Toxic Effects of Bisphenol A and Bisphenol S on Chlorella Pyrenoidosa under Single and Combined Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drug and Microalgal Culture
2.2. Bisphenol A and Bisphenol S Mother Fluids
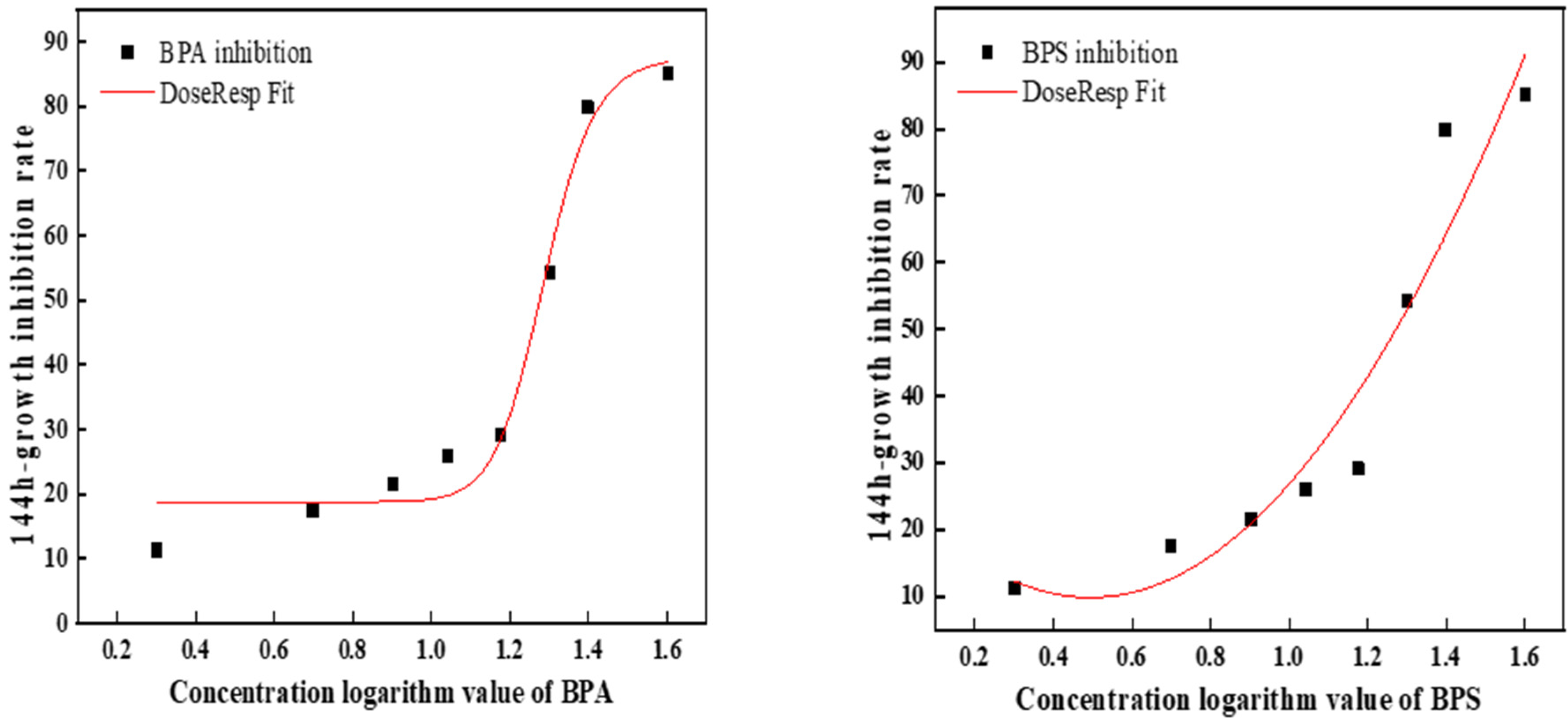
2.3. Median Effective Concentrations of BPA and BPS (EC50)
2.4. Single Toxicity Exposure Experiments for BPA and BPS
2.5. Combined Toxicity Exposure Experiment for BPA and BPS
2.6. Determination of Chlorophyll a Content
2.7. Antioxidant System Analysis
2.7.1. Extraction of Crude Enzyme from Alga Cells
2.7.2. Assays of Oxidative Stress and Antioxidant Enzyme Activities
2.8. Combined Toxic Effect Evaluation
2.9. The Data Processing
3. Results and Discussion
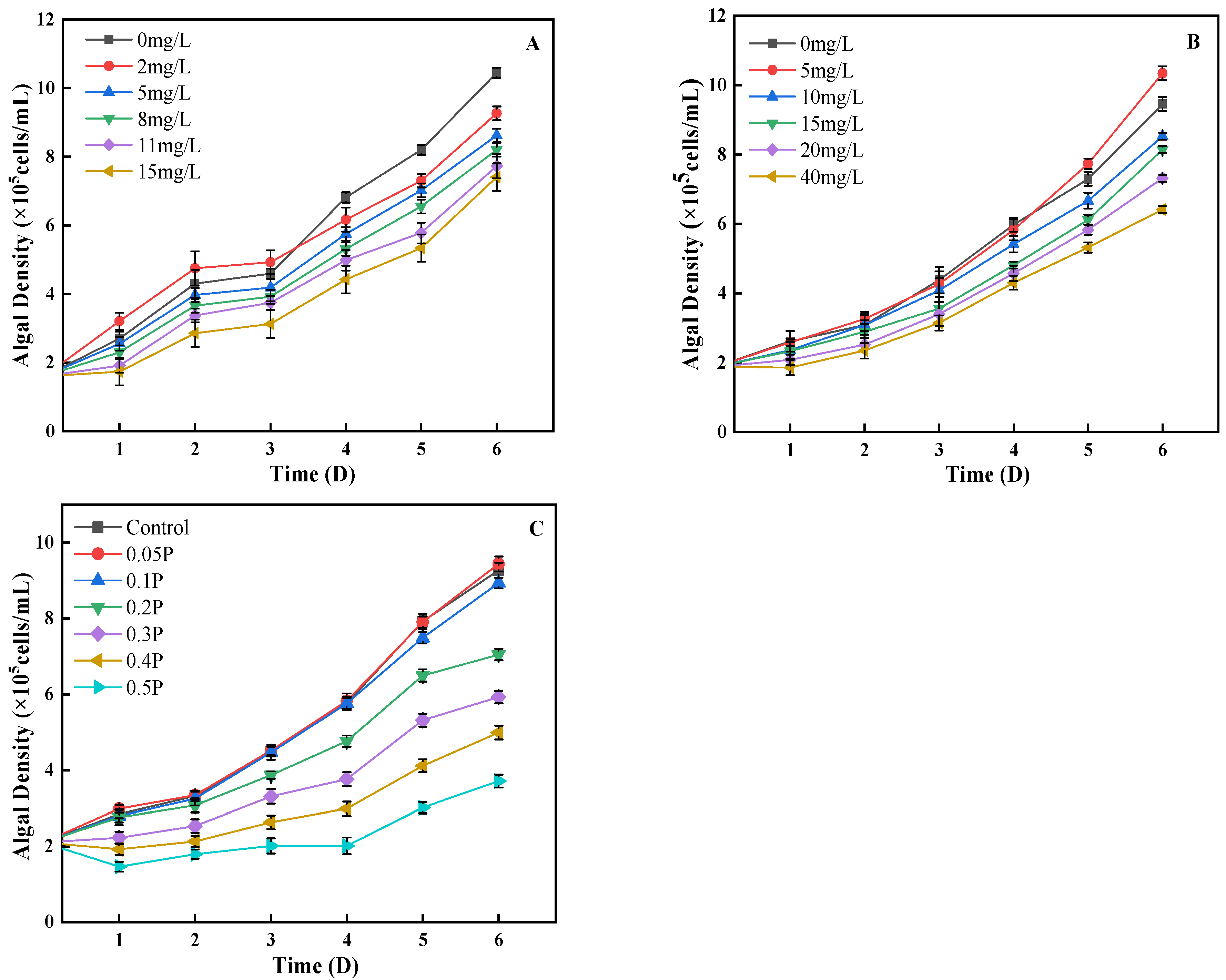
3.1. Effects on Cell Density
3.2. Evaluation of the Toxicity Effects of BPA and BPS on C. pyrenoidosa
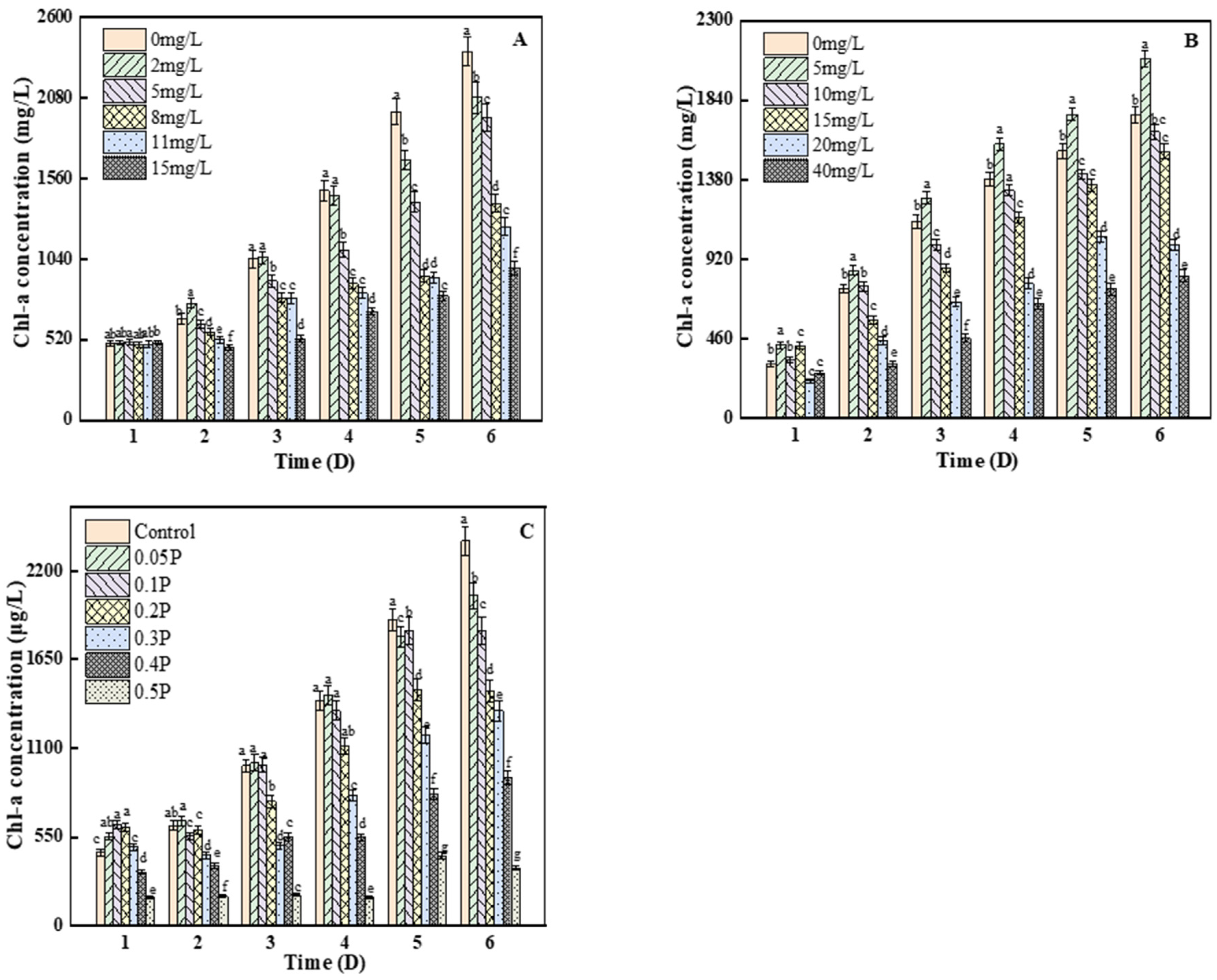
3.3. Effects on Chlorophyll A
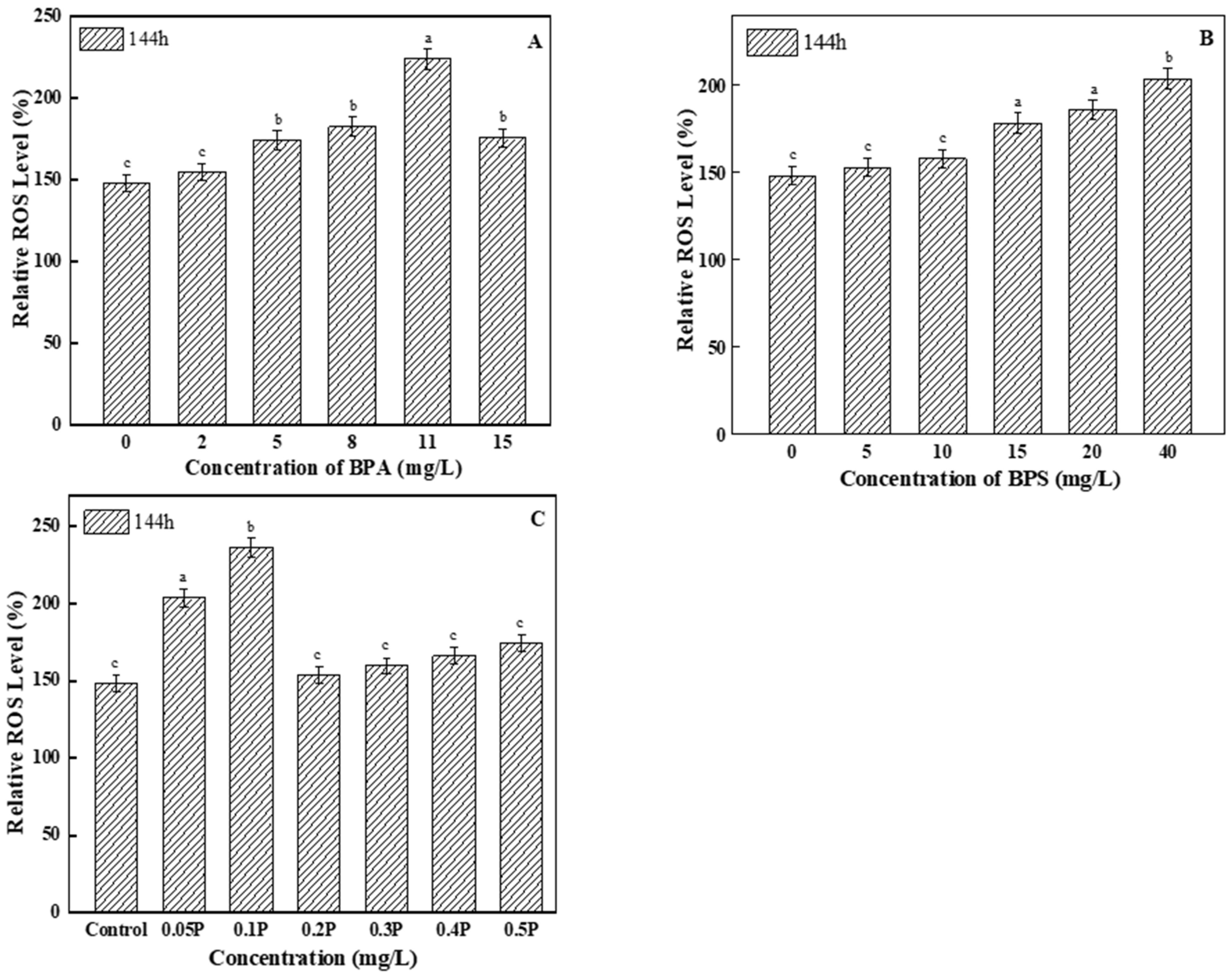
3.4. Oxidative Stress
3.4.1. Analysis of ROS Levels
3.4.2. Analysis of MDA Content, and POD and SOD Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pop, C.E.; Draga, S.; Măciucă, R.; Niță, R.; Crăciun, N.; Wolff, R. Bisphenol A Effects in Aqueous Environment on Lemna minor. Processes 2021, 9, 1512. [Google Scholar] [CrossRef]
- Maria, K.B.; Jacob, D.B.; Ana, B. Bisphenol A and replacements in thermal paper: A review. Chemosphere 2017, 182, 691–706. [Google Scholar]
- Liao, C.; Kannan, K. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch. Environ. Contam. Toxicol. 2014, 67, 50–59. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Hua, W.; Zhou, M.; Wang, Q.; Zhou, Q.; Huang, X. Effects of bisphenol A, an environmental endocrine disruptor, on the endogenous hormones of plants. Environ. Sci. Pollut. Res. Int. 2015, 22, 17653–17662. [Google Scholar] [CrossRef]
- Li, Y.T.; Liang, Y.; Li, Y.N.; Che, X.K.; Zhao, S.J.; Zhang, Z.S.; Gao, H.Y. Mechanisms by which Bisphenol A affect the photosynthetic apparatus in cucumber (Cucumis sativus L.) leaves. Sci. Rep. 2018, 8, 4253. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, L. Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China. Water Res. 2016, 103, 343–351. [Google Scholar] [CrossRef]
- Bich, C.T.; Marie, J.T.; Martine, B.; Fabrice, A.; Marc, C. BPA and phthalate fate in a sewage network and an elementary river of France. Influence of hydroclimatic conditions. Chemosphere 2015, 119, 43–51. [Google Scholar]
- Yang, X.H.; Xue, J.C.; Yao, H.; Wu, Q.; Arjun, K.V.; Rolf, U.H.; Kurunthachalam, K. Occurrence and estrogenic potency of eight bisphenol analogs in sewage sludge from the U.S. EPA targeted national sewage sludge survey. J. Hazard. Mater. 2015, 299, 733–739. [Google Scholar]
- Liao, C.; Liu, F.; Kannan, K. Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ. Sci. Technol. 2012, 46, 6515–6522. [Google Scholar] [CrossRef]
- Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Lam, J.; Lam, P.K.S.; Moon, H.B.; Jeong, Y.; Kannan, P.; Achyuthan, H.; Munuswamy, N.; et al. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 2015, 122, 565–572. [Google Scholar] [CrossRef]
- Ayelet, Z.; Zelieann, R.C.; Wei, W.; Jodi, A.F. Bisphenol A inhibits cultured mouse ovarian follicle growth partially via the aryl hydrocarbon receptor signaling pathway. Reprod. Toxicol. 2013, 42, 58–67. [Google Scholar]
- Amira, M.A.; Lauren, J.; Thomas, F.M.; David, E.C.; Bhramar, M.; John, D.M. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ. Int. 2018, 113, 341–349. [Google Scholar]
- Li, X.Y.; Wang, L.H.; Shen, F.; Zhou, Q.; Huang, X.H. Impacts of exogenous pollutant bisphenol A on characteristics of soybeans. Ecotoxicol. Environ. Saf. 2018, 157, 463–471. [Google Scholar] [CrossRef]
- Xiang, K.L.; Wang, J.J.; Tang, S.; Duan, S.S. Effects of bisphenol A on the disturbance of Platymonas helgolandica. Chin. J. Ecotoxicol. 2015, 10, 262–267. [Google Scholar]
- Yang, C. Effects of Three Environmental Endocrine Disruptors on Growth and Physiology of Microcystis aeruginosa; Shanghai Jiao Tong University: Shanghai, China, 2014. [Google Scholar]
- Yang, C.; Yu, X.J.; Wang, X.Z.; Kong, H.N.; Li, Y.H. Effects of bisphenol A on growth and physiology of Microcystis aeruginosa. Saf. Environ. Eng. 2014, 21, 21–25. [Google Scholar]
- Gattullo, C.E.; Bährs, H.; Steinberg, C.E.; Loffredo, E. Removal of bisphenol A by the freshwater green alga Monoraphidium braunii and the role of natural organic matter. Sci. Total Environ. 2012, 416, 501–506. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- Li, J. Effects of Tetracycline on Growth and Nutrient Removal of Chlamydomonas reinhardtii in Reclaimed Water; Shandong Agricultural University: Taian, China, 2017. [Google Scholar]
- Li, R.; Chen, G.Z.; Tam, N.F.Y.; Luan, T.G.; Shin, P.K.; Cheung, S.G.; Liu, Y. Toxicity of bisphenol A and its bioaccumulation and removal by a marine microalga Stephanodiscus hantzschii. Ecotoxicol. Environ. Saf. 2009, 72, 321–328. [Google Scholar] [CrossRef]
- Nong, Q.Y.; Qin, L.T.; Mo, L.Y.; Liu, Y.A.; Liang, Y.P. Study on long-term toxic interaction between antibiotics and triazole fungicides on Selenastrum capricornutum. Chin. J. Ecotoxicol. 2019, 14, 140–149. [Google Scholar]
- Wu, Y. Effects of Carbon Nanotubes Toxic Stress on Growth and Physiological Characteristics of Microcystis aeruginosa; Sichuan Agricultural University: Ya’an, China, 2017. [Google Scholar]
- Jan, S. Ecological assessments with algae: A review and synthesis. J. Phycol. 2014, 50, 437–461. [Google Scholar]
- Pang, X.Y.; Duan, H.T.; Zhang, Y.C.; Ma, R.H. Comparison of extraction methods of phycocyanin from eutrophic lakes. J. Lake Sci. 2014, 26, 799–806. [Google Scholar]
- Nirmalakhandan, N.; Sun, B.; Arulgnanendran, V.J.; Mohsin, M.; Wang, X.H.; Prakash, J.; Hall, N. Analyzing and modeling toxicity of mixtures of organic chemicals to microorganisms. Water Sci. Technol. 1994, 30, 87–96. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, X.X.; Wang, Y.; Yang, Y.Y.; Zhang, W.; Zhao, Y.C.; Zhang, X.X. ROS changes are responsible for tributyl phosphate (TBP)-induced toxicity in the alga Phaeodactylum tricornutum. Aquat. Toxicol. 2019, 208, 168–178. [Google Scholar] [CrossRef]
- Wang, S.C.; Gao, Z.Y.; Liu, F.F.; Chen, S.Q.; Liu, G.Z. Effects of polystyrene and triphenyl phosphate on growth, photosynthesis and oxidative stress of Chaetoceros meülleri. Sci. Total Environ. 2021, 797, 149180. [Google Scholar] [CrossRef]
- Xiang, R.; Shi, J.; Yu, Y.; Zhang, H.B.; Dong, C.C.; Yang, Y.J.; Wu, Z.X. The Effect of Bisphenol A on Growth, Morphology, Lipid Peroxidation, Antioxidant Enzyme Activity, and PS II in Cylindrospermopsis raciborskii and Scenedesmus quadricauda. Arch. Environ. Contam. Toxicol. 2018, 74, 515–526. [Google Scholar] [CrossRef]
- Kong, W.B.; Yang, S.L.; Guo, B.M.; Wang, H.; Huo, H.R.; Zhang, A.M.; Niu, S.Q. Growth behavior, glucose consumption and phenol removal efficiency of Chlorella vulgaris under the synergistic effects of glucose and phenol. Ecotoxicol. Environ. Saf. 2019, 186, 109762. [Google Scholar] [CrossRef]
- Min-Kyu, J.; Akhil, N.K.; Jaewon, C.; Jae-Hoon, H.; Jung, R.K.; Reda, A.I.A.S.; You-Kwan, O.; Byong-Hun, J. Biodegradation of bisphenol A by the freshwater microalgae Chlamydomonas mexicana and Chlorella vulgaris. Ecol. Eng. 2014, 73, 260–269. [Google Scholar]
- Zhang, J.; Wang, L.; Li, M.; Jiao, L.Y.; Zhou, Q.; Huang, X.H. Effects of bisphenol A on chlorophyll fluorescence in five plants. Environ. Sci. Pollut. Res. Int. 2015, 22, 17724–17732. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, B.; Wang, G. Azoxystrobin-induced excessive reactive oxygen species (ROS) production and inhibition of photosynthesis in the unicellular green algae Chlorella vulgaris. Environ. Sci. Pollut. Res. Int. 2015, 22, 7766–7775. [Google Scholar] [CrossRef]
- Biochemistry; Studies from Nanjing Agricultural University Provide New Data on Biochemistry (Physiological and Biochemical Responses of a Marine Diatom Phaeodactylum tricornutum Exposed to 1-octyl-3-methylimidazolium Bromide). In Chemicals & Chemistry; Nanjing Agricultural University: Nanjing, China, 2016.
- Strašková, A.; Steinbach, G.; Konert, G.; Kotabová, E.; Komend, J.; Tichý, M.; Kaňa, R. Pigment-protein complexes are organized into stable microdomains in cyanobacterial thylakoids. BBA—Bioenergetics 2019, 1860, 148053. [Google Scholar] [CrossRef]
- Li, X.Y.; Luo, Y.R.; Yun, M.X.; Wang, J.; Wang, J.J. Effects of 1-methyl-3-octylimidazolium bromide on the anti-oxidant system of earthworm. Chemosphere 2010, 78, 853–858. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Yin, B. Effects of mixed salt-alkali stress on antioxidant enzyme activity and malondialdehyde content of Alfalfa at seedling stage. Acta Prataculturae Sin. 2009, 18, 46–50. [Google Scholar]
- Wu, X.; Tong, Z.H.; Li, L.L.; Yu, H.Q. Toxic effects of imidazolium-based ionic liquids on Caenorhabditis elegans: The role of reactive oxygen species. Chemosphere 2013, 93, 2399–2404. [Google Scholar] [CrossRef]

| Toxicity Ratio (BPA:BPS) | Concentration Combination (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 P | 0.05 P | 0.1 P | 0.2 P | 0.3 P | 0.4 P | 0.5 P | ||
| 1:1 | BPA | 0 | 0.27 | 0.53 | 1.06 | 1.59 | 2.12 | 2.66 |
| BPS | 0 | 0.99 | 1.98 | 3.96 | 5.94 | 7.92 | 9.90 | |
| The Single Toxicity of 144h-EC50 (mg/L) | The Joint Toxicity of 144h-EC50 (mg/L) | S | AI | Conclusion | |
|---|---|---|---|---|---|
| BPA | 17.7 | 4.8 | 0.543 | 0.842 | Synergy |
| BPS | 66.07 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, Y.; Li, N.; He, Y.; Xiao, H.; Fang, D.; Chen, C. Toxic Effects of Bisphenol A and Bisphenol S on Chlorella Pyrenoidosa under Single and Combined Action. Int. J. Environ. Res. Public Health 2022, 19, 4245. https://doi.org/10.3390/ijerph19074245
Li J, Wang Y, Li N, He Y, Xiao H, Fang D, Chen C. Toxic Effects of Bisphenol A and Bisphenol S on Chlorella Pyrenoidosa under Single and Combined Action. International Journal of Environmental Research and Public Health. 2022; 19(7):4245. https://doi.org/10.3390/ijerph19074245
Chicago/Turabian StyleLi, Junrong, Yingjun Wang, Na Li, Yan He, Hong Xiao, Dexin Fang, and Chao Chen. 2022. "Toxic Effects of Bisphenol A and Bisphenol S on Chlorella Pyrenoidosa under Single and Combined Action" International Journal of Environmental Research and Public Health 19, no. 7: 4245. https://doi.org/10.3390/ijerph19074245
APA StyleLi, J., Wang, Y., Li, N., He, Y., Xiao, H., Fang, D., & Chen, C. (2022). Toxic Effects of Bisphenol A and Bisphenol S on Chlorella Pyrenoidosa under Single and Combined Action. International Journal of Environmental Research and Public Health, 19(7), 4245. https://doi.org/10.3390/ijerph19074245







