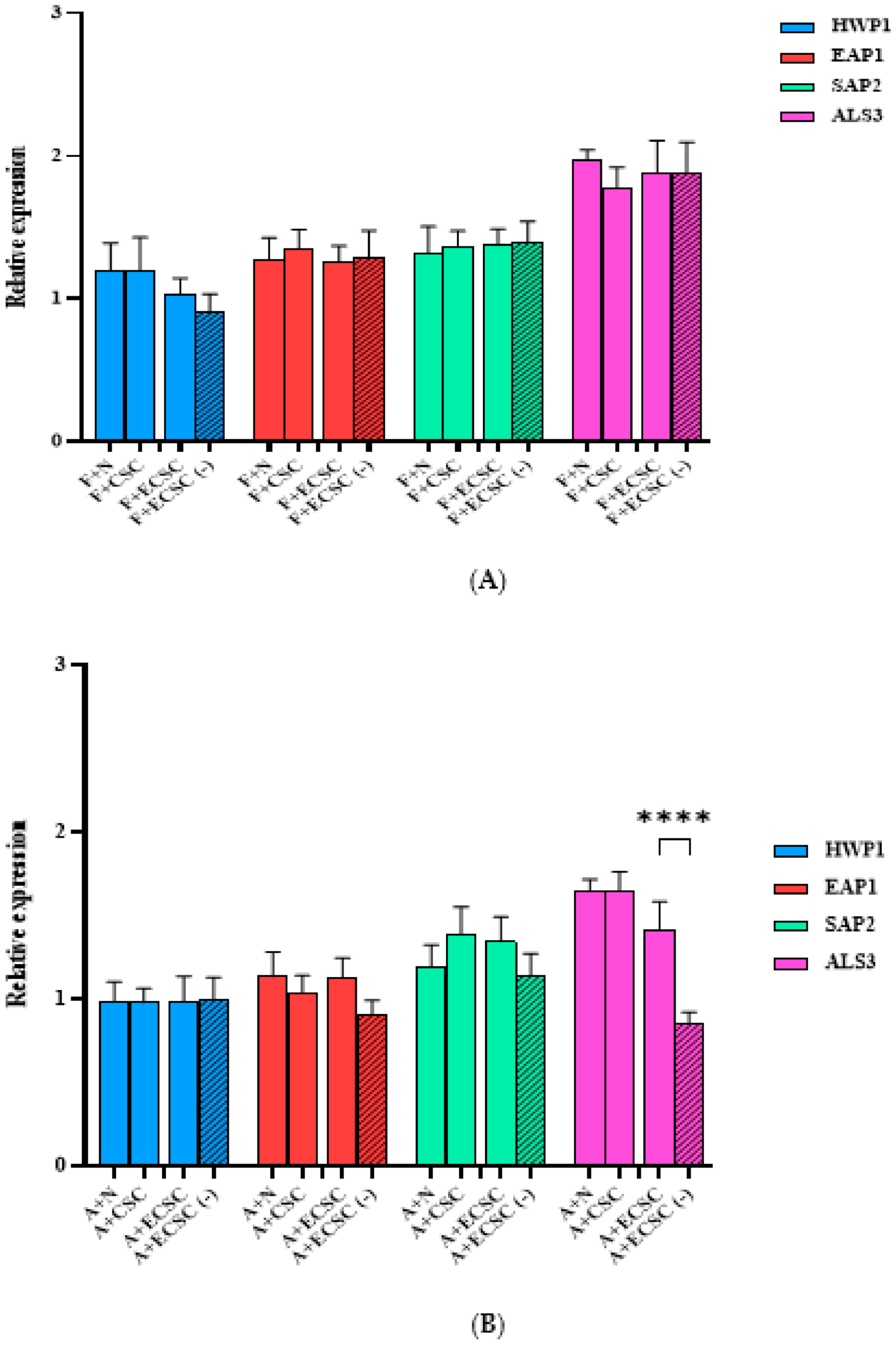
3.3. The Effect of N, CSC, ECSC, and ECSC (−) on Virulence Gene Expression in C. albicans Biofilms
3.4. The Effect of N, CSC, ECSC, and ECSC (−) on the Expression of Virulence Genes in C. albicans Biofilms Treated with Fluconazole and Amphotericin B
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Designation | Sequence (5′-3′) | AT (°C) | Amplicon Size (bp) |
|---|---|---|---|
| HWP1 [22] | F: GCTCAACTTATTGCTATCGCTTATTACA | 54 | 67 |
| R: GACCGTCTACCTGTGGGACAGT | |||
| EAP1 [22] | F: CTGCTCACTCAACTTCAATTGTCG | 60 | 51 |
| R: GAACACATCCACCTTCGGGA | |||
| SAP2 [5] | F: TCCTGATGTTAATGTTGATTGTCAAG | 60 | 82 |
| R: TGGATCATATGTCCCCTTTTGTT | |||
| ALS3 [7] | F: CCACAGCTGCTTCCACTTCT | 57 | 184 |
| R: TGCAGATGGAGCATTACCACC | |||
| ACT1 [5,22] | F: GCTGGTAGAGACTTGACCAACCA | 60 | 87 |
| R: GACAATTTCTCTTTCAGCACTAGTAGTGA |

References
- Feldman, C.; Anderson, R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J. Infect. 2013, 67, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, R.; Gaur, S. Implications of tobacco smoking on the oral health of older adults. Geriatr. Gerontol. Int. 2014, 14, 526–540. [Google Scholar] [CrossRef] [PubMed]
- Baboni, F.B.; Barp, D.; de Azevedo Izidoro, A.C.S.; Samaranayake, L.P.; Rosa, E.A.R. Enhancement of Candida albicans virulence after exposition to cigarette mainstream smoke. Mycopathologia 2009, 168, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Vellappally, S.; Fiala, Z.; Smejkalová, J.; Jacob, V.; Somanathan, R. Smoking related systemic and oral diseases. Acta Medica (Hradec Kralove) 2007, 50, 161–166. [Google Scholar] [CrossRef][Green Version]
- Alanazi, H.; Semlali, A.; Chmielewski, W.; Rouabhia, M. E-cigarettes increase Candida albicans growth and modulate its interaction with gingival epithelial cells. Int. J. Environ. Res. Public Health 2019, 16, 294. [Google Scholar] [CrossRef]
- Talhout, R.; Schulz, T.; Florek, E.; Van Benthem, J.; Wester, P. Hazardous Compounds in Tobacco Smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef]
- Gunasegar, S.; Himratul-Aznita, W.H. Nicotine enhances the thickness of biofilm and adherence of Candida albicans ATCC 14053 and Candida parapsilosis ATCC 22019. FEMS Yeast Res. 2019, 19, foy123. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, W.; Zhang, K.; Zhou, X.; Ren, B.; He, J.; Xu, X.; Cheng, L.; Li, M. Corrigendum to ‘Nicotine Enhances Interspecies Relationship between Streptococcus mutans and Candida albicans. Biomed Res. Int. 2017, 2017, 5803246. [Google Scholar] [CrossRef]
- Wagenknecht, D.R.; BalHaddad, A.R.A.B.; Gregory, R.L. Effects of Nicotine on Oral Microorganisms, Human Tissues, and the Interactions between Them. Curr. Oral Health Rep. 2018, 5, 78–87. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; He, B.; Huang, R.; Li, M. Effect of tobacco on periodontal disease and oral cancer. Tob. Induc. Dis. 2019, 17, 40. [Google Scholar] [CrossRef]
- Liu, S.; Wu, T.; Zhou, X.; Zhang, B.; Huo, S.; Yang, Y.; Zhang, K.; Cheng, L.; Xu, X.; Li, M. Nicotine is a risk factor for dental caries: An in vivo study. J. Dent. Sci. 2018, 13, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S.L.; Hecht, S.S.; Jaspers, I.; Gregory, R.L.; Stepanov, I. Oral Health Effects of Combusted and Smokeless Tobacco Products. Adv. Dent. Res. 2019, 30, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Soysa, N.S.; Ellepola, A.N.B. The impact of cigarette/tobacco smoking on oral candidosis: An overview. Oral Dis. 2005, 11, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Alzayer, Y.M.; Gomez, G.F.; Eckert, G.J.; Levon, J.A.; Gregory, R.L. The Impact of Nicotine and Cigarette Smoke Condensate on Metabolic Activity and Biofilm Formation of Candida albicans on Acrylic Denture Material. J. Prosthodont. 2020, 29, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.; Sandeep, S.; Rodriguez, J. The oral health impact of electronic cigarette use: A systematic review. Crit. Rev. Toxicol. 2020, 50, 97–127. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; Harrell, M.B.; Perry, C.L. Comparing young adults to older adults in e-cigarette perceptions and motivations for use: Implications for health communication. Health Educ. Res. 2016, 31, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Lerner, C.A.; Sundar, I.K.; Watson, R.M.; Elder, A.; Jones, R.; Done, D.; Kurtzman, R.; Osiip, D.J.; Robinson, R.; Mcintosh, S.; et al. Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environ. Pollut. 2015, 198, 100–107. [Google Scholar] [CrossRef]
- Cichońska, D.; Kusiak, A.; Piechowicz, L.; Świetlik, D. A pilot investigation into the influence of electronic cigarettes on oral bacteria. Adv. Dermatol. Allergol. 2021, 38, 1092–1098. [Google Scholar] [CrossRef]
- Cuadra, G.A.; Smith, M.T.; Nelson, J.M.; Loh, E.K.; Palazzolo, D.L. A comparison of flavorless electronic cigarette-generated aerosol and conventional cigarette smoke on the planktonic growth of common oral commensal streptococci. Int. J. Environ. Res. Public Health 2019, 16, 1669. [Google Scholar] [CrossRef]
- Bagale, K.; Paudel, S.; Cagle, H.; Sigel, E.; Kulkarni, R. Electronic cigarette (E-cigarette) vapor exposure alters the streptococcus pneumoniae transcriptome in a nicotine-dependent manner without affecting pneumococcal virulence. Appl. Environ. Microbiol. 2020, 86, e02125-19. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Ross, G.; Pagé, N.; Chakir, J. Interleukin-18 and gamma interferon production by oral epithelial cells in response to exposure to Candida albicans or lipopolysaccharide stimulation. Infect. Immun. 2002, 70, 7073–7080. [Google Scholar] [CrossRef]
- Semlali, A.; Killer, K.; Alanazi, H.; Chmielewski, W.; Rouabhia, M. Cigarette smoke condensate increases C. albicans adhesion, growth, biofilm formation, and EAP1, HWP1 and SAP2 gene expression. BMC Microbiol. 2014, 14, 61. [Google Scholar] [CrossRef]
- Thewes, S.; Moran, G.P.; Magee, B.B.; Schaller, M.; Sullivan, D.J.; Hube, B. Phenotypic screening, transcriptional profiling, and comparative genomic analysis of an invasive and non-invasive strain of Candida albicans. BMC Microbiol. 2008, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Klis, F.M.; Sosinska, G.J.; De Groot, P.W.J.; Brul, S. Covalently linked cell wall proteins of Candida albicans and their role in fitness and virulence. FEMS Yeast Res. 2009, 9, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Dineshshankar, J.; Sivakumar, M.; Karthikeyan, M.; Udayakumar, P.; Shanmugam, K.T.; Kesavan, G. Immunology of oral candidiasis. J. Pharm. Bioallied Sci. 2014, 6, 12–15. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Kullberg, B.J.; Van De Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, F.R.; Littringer, K.; Altmeier, S.; Tran, V.D.; Schoherr, F.; Lemberg, C.; Pagni, M.; Sanglard, D.; Joller, N.; Landmann, S.L. Persistence of Candida albicans in the Oral Mucosa Induces a Curbed Inflammatory Host Response That Is Independent of Immunosuppression. Front. Immunol. 2019, 10, 330. [Google Scholar] [CrossRef]
- Wigginton, B.; Gartner, C.; Rowlands, I.J. Is It Safe to Vape? Analyzing Online Forums Discussing E-Cigarette Use during Pregnancy. Women’s Health 2017, 27, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Gunasegar, S.; Himratul-Aznita, W.H. Influence of nicotine on the adherence of Candida albicans ATCC 14053 and Candida parapsilosis ATCC 22019 and expression of selected binding-related genes. Biotechnol. Biotechnol. Equip. 2017, 31, 807–814. [Google Scholar] [CrossRef]
- Zonuz, A.T.; Rahmati, A.; Mortazavi, H.; Khashabi, E.; Farahani, R.M.Z. Effect of cigarette smoke exposure on the growth of Streptococcus mutans and Streptococcus sanguis: An in vitro study. Nicotine Tob. Res. 2008, 10, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, M.; Gregory, R.L. Effect of nicotine on growth and metabolism of Streptococcus mutans. Eur. J. Oral Sci. 2012, 120, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Mathé, L.; Van Dijck, P. Recent insights into Candida albicans biofilm resistance mechanisms. Curr. Genet. 2013, 59, 251–264. [Google Scholar] [CrossRef]
- Orsi, C.F.; Borghi, E.; Colombari, B.; Neglia, R.G.; Quaglino, D.; Ardizzoni, A.; Morace, G.; Blasi, E. Impact of Candida albicans hyphal wall protein 1 (HWP1) genotype on biofilm production and fungal susceptibility to microglial cells. Microb. Pathog. 2014, 69–70, 20–27. [Google Scholar] [CrossRef]
- Naglik, J.R.; Fostira, F.; Rupari, J.; Staab, J.F.; Challacombe, S.J.; Sundstrom, P. Candida albicans HWP1 gene expression and host antibody responses in colonization and disease. J. Med. Microbiol. 2006, 55, 1323–1327. [Google Scholar] [CrossRef]
- Biswas, S.; Van Dijck, P.; Datta, A. Environmental Sensing and Signal Transduction Pathways Regulating Morphopathogenic Determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 2007, 71, 348–376. [Google Scholar] [CrossRef]
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 2005, 48, 365–377. [Google Scholar] [CrossRef]
- Li, F.; Palecek, S.P. EAP1, a Candida albicans Gene Involved in Binding Human Epithelial Cells. Eukaryot. Cell. 2003, 2, 1266–1273. [Google Scholar] [CrossRef]
- Liu, Y.; Filler, S.G. Candida albicans Als3, a multifunctional adhesin, and invasin. Eukaryot. Cell. 2011, 10, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Phan, Q.T.; Myers, C.L.; Fu, Y.; Sheppard, D.C.; Yeaman, M.R.; Welch, W.H.; Ibrahim, A.S.; Edward, J.E.; Filler, S.G. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007, 5, 0543–0557. [Google Scholar] [CrossRef] [PubMed]



| (A) | ||
| Groups | MIC (mg/mL) | MFC (mg/mL) |
| N | 4 | 8 |
| CSC | 0.25 | 0.5 |
| ECSC | 0.5 | 1 |
| ECSC (−) | - | - |
| (B) | ||
| Groups | MIC (µg/mL) | MFC (µg/mL) |
| Fluconazole | 8 | 16 |
| Amphotericin B | 0.25 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghighi, F.; Andriasian, L.; Tran, N.C.; Lux, R. Effect of Cigarette and E-Cigarette Smoke Condensates on Candida albicans Biofilm Formation and Gene Expression. Int. J. Environ. Res. Public Health 2022, 19, 4626. https://doi.org/10.3390/ijerph19084626
Haghighi F, Andriasian L, Tran NC, Lux R. Effect of Cigarette and E-Cigarette Smoke Condensates on Candida albicans Biofilm Formation and Gene Expression. International Journal of Environmental Research and Public Health. 2022; 19(8):4626. https://doi.org/10.3390/ijerph19084626
Chicago/Turabian StyleHaghighi, Farnoosh, Leah Andriasian, Nini Chaichanasakul Tran, and Renate Lux. 2022. "Effect of Cigarette and E-Cigarette Smoke Condensates on Candida albicans Biofilm Formation and Gene Expression" International Journal of Environmental Research and Public Health 19, no. 8: 4626. https://doi.org/10.3390/ijerph19084626
APA StyleHaghighi, F., Andriasian, L., Tran, N. C., & Lux, R. (2022). Effect of Cigarette and E-Cigarette Smoke Condensates on Candida albicans Biofilm Formation and Gene Expression. International Journal of Environmental Research and Public Health, 19(8), 4626. https://doi.org/10.3390/ijerph19084626






