Genetic Variation in Rhipicephalus sanguineus s.l. Ticks across Arizona
Abstract
:1. Introduction
Genetic Variation of Rhipicephalus sanguineus s.l.
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Isolation and Sequencing
2.3. Primer Design, PCR, and Sequencing
| Primers | Reagent | Volume | Thermocycler Steps | ||
|---|---|---|---|---|---|
| Cycle | Time | ||||
| Standard 12S rRNA | Forward: AAACTAGGATTAGATACCCTATTATTTTAG Reverse: CTATGTAAGCACTTA- TCTTAATAAAGAGTG | PCR Water | 25-sum of other reagents | Initial denaturation | 95 °C for 30 s |
| ThermoPol Buffer (New England BioLabs) | 2.5 | 30 cycles: | |||
| 10 mM dNTPs | 0.5 | Denaturation | 95 °C for 15 s | ||
| 10 µM FWD Primer | 0.5 | Annealing | 50 °C for 30 s | ||
| 10 µM REV Primer | 0.5 | Extension | 68 °C for 60 s | ||
| Taq (New England BioLabs) | 0.125 | Final extension | 68 °C for 60 s | ||
| DNA | 3.0 | Holding | 10°C | ||
| Temperate 12S rRNA | Forward: TTTTAGAGGTAAACA- TTGTT Reverse: GCTTAATTCAAATTGA- CATT | PCR Water | 10-sum of other reagents | Initial denaturation | 95 °C for 30 s |
| ThermoPol Buffer (New England BioLabs) | 1.0 | 25 cycles: | |||
| 10 mM dNTPs | 0.8 | Denaturation | 95 °C for 15 s | ||
| 10 µM FWD Primer | 0.5 | Annealing | 46 °C for 30 s | ||
| 10 µM REV Primer | 0.5 | Extension | 68 °C for 60 s | ||
| Taq (New England BioLabs) | 0.1 | Final extension | 68 °C for 60 s | ||
| DNA | 1.0 | Holding | 10 °C | ||
| Tropical 12S rRNA | Forward: TTTTAGAGCTTAACAT- TGTA Reverse: GCTTAATTCAAATTAA- CATC | PCR Water | 10-sum of other reagents | Initial denaturation | 95 °C for 30 s |
| ThermoPol Buffer (New England BioLabs) | 1.0 | 25 cycles: | |||
| 10 mM dNTPs | 0.8 | Denaturation | 95 °C for 15 s | ||
| 10 µM FWD Primer | 0.5 | Annealing | 46 °C for 30 s | ||
| 10 µM REV Primer | 0.5 | Extension | 68 °C for 60 s | ||
| Taq (New England BioLabs) | 0.1 | Final extension | 68 °C for 60 s | ||
| DNA | 1.0 | Holding | 10 °C | ||
2.4. Phylogenetic and Geospatial Analyses
3. Results
3.1. PCR and Sequencing
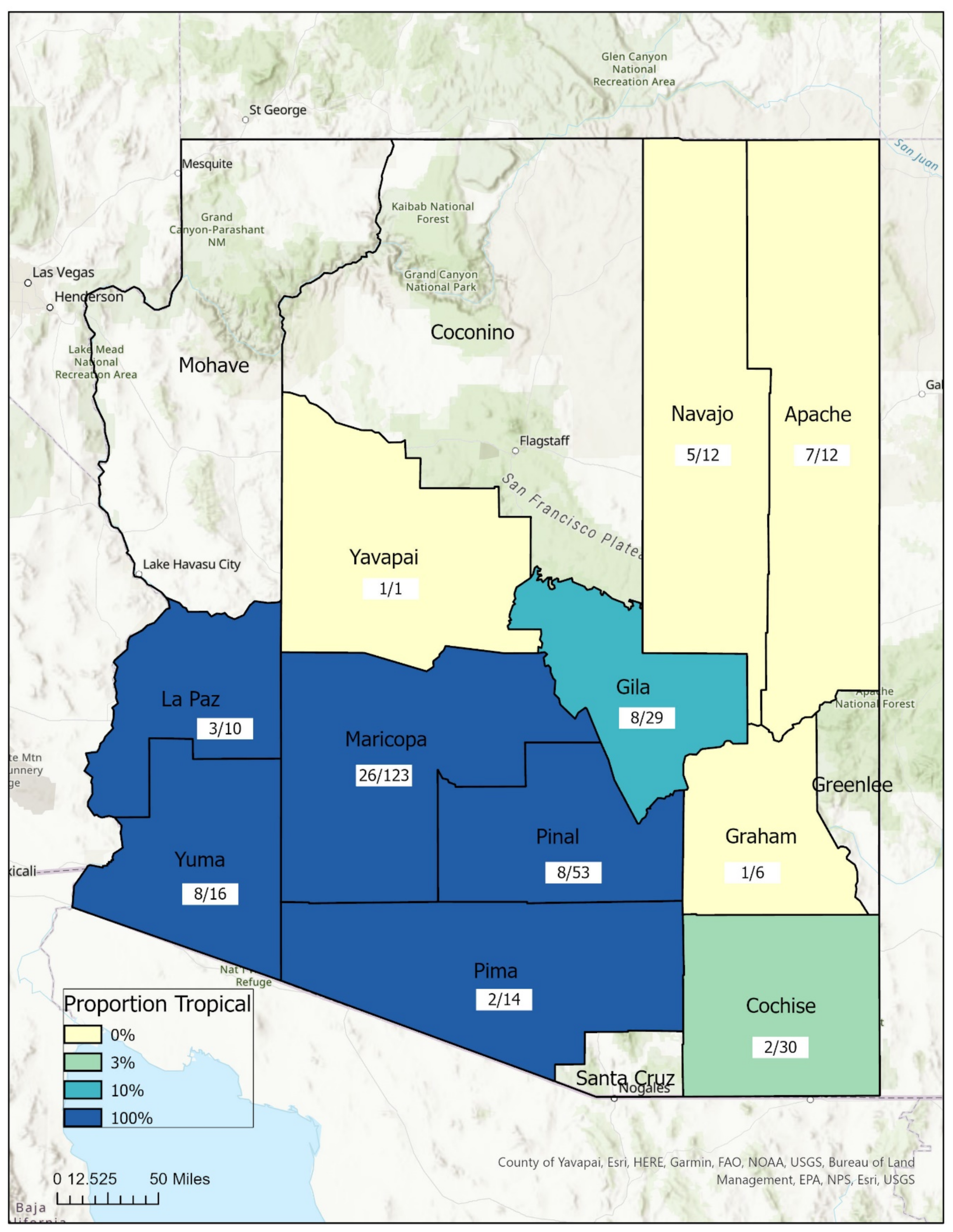
3.2. Geospatial Analysis
| Lineage (# Sites) | Mean (Std) | Min | Max |
|---|---|---|---|
| Temperate (10) | 5054 (1450) | 2650 | 6888 |
| Tropical (10) | 1625 (1316) | 141 | 4633 |

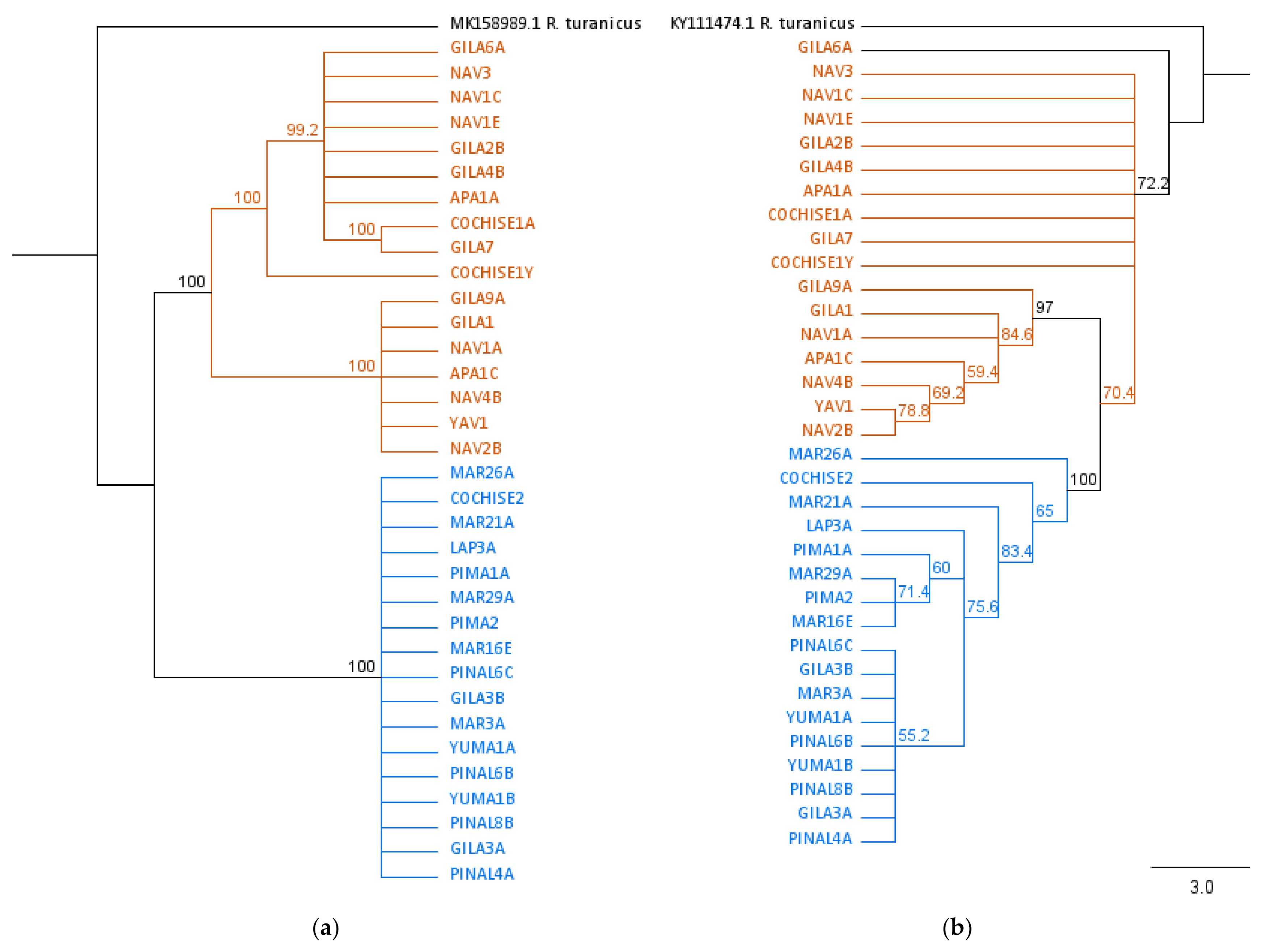
3.3. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McFee, R.B.; Bush, L.; Vazquez-Pertejo, M.T. Tick borne illness-Rocky mountain spotted fever. Dis. Mon. 2018, 64, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R. Vital Signs: Trends in Reported Vectorborne Disease Cases-United States and Territories, 2004–2016. MMWR Morb. Mortal. Wkly Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Tickborne Disease Surveillance Data Summary. 2019. Available online: https://www.cdc.gov/ticks/data-summary/index.html (accessed on 11 December 2019).
- Bustamante, M.; Varela, G. Una nueva rickettsiosis en Mexico: Existencia de la fiebre manchada americana en los estadoes de Sinaloa y Sonora. Rev. Inst. Salubr. Enferm. Trop. 1943, 4, 189–210. [Google Scholar]
- Demma, L.J.; Traeger, M.S.; Nicholson, W.L.; Paddock, C.D.; Blau, D.M.; Eremeeva, M.E.; Dasch, G.; Levin, M.L.; Singleton, J.; Zaki, S.R.; et al. Rocky Mountain Spotted Fever from an Unexpected Tick Vector in Arizona. N. Engl. J. Med. 2005, 353, 587–594. [Google Scholar] [CrossRef]
- Drexler, N.; Miller, M.; Gerding, J.; Todd, S.; Adams, L.; Dahlgren, F.S.; Bryant, N.; Weis, E.; Herrick, K.; Francies, J.; et al. Community-Based Control of the Brown Dog Tick in a Region with High Rates of Rocky Mountain Spotted Fever, 2012–2013. PLoS ONE 2014, 9, e112368. [Google Scholar] [CrossRef] [Green Version]
- Arizona Department of Health Services. Epidemiology & Disease Control: Disease Data, Statistics, & Reports. 2021. Available online: https://azdhs.gov/preparedness/epidemiology-disease-control/index.php#data-stats-past-years (accessed on 10 December 2021).
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites Vectors 2010, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Dantas-Torres, F.; Otranto, D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet. Parasitol. 2014, 208, 9–13. [Google Scholar] [CrossRef]
- Walker, J.B.; Keirans, J.E.; Horak, I.G. The Genus Rhipicephalus (Acari, Ixodidae); Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Gray, J.; Dantas-Torres, F.; Estrada-Peña, A.; Levin, M. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick-Borne Dis. 2013, 4, 171–180. [Google Scholar] [CrossRef]
- Szabó, M.P.; Mangold, A.J.; João, C.F.; Bechara, G.H.; Guglielmone, A.A. Biological and DNA evidence of two dissimilar populations of the Rhipicephalus sanguineus tick group (Acari: Ixodidae) in South America. Vet. Parasitol. 2005, 130, 131–140. [Google Scholar] [CrossRef]
- Nava, S.; Beati, L.; Venzal, J.M.; Labruna, M.B.; Szabó, M.P.; Petney, T.; Saracho-Bottero, M.N.; Tarragona, E.L.; Dantas-Torres, F.; Silva, M.M.S.; et al. Rhipicephalus sanguineus (Latreille, 1806): Neotype designation, morphological re-description of all parasitic stages and molecular characterization. Ticks Tick-Borne Dis. 2018, 9, 1573–1585. [Google Scholar] [CrossRef]
- Chitimia-Dobler, L.; Langguth, J.; Pfeffer, M.; Kattner, S.; Küpper, T.; Friese, D.; Dobler, G.; Guglielmone, A.A.; Nava, S. Genetic analysis of Rhipicephalus sanguineus sensu lato ticks parasites of dogs in Africa north of the Sahara based on mitochondrial DNA sequences. Vet. Parasitol. 2017, 239, 1–6. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Latrofa, M.S.; Annoscia, G.; Giannelli, A.; Parisi, A.; Otranto, D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasites Vectors 2013, 6, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Šlapeta, J.; Chandra, S.; Halliday, B. The tropical lineage of the brown dog tick Rhipicephalus sanguineus sensu lato identified as Rhipicephalus linnaei (Audouin, 1826). Int. J. Parasitol. 2021, 51, 431–436. [Google Scholar] [CrossRef]
- Jones, E.O.; Gruntmeir, J.M.; Hamer, S.A.; Little, S.E. Temperate and tropical lineages of brown dog ticks in North America. Vet. Parasitol. Reg. Stud. Rep. 2017, 7, 58–61. [Google Scholar] [CrossRef]
- Eremeeva, M.E.; Zambrano, M.L.; Anaya, L.; Beati, L.; Karpathy, S.E.; Santos-Silva, M.M.; Salceda, B.; Macbeth, D.; Olguin, H.; Dasch, G.; et al. Rickettsia rickettsii in Rhipicephalus Ticks, Mexicali, Mexico. J. Med. Èntomol. 2011, 48, 418–421. [Google Scholar] [CrossRef]
- Villarreal, Z.; Stephenson, N.; Foley, J. Possible Northward Introgression of a Tropical Lineage ofRhipicephalus sanguineusTicks at a Site of Emerging Rocky Mountain Spotted Fever. J. Parasitol. 2018, 104, 240–245. [Google Scholar] [CrossRef]
- Hornok, S.; Sándor, A.D.; Tomanović, S.; Beck, R.; D’Amico, G.; Kontschán, J.; Takács, N.; Görföl, T.; Bendjeddou, M.L.; Földvári, G.; et al. East and west separation of Rhipicephalus sanguineus mitochondrial lineages in the Mediterranean Basin. Parasites Vectors 2017, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Montes, S. Rhipicephalus sanguineus Complex in the Americas: Systematic, Genetic Diversity, and Geographic Insights. Pathogens 2021, 10, 1118. [Google Scholar] [CrossRef]
- Zemtsova, G.E.; Apanaskevich, D.A.; Reeves, W.K.; Hahn, M.; Snellgrove, A.; Levin, M.L. Phylogeography of Rhipicephalus sanguineus sensu lato and its relationships with climatic factors. Exp. Appl. Acarol. 2016, 69, 191–203. [Google Scholar] [CrossRef] [Green Version]
- René-Martellet, M.; Minard, G.; Massot, R.; Van, V.T.; Moro, C.V.; Chabanne, L.; Mavingui, P. Bacterial microbiota associated with Rhipicephalus sanguineus (s.l.) ticks from France, Senegal and Arizona. Parasites Vectors 2017, 10, 416. [Google Scholar] [CrossRef]
- Solis, A. Prevalence and Diversity of Spotted Fever Group Rickettsia in Rhipicephalus sanguineus sensu lato Ticks from Arizona. Ph.D. Dissertation, Northern Arizona University, Flagstaff, AZ, USA, December 2017. [Google Scholar]
- Inokuma, H.; Beppu, T.; Okuda, M.; Shimada, Y.; Sakata, Y. Epidemiological survey of Anaplasma platys and Ehrlichia canis using ticks collected from dogs in Japan. Vet. Parasitol. 2003, 115, 343–348. [Google Scholar] [CrossRef]
- Sanogo, Y.O.; Davoust, B.; Inokuma, H.; Camicas, J.-L.; Parola, P.; Brouqui, P. First evidence of Anaplasma platys in Rhipicephalus sanguineus (Acari: Ixodida) collected from dogs in Africa. Onderstepoort J. Vet. Res. 2003, 70, 205–212. [Google Scholar]
- Ramos, R.A.N.; Giannelli, A.; Torres, F.D.; Otranto, D. Effect of egg clustering on the fitness of Rhipicephalus sanguineus larvae. Parasitol. Res. 2012, 112, 1795–1797. [Google Scholar] [CrossRef]
- Demoner Lde, C. Investigation of tick vectors of Hepatozoon canis in Brazil, Ticks Tick. Borne. Dis. 2013, 4, 542–546. [Google Scholar]
- Moraes-Filho, J.; Krawczak, F.D.S.; Costa, F.B.; Soares, J.F.; Labruna, M.B. Comparative Evaluation of the Vector Competence of Four South American Populations of the Rhipicephalus sanguineus Group for the Bacterium Ehrlichia canis, the Agent of Canine Monocytic Ehrlichiosis. PLoS ONE 2015, 10, e0139386. [Google Scholar] [CrossRef]
- Venzal, J.M.; Pena, A.E.; Castro, O.; De Souza, C.G.; Portillo, A.; Oteo, J.A. Study on seasonal activity in dogs and ehrlichial infection in Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) from southern Uruguay. Parasitol. Latinoam. 2007, 62, 23–26. [Google Scholar] [CrossRef]
- Dabert, M. DNA markers in the phylogenetics of the Acari. Biol. Lett. 2006, 43, 97–107. [Google Scholar]
- Cruickshank, R.H. Molecular markers for the phylogenetics of mites and ticks. Syst. Appl. Acarol. 2002, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Levin, M.L.; Studer, E.; Killmaster, L.; Zemtsova, G.; Mumcuoglu, K.Y. Crossbreeding between different geographical populations of the brown dog tick, Rhipicephalus sanguineus (Acari: Ixodidae). Exp. Appl. Acarol. 2012, 58, 51–68. [Google Scholar] [CrossRef] [Green Version]
- Burlini, L.; Teixeira, K.R.S.; Szabó, M.P.J.; Famadas, K.M. Molecular dissimilarities of Rhipicephalus sanguineus (Acari: Ixodidae) in Brazil and its relation with samples throughout the world: Is there a geographical pattern? Exp. Appl. Acarol. 2009, 50, 361–374. [Google Scholar] [CrossRef]
- Sanches, G.S.; Évora, P.M.; Mangold, A.J.; Jittapalapong, S.; Rodriguez-Mallon, A.; Guzmán, P.E.; Bechara, G.H.; Camargo-Mathias, B. Molecular, biological, and morphometric comparisons between different geographical populations of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae). Vet. Parasitol. 2016, 215, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangold, A.J.; Bargues, M.D.; Mas-Coma, S. Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and other tick genera among Metastriata (Acari: Ixodidae). Parasitol. Res. 1998, 84, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Hekimoğlu, O.; Sağlam, I.; Özer, N.; Peña, A.E. New molecular data shed light on the global phylogeny and species limits of the Rhipicephalus sanguineus complex. Ticks Tick-Borne Dis. 2016, 7, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Díaz, F.E.; Martínez-Valdebenito, C.; López, J.; Weitzel, T.; Abarca, K. Geographical distribution and phylogenetic analysis of Rhipicephalus sanguineus sensu lato in northern and central Chile. Ticks Tick. Borne. Dis. 2018, 9, 792–797. [Google Scholar] [CrossRef]
- Abdullah, H.H.A.M.; El-Molla, A.; Salib, F.A.; Allam, N.A.T.; Ghazy, A.A.; Abdel-Shafy, S. Morphological and molecular identification of the brown dog tick Rhipicephalus sanguineus and the camel tick Hyalomma dromedarii (Acari: Ixodidae) vectors of Rickettsioses in Egypt. Vet. World 2016, 9, 1087–1101. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Mastrantonio, V.; Latrofa, M.S.; Porretta, D.; Lia, R.P.; Parisi, A.; Iatta, R.; Torres, F.D.; Otranto, D.; Urbanelli, S. Paternal leakage and mtDNA heteroplasmy in Rhipicephalus spp. ticks. Sci. Rep. 2019, 9, 1460. [Google Scholar] [CrossRef]
- Labruna, M.B.; Gerardi, M.; Krawczak, F.S.; Moraes-Filho, J. Comparative biology of the tropical and temperate species of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) under different laboratory conditions. Ticks Tick-Borne Dis. 2016, 8, 146–156. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Dantas-Torres, F.; Latrofa, M.S.; Ramos, R.A.N.; Lia, R.P.; Capelli, G.; Parisi, A.; Porretta, D.; Urbanelli, S.; Otranto, D. Biological compatibility between two temperate lineages of brown dog ticks, Rhipicephalus sanguineus (sensu lato). Parasites Vectors 2018, 11, 398. [Google Scholar] [CrossRef]
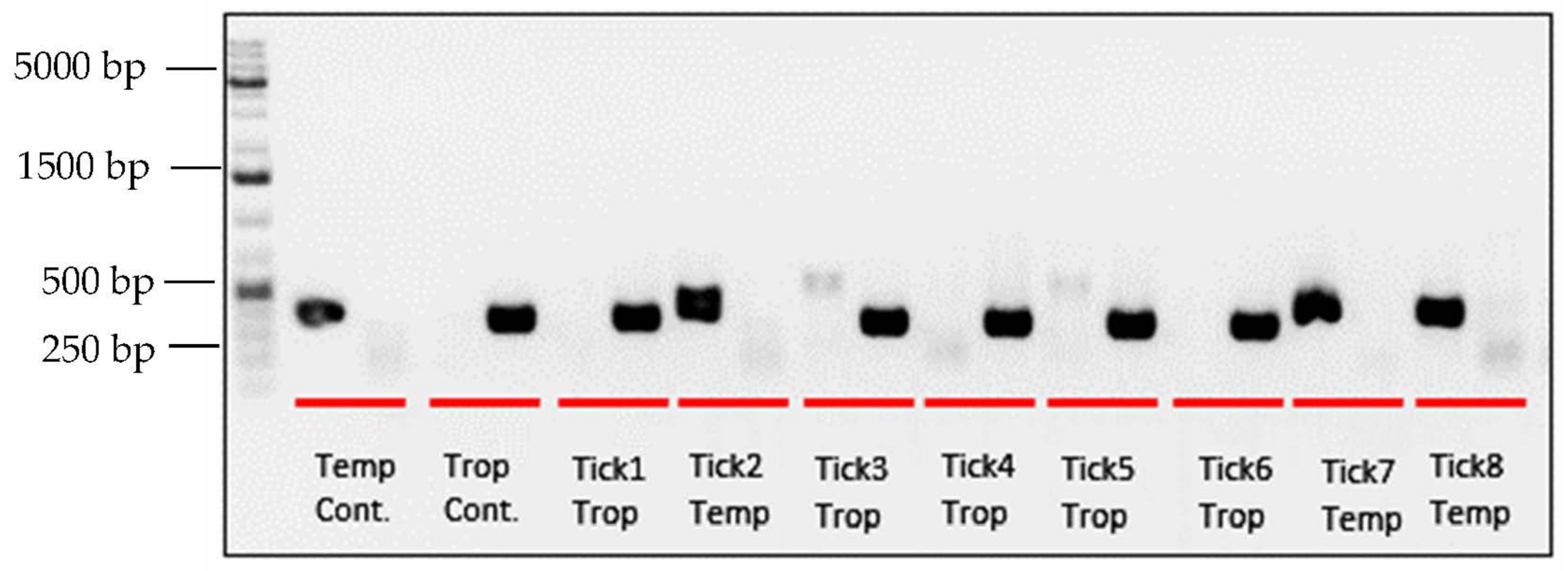
| Life Stage | n (%) |
|---|---|
| Larva | 4 (1.3) |
| Nymph | 44 (14.4) |
| Adult | 258 (84.3) |
| Male | 144 (55.8) |
| Female | 114 (44.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brophy, M.; Riehle, M.A.; Mastrud, N.; Ravenscraft, A.; Adamson, J.E.; Walker, K.R. Genetic Variation in Rhipicephalus sanguineus s.l. Ticks across Arizona. Int. J. Environ. Res. Public Health 2022, 19, 4223. https://doi.org/10.3390/ijerph19074223
Brophy M, Riehle MA, Mastrud N, Ravenscraft A, Adamson JE, Walker KR. Genetic Variation in Rhipicephalus sanguineus s.l. Ticks across Arizona. International Journal of Environmental Research and Public Health. 2022; 19(7):4223. https://doi.org/10.3390/ijerph19074223
Chicago/Turabian StyleBrophy, Maureen, Michael A. Riehle, Nikki Mastrud, Alison Ravenscraft, Johnathan E. Adamson, and Kathleen R. Walker. 2022. "Genetic Variation in Rhipicephalus sanguineus s.l. Ticks across Arizona" International Journal of Environmental Research and Public Health 19, no. 7: 4223. https://doi.org/10.3390/ijerph19074223
APA StyleBrophy, M., Riehle, M. A., Mastrud, N., Ravenscraft, A., Adamson, J. E., & Walker, K. R. (2022). Genetic Variation in Rhipicephalus sanguineus s.l. Ticks across Arizona. International Journal of Environmental Research and Public Health, 19(7), 4223. https://doi.org/10.3390/ijerph19074223







