Removal of Sulfonamide Resistance Genes in Fishery Reclamation Mining Subsidence Area by Zeolite
Abstract
:1. Introduction
2. Materials and Methods
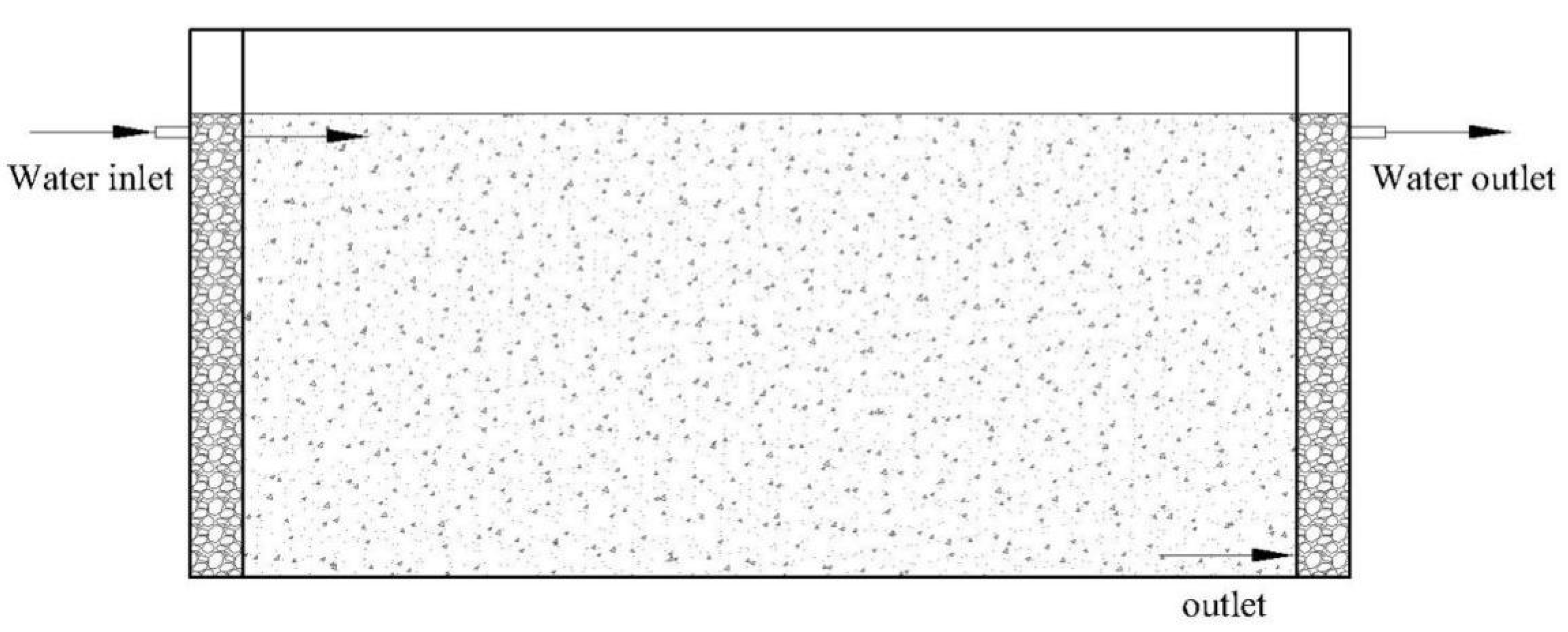
2.1. Experimental Device
2.2. Source and Quality of Experimental Water
2.3. Experiment Operation and Sample Collection
2.4. Methods
- Determination of conventional pollutants
- 2.
- Determination of gene abundance of ARGs and 16S rRNA
- 3.
- Determination of microbial diversity
3. Results
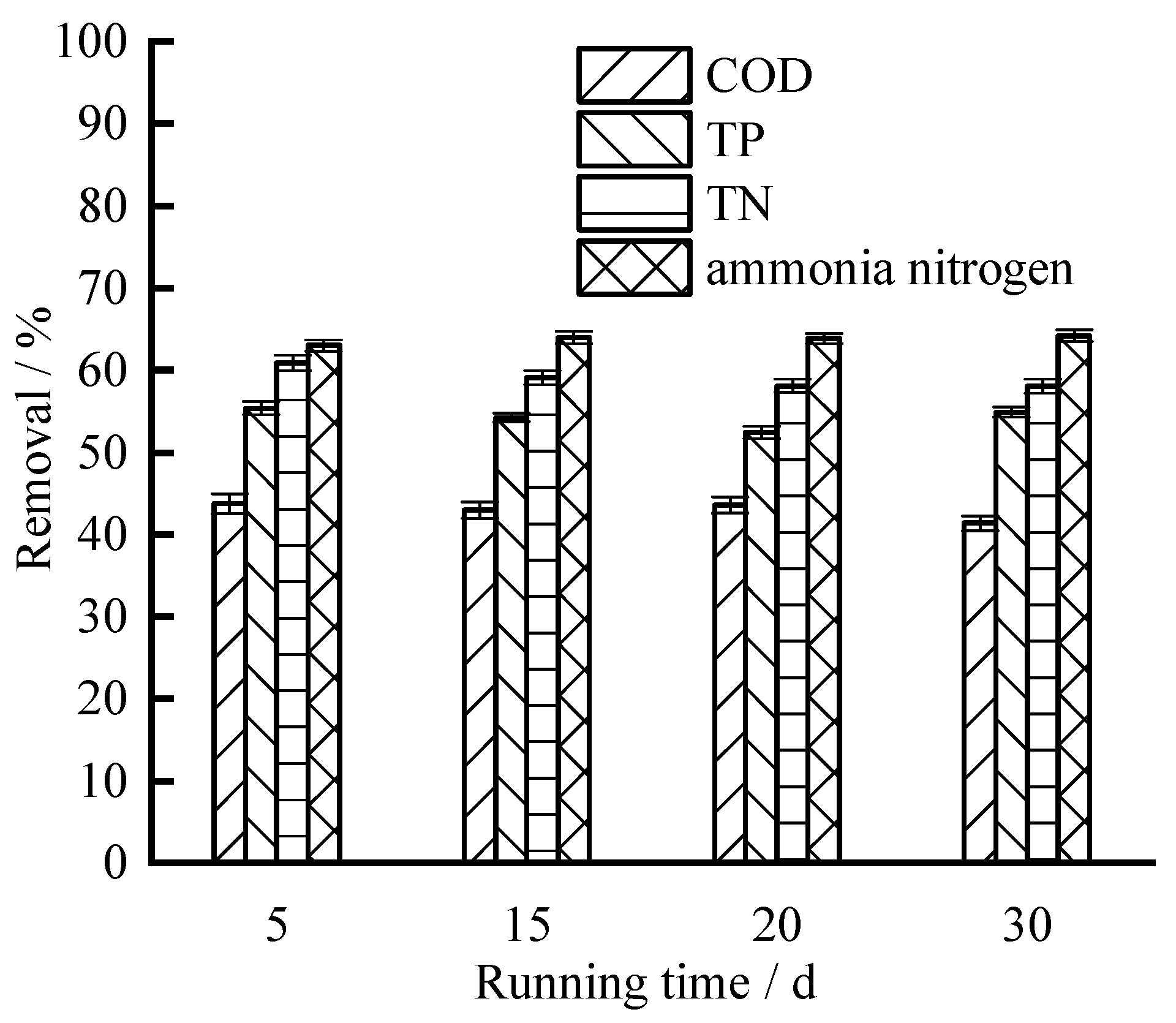
3.1. Removals of Conventional Pollutants
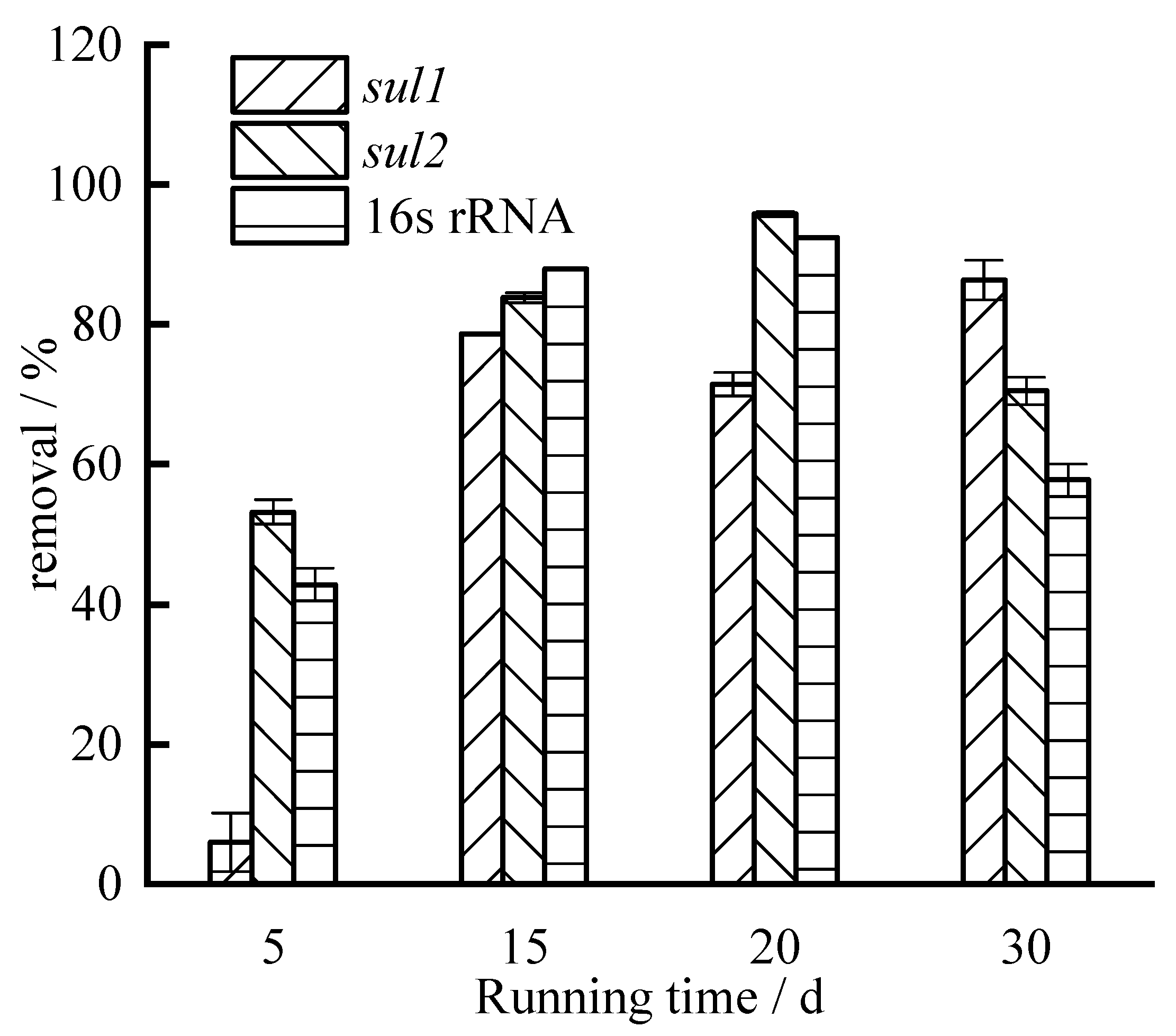
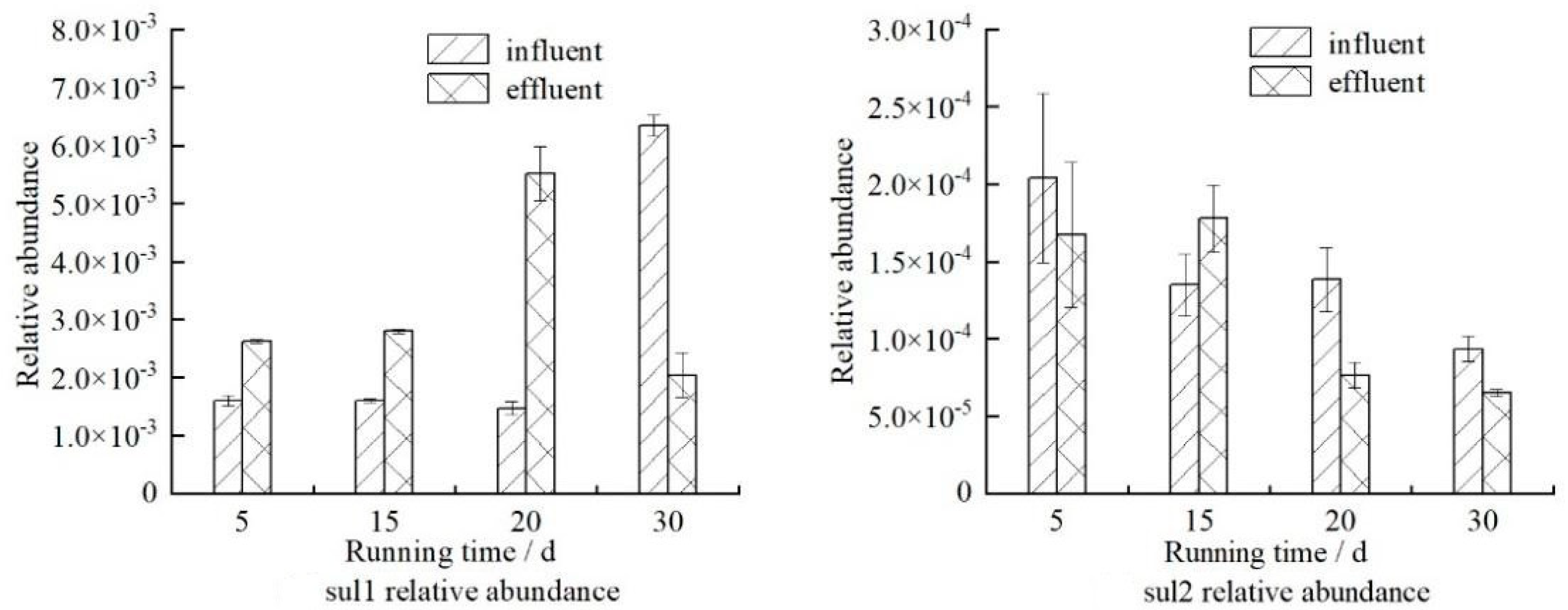
3.2. Removals of Sulfonamide ARGs
3.3. Changes in Microbial Community Structure
4. Analysis and Discussion
5. Conclusions
- (1)
- Zeolite can reduce the absolute abundance of sulfonamides ARGs and as high as 90% can be removed; the average removal of sul1 is lower than that of sul2.
- (2)
- There is a big difference in the microbial community structure of the influent and effluent. The zeolite treatment affects the microbial community structure of the effluent, and the change of the microbial structure indirectly affects the removal of ARGs.
- (3)
- The experimental results found that the device can only remove part of the drug-resistant bacteria, so there is still a risk of aggravating the drug resistance of the bacterial community.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Q.; Liu, H.L.; Zhao, H.Q.; Zhang, M.; Liu, S.Q.; Zeng, Y.F. Discussion on the nine aspects of addressing environmental problems of mining. J. China Coal Soc. 2019, 44, 10–22. (In Chinese) [Google Scholar]
- Cheng, S.; Lu, P.; Feng, Q.Y. Distribution of Antibiotic Resistance Genes and Microbial Communities in a Fishery Reclamation Mining Subsidence Area. Chin. J. Environ. Sci. 2021, 42, 2541–2549. (In Chinese) [Google Scholar]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Wu, J.J.; Zhu, C. Analysis of microbial antibiotic resistance genes in sediment of aquaculture pond. Acta Sci. Circumst. 2017, 37, 3649. (In Chinese) [Google Scholar]
- Muziasari, W.I.; Pärnänen, K.; Johnson, T.A.; Lyra, C.; Karkman, A.; Stedtfeld, R.D.; Tamminen, M.; Tiedje, J.M.; Virta, M. Aquaculture changes the profile of antibiotic resistance and mobile genetic element associated genes in Baltic Sea sediments. FEMS Microbiol. Ecol. 2016, 92, fiw052. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Hoa, P. Distribution of quinolones, sulfonamides, tetracyclines in aquatic environment and antibiotic resistance in Indochina. Front. Microbiol. 2012, 3, 67. [Google Scholar] [CrossRef] [Green Version]
- Monika, H.; Ewa, K.; Iwona, G. The impact of a freshwater fish farm on the community of tetracycline resistant bacteria and the structure of tetracycline resistance genes in river water. Chemosphere 2014, 128, 134–141. [Google Scholar]
- Nakayama, T.; Tran, T.T.H.; Harada, K.; Warisaya, M.; Asayama, M.; Hinenoya, A.; Lee, J.W.; Phu, T.M.; Ueda, S.; Sumimura, Y.; et al. Water metagenomic analysis reveals low bacterial diversity and the presence of antimicrobial residues and resistance genes in a river containing wastewater from backyard aquacultures in the Mekong Delta, Vietnam. Environ. Pollut. 2017, 222, 294–306. [Google Scholar] [CrossRef]
- Wu, J.J.; Su, Y.L.; Deng, Y.Q.; Guo, Z.X.; Mao, C.; Liu, G.F.; Xu, L.W.; Cheng, C.H.; Bei, L.; Feng, J. Prevalence and distribution of antibiotic resistance in marine fish farming areas in Hainan, China. Sci. Total Environ. 2019, 653, 605–611. [Google Scholar] [CrossRef]
- Gao, P.P.; Mao, D.Q.; Luo, Y.; Wang, L.M.; Xu, B.J.; Xu, L. Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef]
- Yuan, J.L.; Ni, M.; Liu, M.; Zheng, Y.; Gu, Z.M. Occurrence of antibiotics and antibiotic resistance genes in a typical estuary aquaculture region of Hangzhou Bay, China. Mar. Pollut. Bull. 2019, 138, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.M.; Nie, X.P.; Shi, Z. Preliminary studies on the occurrence of antibiotic resistance genes in typical aquaculture area of the Pearl River Estuary. Chin. J. Environ. Sci. 2013, 34, 4073–4080. (In Chinese) [Google Scholar]
- Wang, J.H.; Lu, J.; Zhang, Y.X.; Wu, J.; Luo, Y.M.; Liu, H. Metagenomic analysis of antibiotic resistance genes in coastal industrial mariculture systems. Bioresour. Technol. 2018, 253, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Q.; Su, M.; Zhang, T.; Xue, G.L. Adsorption characteristics of sulfa antibiotics in the river sediments. J. Saf. Environ. 2016, 16, 278. (In Chinese) [Google Scholar]
- Li, Y.N.; Zhang, L.H.; Wu, P.; Sheng, H.K.; Gao, X.Y.; Liu, P.X.; Li, G.D. Transfer and transformation of sulfonamides in soil and effects of plants. J. Saf. Environ. 2020, 19, 1425. (In Chinese) [Google Scholar]
- Li, Z.B.; Han, F.; Zeng, S.N.; Xie, F.F.; Ye, P.; Sun, Z.X. A review on the factors affecting the removal of sulfonamides from breeding wastewater in constructed wetlands. Asian J. Ecotoxicol. 2020, 15, 49–58. (In Chinese) [Google Scholar]
- Chen, J.; Michel, F.C.; Sreevatsan, S.; Morrison, M.; Yu, Z. Occurrence and persistence of erythromycin resistance genes (erm) and tetracycline resistance genes (tet) in waste treatment systems on swine farms. Microb. Ecol. 2010, 60, 479–486. [Google Scholar] [CrossRef]
- Hultman, J.; Tamminen, M.; Parnanen, K.; Cairns, J.; Karkman, A.; Virta, M. Host range of antibiotic resistance genes in wastewater treatment plant influent and effluent. FEMS Microbiol. Ecol. 2018, 94, fiy038. [Google Scholar] [CrossRef]
- Breitholtz, M.; Naslund, M.; Strad, D.; Borg, H.; Grabic, R.; Fick, J. An evaluation of free water surface wetlands as tertiary sewage water treatment of micropollutants. Ecotoxicol. Environ. Saf. 2012, 78, 63–71. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, Y.C.; Wang, X.C.; Jia, C.; Zhao, M.Y. Mechanism of different scales subsurface flow constructed wetlands for purifying polluted river water. Chin. J. Environ. Eng. 2018, 12, 1667–1675. (In Chinese) [Google Scholar]
- Younger, P.L.; Henderson, R. Synergistic wetland treatment of sewage and mine water: Pollutant removal performance of the first full-scale system. Water Res. 2014, 55, 74–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papaevangelou, V.A.; Gikas, G.D.; Tsihrintzis, V.A.; Antonopoulou, M.; Konstantinou, I.K. Removal of Endocrine Disrupting Chemicals in HSF and VF pilot-scale constructed wetlands. Chem. Eng. J. 2016, 294, 146–156. [Google Scholar] [CrossRef]
- Lamori, J.G.; Xue, J.; Rachmadi, A.T.; Lopez, G.U.; Kitajima, M.; Gerba, C.P.; Pepper, I.L.; Brooks, J.P.; Sherchan, S. Removal of fecal indicator bacteria and antibiotic resistant genes in constructed wetlands. Environ. Sci. Pollut. Res. 2019, 26, 10188–10197. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Garcia-galan, M.J.; Day, J.W.; Boopathy, R.; White, J.R.; Wallace, S.; Hunter, R.G. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: Increasing removal with wetlands and reducing environmental impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Guo, X.C.; Liu, Y.; Lu, S.Y.; Xi, B.D.; Zhang, J.; Wang, Z.; Bi, B. A review on removing antibiotics and antibiotic resistance genes from wastewater by constructed wetlands: Performance and microbial response. Environ. Pollut. 2019, 254, 112996. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, C.X.; Zheng, J.Y.; Huang, X.; Wang, Z.; Liu, Y.H.; Zhu, G.F. Elimination of veterinary antibiotics and antibiotic resistance genes from swine wastewater in the vertical flow constructed wetlands. Chemosphere 2013, 91, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, W.J.; Liu, Y.S.; Hu, L.X.; He, L.Y.; Zhao, J.L.; Wang, T.T.; Ying, G.G. Fate and removal of antibiotics and antibiotic resistance genes in hybrid constructed wetlands. Environ. Pollut. 2019, 249, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.L.; Shao, X.X.; Wu, M.; Jiang, X.S.; Lu, L.Y. Effects of different substrates and particle sizes on wastewater purification. Chin. J. Environ. Sci. 2018, 39, 4236–4241. (In Chinese) [Google Scholar]
- Lu, S.Y.; Wan, Z.F.; Li, F.M.; Zhang, X.Q. Ammonia nitrogen adsorption and desorption characteristics of twenty-nine kinds of constructed wetland substrates. Res. Environ. Sci. 2016, 29, 1187–1194. (In Chinese) [Google Scholar]
- Pelissari, C.; Guivernau, M.; Vinas, M.; García, J.; Velasco-Galilea, M.; Souza, S.S.; Sezerino, P.H.; Ávila, C. Effects of partially saturated conditions on the metabolically active microbiome and on nitrogen removal in vertical subsurface flow constructed wetlands. Water Res. 2018, 141, 185–195. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Liu, S.W.; Zhang, L. Removal of sulfonamide resistance genes in livestock farms with constructed wetland. Environ. Sci. Manag. 2016, 41, 89. (In Chinese) [Google Scholar]
- Liu, L.; Liu, Y.H.; Wang, Z.; Liu, C.X.; Huang, X.; Zhu, G.F. Behavior of tetracycline and sulfamethazine with corresponding resistance genes from swine wastewater in pilot-scale constructed wetlands. J. Hazard. Mater. 2014, 278, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Zhang, Z.; Sun, L.; Yu, L.J.; Li, Y.J. Purification effects and microbial community differences of the surface-flow constructed wetlands with different vegetation plantation. Chin. J. Environ. Eng. 2019, 13, 1918–1929. (In Chinese) [Google Scholar]
- Yi, X.Z.; Tran, N.H.; Yin, T.R.; He, Y.L.; Gin, K.Y.H. Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system. Water Res. 2017, 121, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Song, H.L.; Zhang, S.; Guo, J.H.; Yang, Y.L.; Zhang, L.M.; Li, H.; Yang, X.L.; Liu, X. Vertical up-flow constructed wetlands exhibited efficient antibiotic removal but induced antibiotic resistance genes in effluent. Chemosphere 2018, 203, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wei, X.D.; Liu, Y.S.; Ying, G.G.; Liu, S.S.; He, L.Y.; Su, H.C.; Hu, L.X.; Chen, F.R.; Yang, Y.Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Optimization of wetland substrates and hydraulic loading. Sci. Total Environ. 2016, 565, 240–248. [Google Scholar] [CrossRef]
- Huang, X.; Liu, C.X.; Li, K.; Su, J.Q.; Zhu, G.F.; Liu, L. Performance of vertical up-flow constructed wetlands on swine wastewater containing tetracyclines and tet genes. Water Res. 2015, 70, 109–117. [Google Scholar] [CrossRef]
- Zhang, S.; Song, H.L.; Yang, X.L.; Yang, K.Y.; Wang, X.Y. Effect of electrical stimulation on the fate of sulfamethoxazole and tetracycline with their corresponding resistance genes in three-dimensional biofilm-electrode reactors. Chemosphere 2016, 164, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Antunes, P.; Machado, J.; Sousa, J.C.; Peixe, L. Dissemination of sulfonamide resistance genes (sul1, sul2, and sul3) in Portuguese Salmonella enterica strains and relation with integrons. Antimicrob. Agents Chemother. 2005, 49, 836–839. [Google Scholar] [CrossRef] [Green Version]
- Enne, V.I.; Livermore, D.M.; Stephens, T.P.; Hall, L.M. Persistence of sulfonamide resistance in Escherichia coli in the UK despite national prescribing restriction. Lancet 2001, 357, 1325–1328. [Google Scholar] [CrossRef]
- Gu, X.; Zhai, H.Y.; Cheng, S.Z. Fate of antibiotics and antibiotic resistance genes in home water purification systems. Water Res. 2021, 190, 116762. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.M.; Xu, H.T.; Xiao, W.S.; Lu, J.K.; Lu, D.; Chen, X.Y.; Zheng, X.Y.; Jeppesen, E.; Zhang, W.; Wang, L.Q. Ecotoxicological effects of sulfonamide on and its removal by the submerged plant Vallisneria natans (Lour). Hara. Water Res. 2020, 170, 115354. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Gao, J.F.; Zhao, Y.F.; Dai, H.H.; Jia, J.X.; Zhang, D. Plastisphere enrich antibiotic resistance genes and potential pathogenic bacteria in sewage with pharmaceuticals. Sci. Total Environ. 2021, 768, 144663. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.Q.; Luo, Y.; Mathieu, J.; Wang, Q.; Feng, L.; Mu, Q.H.; Feng, C.Y.; Alvarez, P.J. Persistence of extracellular DNA in river sediment facilitates antibiotic resistance gene propagation. Environ. Sci. Technol. 2014, 48, 71–78. [Google Scholar] [CrossRef]

| Gene Name | Primer Name | Primer Sequence (5′ → 3′) | Product Size/bp | Annealing Temperature/°C |
|---|---|---|---|---|
| sul1 | sul1-F | CACCGGAAACATCGCTGCA | 159 | 58 |
| sul1-R | AAGTTCCGCCGCAAGGCT | |||
| sul2 | sul2-F | CTCCGATGGAGGCCGGTAT | 191 | 58 |
| sul2-R | GGGAATGCCATCTGCCTTGA | |||
| 16S rRNA | 16S-F | TGTGTAGCGGTGAAATGCG | 140 | 62 |
| 16S-R | CATCGTTTACGGCGTGGAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, T.; Lin, Z.-B.; Cheng, S.; Wang, R.; Lu, P. Removal of Sulfonamide Resistance Genes in Fishery Reclamation Mining Subsidence Area by Zeolite. Int. J. Environ. Res. Public Health 2022, 19, 4281. https://doi.org/10.3390/ijerph19074281
Yuan T, Lin Z-B, Cheng S, Wang R, Lu P. Removal of Sulfonamide Resistance Genes in Fishery Reclamation Mining Subsidence Area by Zeolite. International Journal of Environmental Research and Public Health. 2022; 19(7):4281. https://doi.org/10.3390/ijerph19074281
Chicago/Turabian StyleYuan, Tao, Zi-Bo Lin, Sen Cheng, Rui Wang, and Ping Lu. 2022. "Removal of Sulfonamide Resistance Genes in Fishery Reclamation Mining Subsidence Area by Zeolite" International Journal of Environmental Research and Public Health 19, no. 7: 4281. https://doi.org/10.3390/ijerph19074281
APA StyleYuan, T., Lin, Z.-B., Cheng, S., Wang, R., & Lu, P. (2022). Removal of Sulfonamide Resistance Genes in Fishery Reclamation Mining Subsidence Area by Zeolite. International Journal of Environmental Research and Public Health, 19(7), 4281. https://doi.org/10.3390/ijerph19074281






