Improved Water and Waste Management Practices Reduce Diarrhea Risk in Children under Age Five in Rural Tanzania: A Community-Based, Cross-Sectional Analysis
Abstract
:1. Introduction
2. Materials and Methods
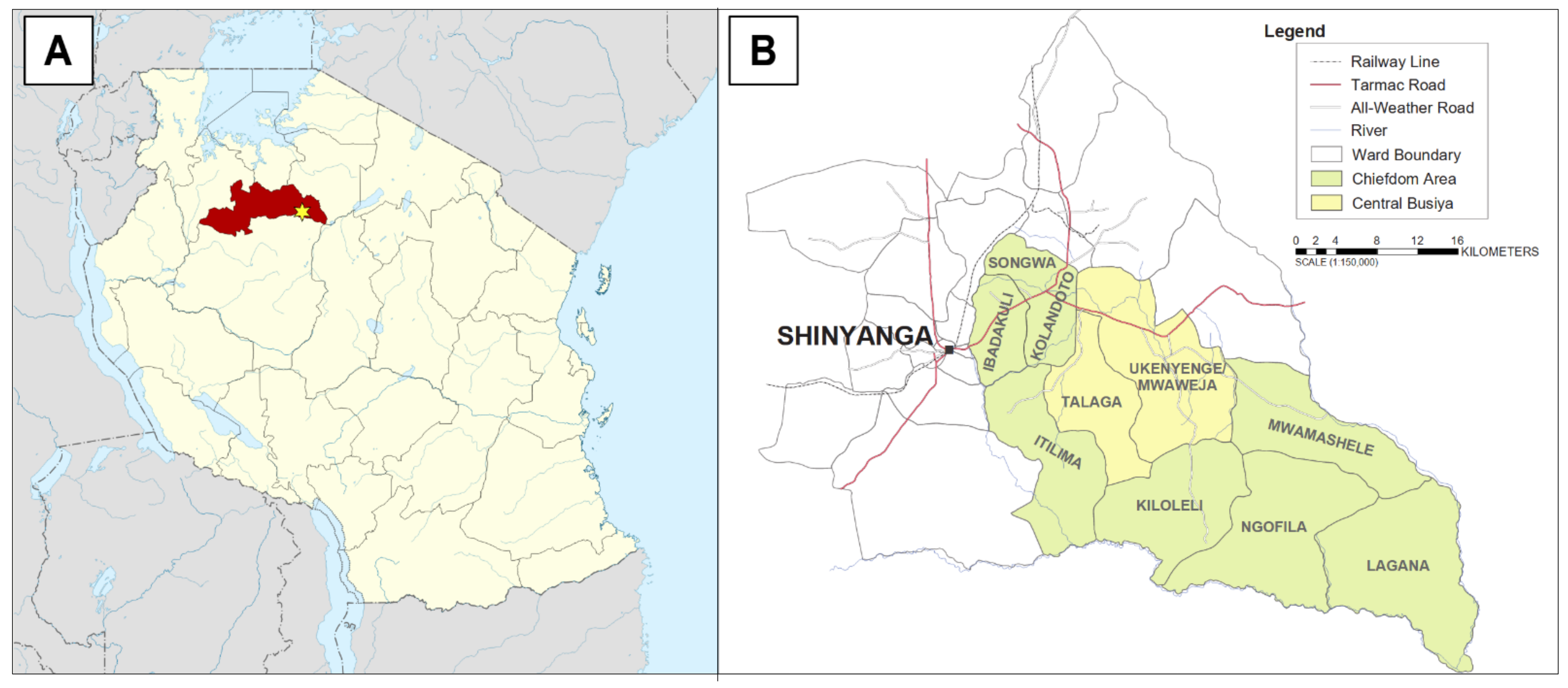
2.1. Study Area and Population
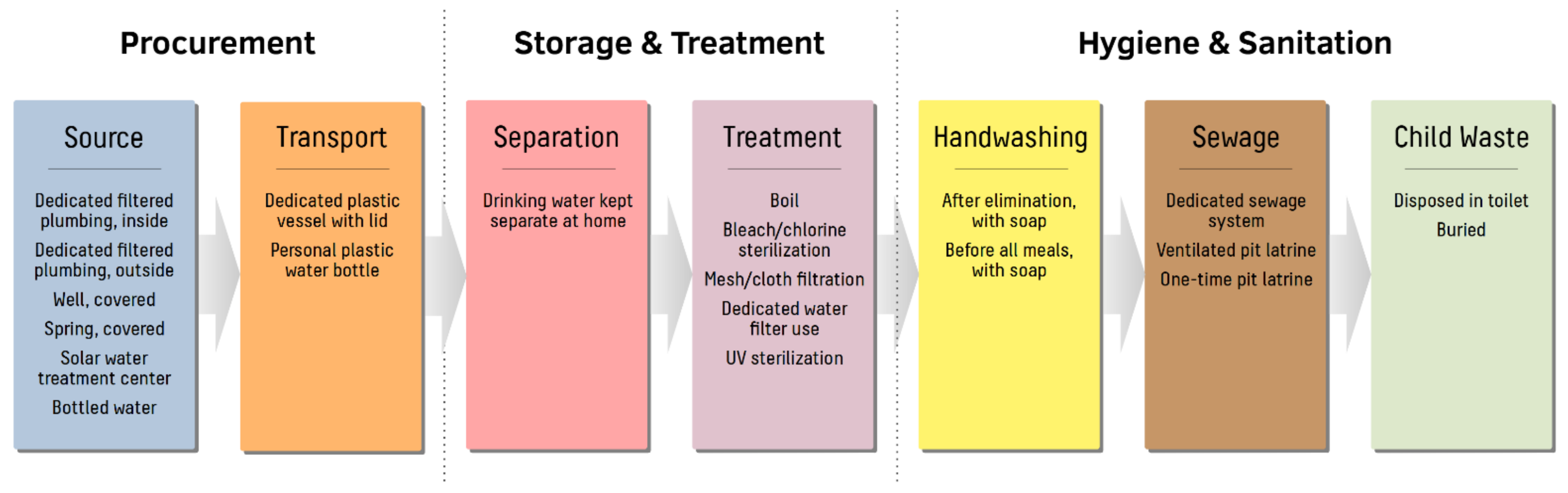
2.2. Previous Interventions and Current Clean Water Practices
2.3. Study Design and Variables
2.4. Survey Implementation and Ethical Clearance
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics
3.2. Drinking Water Sources and Sanitation
3.3. Prevalence and Treatment of Diarrhea in Under-Five Children
3.4. Predictive Factors Leading to Diarrhea in Under-Five Children
4. Discussion
4.1. Prevalence of Under-Five Diarrhea in Rural Tanzania
4.2. Water Procurement Strategies for Preventing Under-Five Diarrhea
4.3. Effects of Point-of-Use Water Treatment and WASH Practices on Under-Five Diarrhea
4.4. Influence of Social Factors on Under-Five Diarrhea
4.5. Treatment of Diarrhea in Under-Five Children
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Cherunya, P.C.; Janezic, C.; Leuchner, M. Sustainable Supply of Safe Drinking Water for Underserved Households in Kenya: Investigating the Viability of Decentralized Solutions. Water 2015, 7, 5437–5457. [Google Scholar] [CrossRef] [Green Version]
- Mshida, H.A.; Kassim, N.; Kimanya, M.E.; Mpolya, E. Influence of Water, Sanitation, and Hygiene Practices on Common Infections among Under-Five Children in Longido and Monduli Districts of Arusha, Tanzania. J. Environ. Public Health 2017, 2017, 9235168. [Google Scholar] [CrossRef] [PubMed]
- Diouf, K.; Tabatabai, P.; Rudolph, J.; Marx, M. Diarrhoea Prevalence in Children under Five Years of Age in Rural Burundi: An Assessment of Social and Behavioural Factors at the Household Level. Glob. Health Action 2014, 7, 24895. [Google Scholar] [CrossRef] [PubMed]
- Andreolli, M.; Giovannini, M.; Fatone, F.; Kyamunyogonya, M.; Yatuha, J. A Basic Bottom-up Approach for Small Systems of Safe-Water Supply: A Decentralized Case Study in Uganda. J. Water Supply Res. Technol.-AQUA 2015, 64, 105–116. [Google Scholar] [CrossRef]
- Esrey, S.A.; Habicht, J.P.; Latham, M.C.; Sisler, D.G.; Casella, G. Drinking Water Source, Diarrheal Morbidity, and Child Growth in Villages with Both Traditional and Improved Water Supplies in Rural Lesotho, Southern Africa. Am. J. Public Health 1988, 78, 1451–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, R.; Fontaine, O.; Lamberti, L.; Bhan, M.; Huicho, L.; El Arifeen, S.; Masanja, H.; Walker, C.F.; Mengestu, T.K.; Pearson, L.; et al. Drivers of the Reduction in Childhood Diarrhea Mortality 1980-2015 and Interventions to Eliminate Preventable Diarrhea Deaths by 2030. J. Glob. Health 2019, 9, 20801. [Google Scholar] [CrossRef]
- United Nations. The Millennium Development Goals Report 2015; United Nations: New York, NY, USA, 2015; pp. 1–75. Available online: https://www.un.org/millenniumgoals/2015_MDG_Report/pdf/MDG%202015%20rev%20(July%201).pdf (accessed on 13 July 2021).
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. 2015. Available online: https://sdgs.un.org/sites/default/files/publications/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf (accessed on 13 July 2021).
- Chopra, M.; Mason, E.; Borrazzo, J.; Campbell, H.; Rudan, I.; Liu, L.; Black, R.E.; Bhutta, Z.A. Ending of Preventable Deaths from Pneumonia and Diarrhoea: An Achievable Goal. Lancet 2013, 381, 1499–1506. [Google Scholar] [CrossRef]
- Curtis, V.; Cairncross, S.; Yonli, R. Review: Domestic Hygiene and Diarrhoea—Pinpointing the Problem. Trop. Med. Int. Health 2000, 5, 22–32. [Google Scholar] [CrossRef]
- Eisenberg, J.N.S.; Trostle, J.; Sorensen, R.J.D.; Shields, K.F. Toward a Systems Approach to Enteric Pathogen Transmission: From Individual Independence to Community Interdependence. Annu. Rev. Public Health 2012, 33, 239–257. [Google Scholar] [CrossRef] [Green Version]
- Kamara, J.K.; Galukande, M.; Maeda, F.; Luboga, S.; Renzaho, A.M.N. Understanding the Challenges of Improving Sanitation and Hygiene Outcomes in a Community Based Intervention: A Cross-Sectional Study in Rural Tanzania. Int. J. Environ. Res. Public Health 2017, 14, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, S.; Mosler, H.-J. Differences in Influence Patterns between Groups Predicting the Adoption of a Solar Disinfection Technology for Drinking Water in Bolivia. Soc. Sci. Med. 2008, 67, 497–504. [Google Scholar] [CrossRef]
- Rajasingham, A.; Harvey, B.; Taye, Y.; Kamwaga, S.; Martinsen, A.; Sirad, M.; Aden, M.; Gallagher, K.; Handzel, T. Improved Chlorination and Rapid Water Quality Assessment in Response to an Outbreak of Acute Watery Diarrhea in Somali Region, Ethiopia. J. Water Sanit. Hyg. Dev. 2020, 10, 596–602. [Google Scholar] [CrossRef]
- Mashoto, K.O.; Malebo, H.M.; Msisiri, E.; Peter, E. Prevalence, One Week Incidence and Knowledge on Causes of Diarrhea: Household Survey of under-Fives and Adults in Mkuranga District, Tanzania. BMC Public Health 2014, 14, 985. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, M.; Reller, M.E.; Baier, K.G.; Hoekstra, R.M.; Keswick, B.H.; Mendoza, C.E.; Luby, S.P.; Lopez, M.B.; Olson, C.A. A Randomized Controlled Trial of Household-Based Flocculant-Disinfectant Drinking Water Treatment for Diarrhea Prevention in Rural Guatemala. Am. J. Trop. Med. Hyg. 2003, 69, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Francis, M.R.; Sarkar, R.; Roy, S.; Jaffar, S.; Mohan, V.R.; Kang, G.; Balraj, V. Effectiveness of Membrane Filtration to Improve Drinking Water: A Quasi-Experimental Study from Rural Southern India. Am. J. Trop. Med. Hyg. 2016, 95, 1192–1200. [Google Scholar] [CrossRef] [Green Version]
- Sima, L.C.; Desai, M.M.; McCarty, K.M.; Elimelech, M. Relationship between Use of Water from Community-Scale Water Treatment Refill Kiosks and Childhood Diarrhea in Jakarta. Am. J. Trop. Med. Hyg. 2012, 87, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilungo, A.; Powers, L.; Arnold, N.; Whelan, K.; Paterson, K.; Young, D. Evaluation of Well Designs to Improve Access to Safe and Clean Water in Rural Tanzania. Int. J. Environ. Res. Public Health 2018, 15, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masanja, H.; Mongi, P.; Baraka, J.; Jackson, B.; Kisisiwe, Y.; Manji, K.; Iriya, N.; John, T.; Kimatta, S.; Walker, N.; et al. Factors Associated with the Decline in under Five Diarrhea Mortality in Tanzania from 1980–2015. J. Glob. Health 2019, 9, 20806. [Google Scholar] [CrossRef]
- Dadonaite, B.; Ritchie, H.; Roser, M. Diarrheal Diseases. Our World Data. 2018. Available online: https://ourworldindata.org/diarrheal-diseases (accessed on 13 July 2021).
- Malebo, H.; Makundi, E.; Mussa, R.; Mushi, A.; Munga, M.; Mrisho, M.; Senkoro, K.; Mcharo, J.; Urassa, J.; Msimbe, V.; et al. Outcome and Impact Monitoring for Scaling Up Mtumba Sanitation and Hygiene Participatory Approach in Tanzania. Nat’l Inst. Med. Res. 2012, 1, 1–70. [Google Scholar]
- Leach, V.; Kilama, B. Preventing Malnutrition in Tanzania: A Focused Strategy to Improve Nutrition in Young Children. Special Paper no.15. Research on Poverty Alleviation. 2009. Available online: https://opendocs.ids.ac.uk/opendocs/handle/20.500.12413/1816 (accessed on 13 July 2021).
- Ministry of Health, Community Development, Gender, Elderly and Children (MoHCDGEC); National Bureau of Statistics (NBS); Office of the Chief Government Statistician (OCGS). Tanzania Demographic and Health Survey and Malaria Indicator Survey (TDHS-MIS) 2015–16. 2016. Available online: https://www.dhsprogram.com/pubs/pdf/FR321/FR321.pdf (accessed on 13 July 2021).
- Data Africa. Shinyanga, United Republic of Tanzania. Available online: https://dataafrica.io/profile/shinyang-tza (accessed on 16 June 2021).
- Tanzania National Bureau of Statistics. Population Distribution by Age and Sex. Ministry of Finance, Dar es Salaam, Tanzania. 2013. Available online: https://www.nbs.go.tz/index.php/en/census-surveys/population-and-housing-census/163-phc-2012-population-distribution-by-age-and-sex-report (accessed on 13 July 2021).
- Tanzania National Bureau of Statistics. Bureau of Statistics. Basic Demographic and Socio-Economic Profile Report, Tanzania Mainland. Ministry of Finance, Dar es Salaam, Tanzania. 2014. Available online: https://www.tanzania.go.tz/egov_uploads/documents/TANZANIA_MAINLAND_SOCIO_ECONOMIC_PROFILE_sw.pdf (accessed on 13 July 2021).
- Tanzania National Bureau of Statistics. 2012 Population and Housing Census: Population Distribution by Administrative Areas. Ministry of Finance, Dar es Salaam, Tanzania. 2013. Available online: https://www.nbs.go.tz/index.php/en/census-surveys/population-and-housing-census/162-2012-phc-population-distribution-by-administrative-areas (accessed on 13 July 2021).
- UNICEF. Strategy for Water, Sanitation, and Hygiene 2016–2030. Programme Div. UNICEF N. Y. 2016. Available online: https://www.unicef.org/documents/unicef-strategy-water-sanitation-and-hygiene-2016–2030 (accessed on 13 July 2021).
- Innovative Water Technologies, Inc. SunSpring® Information Brochure 2017. 2017. Available online: https://innovativeh2o.com/sunspring-hybrid-2/ (accessed on 13 July 2021).
- WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. Core Questions on Drinking-Water and Sanitation for Household Surveys. World Health Organanization UNICEF. 2009. Available online: https://www.who.int/water_sanitation_health/monitoring/oms_brochure_core_questionsfinal24608.pdf (accessed on 13 July 2021).
- World Health Organization. WHO Fact Sheet: Diarrhoeal Disease. 2 May 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 13 July 2021).
- Edwin, P.; Azage, M. Geographical Variations and Factors Associated with Childhood Diarrhea in Tanzania: A National Population Based Survey 2015–2016. Ethiop. J. Health Sci. 2019, 29, 513–524. [Google Scholar] [CrossRef]
- Kabhele, S.; New-Aaron, M.; Kibusi, S.M.; Gesase, A.P. Prevalence and Factors Associated with Diarrhoea among Children between 6 and 59 Months of Age in Mwanza City Tanzania. J. Trop. Pediatr. 2018, 64, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Kang, D.; Tuffuor, B.; Lee, G.; Cho, J.; Chung, J.; Kim, M.; Lee, H.; Lee, J.; Oh, C. The Effect of Improved Water Supply on Diarrhea Prevalence of Children under Five in the Volta Region of Ghana: A Cluster-Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2015, 12, 12127–12143. [Google Scholar] [CrossRef] [Green Version]
- Soboksa, N.E. Associations Between Improved Water Supply and Sanitation Usage and Childhood Diarrhea in Ethiopia: An Analysis of the 2016 Demographic and Health Survey. Environ. Health Insights 2021, 15, 1–10. [Google Scholar] [CrossRef]
- Hendrickson, C.; Oremo, J.; Akello, O.O.; Bunde, S.; Rayola, I.; Akello, D.; Akwiri, D.; Park, S.-J.; Dorevitch, S. Decentralized Solar-Powered Drinking Water Ozonation in Western Kenya: An Evaluation of Disinfection Efficacy. Gates Open Res. 2020, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Hunter, P.R.; Freeman, M.C.; Cumming, O.; Clasen, T.; Bartram, J.; Higgins, J.P.T.; Johnston, R.; Medlicott, K.; Boisson, S.; et al. Impact of Drinking Water, Sanitation and Handwashing with Soap on Childhood Diarrhoeal Disease: Updated Meta-Analysis and Meta-Regression. Trop. Med. Int. Health TMIH 2018, 23, 508–525. [Google Scholar] [CrossRef] [PubMed]
- Geremew, A.; Damtew, Y.T. Household Water Treatment Using Adequate Methods in Sub-Saharan Countries: Evidence from 2013–2016 Demographic and Health Surveys. J. Water Sanit. Hyg. Dev. 2019, 10, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.; Colford, J.M. Effects of Boiling Drinking Water on Diarrhea and Pathogen-Specific Infections in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2017, 97, 1362–1377. [Google Scholar] [CrossRef] [Green Version]
- Oloruntoba, E.O.; Folarin, T.B.; Ayede, A.I. Hygiene and Sanitation Risk Factors of Diarrhoeal Disease among Under-Five Children in Ibadan, Nigeria. Afr. Health Sci. 2014, 14, 1001–1011. [Google Scholar] [CrossRef]
- Getahun, W.; Adane, M. Prevalence of Acute Diarrhea and Water, Sanitation, and Hygiene (WASH) Associated Factors among Children under Five in Woldia Town, Amhara Region, Northeastern Ethiopia. BMC Pediatr. 2021, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Bennion, N.; Mulokozi, G.; Allen, E.; Fullmer, M.; Kleinhenz, G.; Dearden, K.; Linehan, M.; Torres, S.; West, J.; Crookston, B.; et al. Association between WASH-Related Behaviors and Knowledge with Childhood Diarrhea in Tanzania. Int. J. Environ. Res. Public Health 2021, 18, 4681. [Google Scholar] [CrossRef]
- Solomon, E.T.; Gari, S.R.; Kloos, H.; Alemu, B.M. Handwashing Effect on Diarrheal Incidence in Children under 5 Years Old in Rural Eastern Ethiopia: A Cluster Randomized Controlled Trial. Trop. Med. Health 2021, 49, 26. [Google Scholar] [CrossRef]
- Hashi, A.; Kumie, A.; Gasana, J. Hand Washing with Soap and WASH Educational Intervention Reduces Under-Five Childhood Diarrhoea Incidence in Jigjiga District, Eastern Ethiopia: A Community-Based Cluster Randomized Controlled Trial. Prev. Med. Rep. 2017, 6, 361–368. [Google Scholar] [CrossRef]
- Null, C.; Stewart, C.P.; Pickering, A.J.; Dentz, H.N.; Arnold, B.F.; Arnold, C.D.; Benjamin-Chung, J.; Clasen, T.; Dewey, K.G.; Fernald, L.C.H.; et al. Effects of Water Quality, Sanitation, Handwashing, and Nutritional Interventions on Diarrhoea and Child Growth in Rural Kenya: A Cluster-Randomised Controlled Trial. Lancet Glob. Health 2018, 6, e316–e329. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, J.H.; Mbuya, M.N.N.; Ntozini, R.; Moulton, L.H.; Stoltzfus, R.J.; Tavengwa, N.V.; Mutasa, K.; Majo, F.; Mutasa, B.; Mangwadu, G.; et al. Independent and Combined Effects of Improved Water, Sanitation, and Hygiene, and Improved Complementary Feeding, on Child Stunting and Anaemia in Rural Zimbabwe: A Cluster-Randomised Trial. Lancet Glob. Health 2019, 7, e132–e147. [Google Scholar] [CrossRef] [Green Version]
- Rogawski McQuade, E.T.; Platts-Mills, J.A.; Gratz, J.; Zhang, J.; Moulton, L.H.; Mutasa, K.; Majo, F.D.; Tavengwa, N.; Ntozini, R.; Prendergast, A.J.; et al. Impact of Water Quality, Sanitation, Handwashing, and Nutritional Interventions on Enteric Infections in Rural Zimbabwe: The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial. J. Infect. Dis. 2020, 221, 1379–1386. [Google Scholar] [CrossRef]
- Demissie, G.D.; Yeshaw, Y.; Aleminew, W.; Akalu, Y. Diarrhea and Associated Factors among under Five Children in Sub-Saharan Africa: Evidence from Demographic and Health Surveys of 34 Sub-Saharan Countries. PLoS ONE 2021, 16, e0257522. [Google Scholar] [CrossRef]

| Study Variable | Central Busiya (Total) | Nhobola | Negezi | Ngunga | Ubata |
|---|---|---|---|---|---|
| Total households, n | 779 | 261 | 164 | 156 | 198 |
| Total individuals, n | 4702 | 1747 | 1077 | 1033 | 845 |
| Individuals per household, median (IQR) | 6 (4–7) | 6 (5–8) | 6 (4–8) | 6 (5–8) | 4 (3–5) |
| Employed as subsistence farmer, n (%) | 729/761 (95.8%) | 242/260 (93.1%) | 157/162 (96.9%) | 148/149 (99.3%) | 182/190 (95.8%) |
| Highest level of education in the household | |||||
| None | 77/723 (10.7%) | 10/240 (4.2%) | 6/148 (4.1%) | 21/147 (14.3%) | 40/188 (21.3%) |
| Primary | 595/723 (82.3%) | 219/240 (91.3%) | 125/148 (84.5%) | 122/147 (83.0%) | 129/188 (68.6%) |
| Secondary | 41/723 (5.7%) | 4/240 (1.7%) | 16/148 (10.8%) | 3/147 (2.0%) | 18/188 (9.6%) |
| Vocational/College | 10/723 (1.4%) | 7/240 (2.9%) | 1/148 (0.7%) | 1/147 (0.7%) | 1/188 (0.5%) |
| Child demographics | |||||
| Total children, n (%) | 1338/4702 (28.5%) | 453/1747 (25.9%) | 274/1077 (25.4%) | 269/1033 (26.0%) | 342/845 (40.5%) |
| Children per household, median (IQR) | 2 (1–2) | 2 (1–2) | 2 (1–2) | 1.5 (1–2) | 2 (1–2) |
| Child age, median (IQR) | 3 (2–4) | 3 (2–4) | 4 (2–4) | 4 (2–4) | 3 (2–4) |
| Number of females, n (%) | 678/1334 (50.8%) | 234/450 (52.0%) | 144/274 (52.6%) | 132/269 (49.1%) | 168/341 (49.3%) |
| WASH compliance at household level | |||||
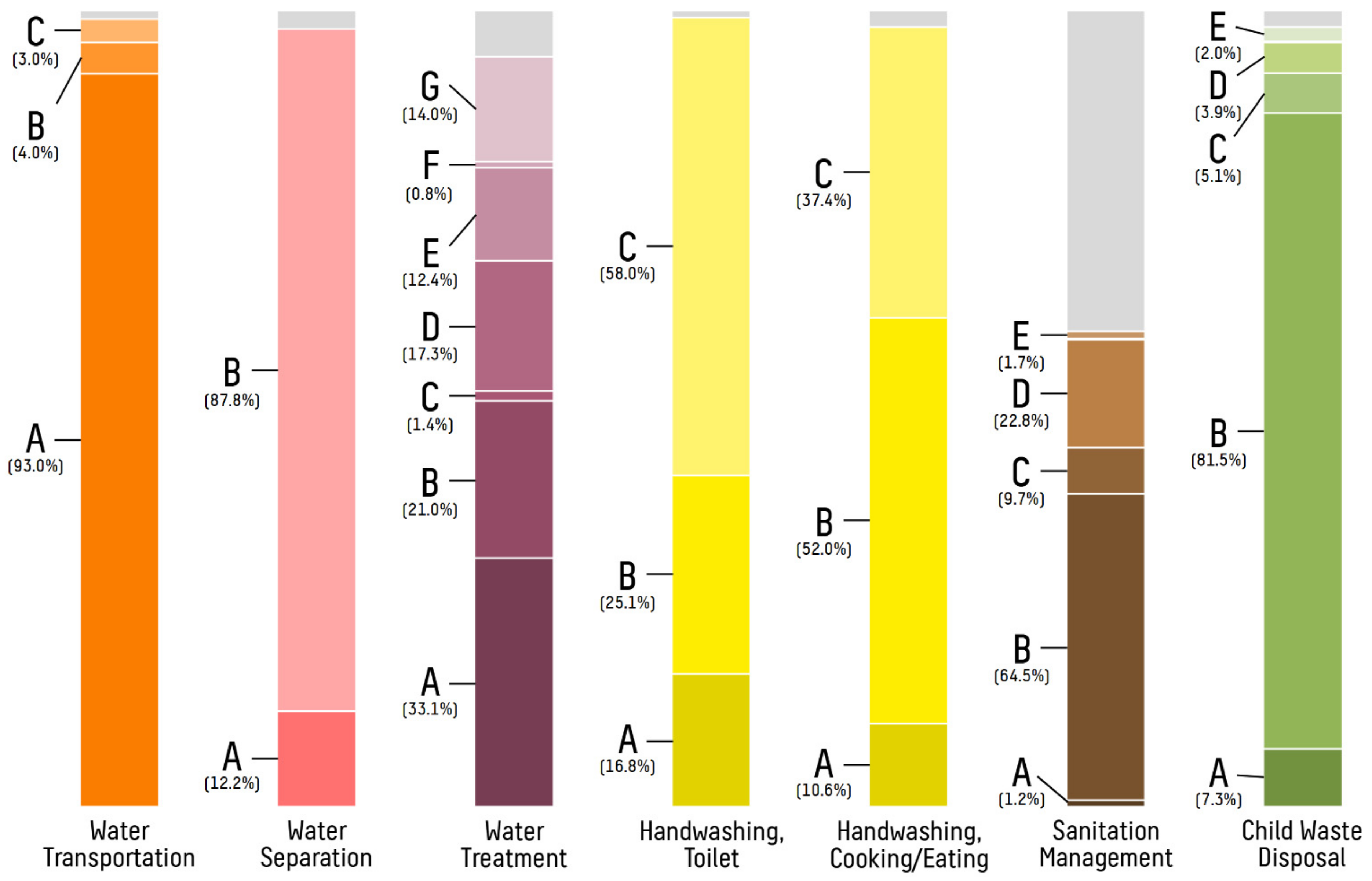
| Clean/improved source of drinking water, n (%) | 270/779 (34.7%) | 54/261 (20.7%) | 45/164 (27.4%) | 111/156 (71.2%) | 60/198 (30.3%) |
| Clean/improved source of non-drinking water, n (%) | 235/779 (30.2%) | 99/261 (37.9%) | 45/164 (27.4%) | 52/156 (33.3%) | 39/198 (19.7%) |
| Dedicated transportation vessel for drinking water, n (%) | 648/771 (84.0%) | 240/259 (92.7%) | 118/162 (72.8%) | 121/153 (79.1%) | 169/197 (85.8%) |
| Dedicated transportation vessel for non-drinking water, n (%) | 590/776 (76.0%) | 193/261 (73.9%) | 111/161 (68.9%) | 121/156 (77.6%) | 165/198 (83.3%) |
| Water procurement time <2 h, n (%) | 396/775 (51.1%) | 203/257 (79.0%) | 85/164 (51.8%) | 57/156 (36.5%) | 51/198 (25.8%) |
| Times visiting water source per week, median (IQR) * | 4 (3–7) | 4 (3–6) | 7 (1–7) | 7 (6.5–21) | 3 (2–5) |
| Amount of water returned per trip in liters, median (IQR) † | 100 (40–140) | 120 (100–200) | 40 (40–40) | 100 (60–130) | 120 (60–160) |
| Storage of water in a different location than transport, n (%) | 574/753 (76.2%) | 244/247 (98.8%) | 97/158 (61.4%) | 143/154 (92.9%) | 90/194 (46.4%) |
| Separation of drinking water, n (%) | 668/761 (87.8%) | 240/258 (93.0%) | 138/158 (87.3%) | 153/155 (98.7%) | 137/194 (70.6%) |
| Point-of-use drinking water treatment at home, n (%) | 334/779 (42.9%) | 88/261 (33.7%) | 82/164 (50.0%) | 114/156 (73.1%) | 50/198 (25.3%) |
| Handwashing with soap after urination or defecation, n (%) | 448/772 (58.0%) | 138/258 (53.5%) | 124/162 (76.5%) | 109/155 (70.3%) | 77/197 (39.1%) |
| Handwashing with soap before eating or cooking, n (%) | 285/763 (37.4%) | 69/250 (27.6%) | 88/162 (54.3%) | 55/154 (35.7%) | 73/197 (37.1%) |
| Adequate sewage management at home, n (%) | 348/779 (44.7%) | 250/261 (95.8%) | 63/164 (38.4%) | 29/156 (18.6%) | 6/198 (3.0%) |
| Adequate disposal of child waste, n (%) | 627/779 (80.5%) | 224/261 (85.8%) | 114/164 (69.5%) | 111/156 (71.2%) | 178/198 (89.9%) |
| Study Variable | Central Busiya (Total) | Nhobola | Negezi | Ngunga | Ubata |
|---|---|---|---|---|---|
| Households reporting a child with diarrhea, n (%) | 250/779 (32.1%) | 57/261 (21.8%) | 40/164 (24.4%) | 41/156 (26.3%) | 112/198 (56.6%) |
| Diarrhea among children | |||||
| Frequency, n (%) | 343/1338 (25.6%) | 70/453 (15.5%) | 57/274 (20.8%) | 45/269 (16.7%) | 171/342 (50.0%) |
| Child age, median (IQR) | 2 (1–4) | 1 (1–3) | 2 (1–4) | 3 (2–4) | 2 (1–4) |
| Number of females, n (%) | 173/343 (50.4%) | 38/70 (54.2%) | 31/57 (54.3%) | 18/45 (40.0%) | 86/171 (50.3%) |
| Quality of diarrhea among children | |||||
| Mucoid, n (%) | 102/321 (31.8%) | 12/68 (17.6%) | 5/53 (9.4%) | 13/44 (29.5%) | 72/156 (46.2%) |
| Watery, n (%) | 195/321 (60.7%) | 47/68 (69.1%) | 40/53 (70.2%) | 26/44 (59.1%) | 82/156 (52.6%) |
| Bloody, n (%) | 9/321 (2.8%) | 0/68 (0.0%) | 7/53 (13.2%) | 1/44 (2.3%) | 1/156 (0.6%) |
| Ova and Parasites, n (%) | 15/321 (4.7%) | 9/68 (13.2%) | 1/53 (1.9%) | 4/44 (9.1%) | 1/156 (0.6%) |
| Clinic visits for diarrhea, n (%) | 285/343 (83.1%) | 40/70 (57.1%) | 42/57 (73.9%) | 37/45 (82.2%) | 166/171 (97.1%) |
| Treatment of diarrhea among children, n (%) | 300/343 (87.5%) | 45/70 (64.3%) | 47/57 (82.5%) | 41/45 (91.1%) | 167/171 (97.7%) |
| Type of treatment for diarrhea among children | |||||
| Antibiotics, n (%) | 132/300 (44.0%) | 1/45 (2.2%) | 22/47 (46.8%) | 8/41 (19.5%) | 101/167 (60.5%) |
| Antiparasitics, n (%) | 53/300 (17.7%) | 11/45 (24.4%) | 7/47 (14.9%) | 10/41 (24.4%) | 25/167 (15.0%) |
| Oral rehydration solution, n (%) | 82/300 (27.3%) | 24/45 (53.3%) | 7/47 (14.9%) | 18/41 (43.9%) | 33/167 (19.8%) |
| Traditional and other medicines, n (%) | 33/300 (11.0%) | 9/45 (20.0%) | 11/47 (23.4%) | 5/41 (12.2%) | 8/167 (4.8%) |
| Household | Child | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||||||
| Model Variable | OR | 95% CI | p-Value § | aOR | 95% CI | p-Value § | OR | 95% CI | p-Value § | aOR | 95% CI | p-Value § |
| Clean/improved source of drinking water | 0.72 | 0.52–0.99 | 0.04 | 0.60 | 0.421–0.842 | 0.0034 | 0.83 | 0.65–1.05 | 0.13 | |||
| Clean/improved source of nondrinking water | 0.76 | 0.55–1.07 | 0.12 | 0.78 | 0.61–1.00 | 0.05 | ||||||
| Dedicated transportation vessel for drinking water * | 0.54 | 0.36–0.78 | 0.0011 | 0.51 | 0.37–0.68 | <0.0001 | ||||||
| Dedicated transportation vessel for nondrinking water * | 0.53 | 0.38–0.75 | 0.0003 | 0.61 | 0.46–0.80 | 0.0004 | ||||||
| Water procurement time <2 h | 0.49 | 0.36–0.67 | <0.0001 | 0.59 | 0.420–0.816 | 0.0016 | 0.54 | 0.43–0.68 | <0.0001 | 0.69 | 0.55–0.86 | 0.0012 |
| Times visiting water source per week † | 0.92 | 0.88–0.96 | 0.0002 | 0.94 | 0.92–0.96 | <0.0001 | ||||||
| Volume of water returned per trip, liters | 1.00 | 1.00–1.00 | 0.33 | 1.00 | 0.99–1.00 | 0.56 | ||||||
| Storage water in a different location than transport * | 0.25 | 0.18–0.36 | <0.0001 | 0.35 | 0.29–0.43 | <0.0001 | ||||||
| Separation of drinking water * | 0.38 | 0.24–0.59 | <0.0001 | 0.56 | 0.44–0.71 | <0.0001 | ||||||
| Point-of-use drinking water treatment at home | 1.00 | 0.74–1.35 | 0.98 | 0.92 | 0.74–1.15 | 0.46 | ||||||
| Handwashing with soap after urination or defecation | 0.55 | 0.41–0.75 | 0.0002 | 0.62 | 0.410–0.939 | 0.0238 | 0.66 | 0.53–0.82 | 0.0002 | 0.66 | 0.54–0.82 | 0.0001 |
| Handwashing with soap before eating or cooking | 0.68 | 0.49–0.93 | 0.02 | 0.81 | 0.519–1.247 | 0.331 | 0.85 | 0.57–1.08 | 0.18 | |||
| Adequate sewage management | 0.37 | 0.27–0.51 | <0.0001 | 0.40 | 0.281–0.561 | <0.0001 | 0.38 | 0.30–0.49 | <0.0001 | 0.42 | 0.32–0.54 | 0.0001 |
| Adequate disposal of child waste * | 0.67 | 0.46–0.96 | 0.03 | 0.86 | 0.67–1.10 | 0.24 | ||||||
| Solar water treatment center knowledge ‡ | 0.71 | 0.50–1.02 | 0.06 | 0.73 | 0.55–0.97 | 0.03 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McClelland, P.H.; Kenney, C.T.; Palacardo, F.; Roberts, N.L.S.; Luhende, N.; Chua, J.; Huang, J.; Patel, P.; Sanchez, L.A.; Kim, W.J.; et al. Improved Water and Waste Management Practices Reduce Diarrhea Risk in Children under Age Five in Rural Tanzania: A Community-Based, Cross-Sectional Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4218. https://doi.org/10.3390/ijerph19074218
McClelland PH, Kenney CT, Palacardo F, Roberts NLS, Luhende N, Chua J, Huang J, Patel P, Sanchez LA, Kim WJ, et al. Improved Water and Waste Management Practices Reduce Diarrhea Risk in Children under Age Five in Rural Tanzania: A Community-Based, Cross-Sectional Analysis. International Journal of Environmental Research and Public Health. 2022; 19(7):4218. https://doi.org/10.3390/ijerph19074218
Chicago/Turabian StyleMcClelland, Paul H., Claire T. Kenney, Federico Palacardo, Nicholas L. S. Roberts, Nicholas Luhende, Jason Chua, Jennifer Huang, Priyanka Patel, Leonardo Albertini Sanchez, Won J. Kim, and et al. 2022. "Improved Water and Waste Management Practices Reduce Diarrhea Risk in Children under Age Five in Rural Tanzania: A Community-Based, Cross-Sectional Analysis" International Journal of Environmental Research and Public Health 19, no. 7: 4218. https://doi.org/10.3390/ijerph19074218
APA StyleMcClelland, P. H., Kenney, C. T., Palacardo, F., Roberts, N. L. S., Luhende, N., Chua, J., Huang, J., Patel, P., Sanchez, L. A., Kim, W. J., Kwon, J., Christos, P. J., & Finkel, M. L. (2022). Improved Water and Waste Management Practices Reduce Diarrhea Risk in Children under Age Five in Rural Tanzania: A Community-Based, Cross-Sectional Analysis. International Journal of Environmental Research and Public Health, 19(7), 4218. https://doi.org/10.3390/ijerph19074218







