Abstract
The price of cancer drugs has skyrocketed, yet it is not clear whether their value is commensurate with their price. More cancer drugs are approved under expedited review, which considers less rigorous clinical evidence, yet only 20% of them show an overall survival gain in the confirmatory trial. Moreover, clinical data are often generated based on small, single-arm studies with surrogate outcomes, challenging economic evaluation. With their high price and uncertain (marginal) clinical value, cancer drugs are frequently rejected by health technology assessment (HTA) bodies. Therefore, agencies, including the UK’s National Institute for Health and Care Excellence (NICE), have adopted cancer drug funds (CDF) or risk-sharing schemes to provide extra access for expensive cancer drugs which fail to meet NICE’s cost effectiveness threshold. With rising pricing and fewer new cancer medications with novel mechanisms of action, it is unclear if newly marketed cancer therapies address unmet clinical needs or whether we are paying too much. Transparency, equity, innovativeness, and sustainability are all harmed by a “special” approach for cancer medications. If early access is allowed, confirmatory trials within a certain time frame and economic evaluation should be conducted, and label changes or disinvestment should be carried out based on those evaluations.
1. Introduction
Global spending on cancer drugs rose to USD 164 billion in 2020, with the average annual growth rate being 14.3% over the past 5 years [1]. For drugs approved by the US Food and Drug Administration (FDA) in 2018, the average price per patient per course was USD 150,384 [2] and is expected to increase even further with the introduction of more advanced treatment options (such as chimeric antigen receptor (CAR)-engineered T cell (CAR-T cell) therapy). The financial toxicity of expensive cancer drugs and concerns about affordability and sustainability have been raised in many developed and developing countries [3,4,5,6], while patients have spoken out in favor of quick access to cancer drugs [7].
Yet, it is not clear whether the clinical benefit of cancer drugs corresponds to their high price. The average incremental gain in the overall survival (OS) of the new cancer drugs approved by the FDA and European Medicine Agency (EMA) between 2003 and 2013 was 3.43 months [8]. In Europe, Australia, and the United States, the price of cancer drugs was weakly [9] or not significantly [10,11] associated with their clinical benefit [12]. However, their clinical and economic value is not rigorously examined, such as by expediting regulatory review or waiving economic evaluation.
In general, a rigorous review process of efficacy and side effects is applied to drug approval. Following the thalidomide tragedy in 1962, which was shown to cause severe congenital disorders, the United States Congress legally required evidence of efficacy before approving a drug through the Kefauver–Harris Drug Amendments to the Federal Food, Drug, and Cosmetic Act (FDCA) [13]. This thorough process extended FDA review times to more than 30 months in the early 1980s, and manufacturers and industry organizations voiced worry that it was taking too long to approve lifesaving treatments. Since then, the approval and regulatory process has been added to special programs such as the Orphan Drug Act in 1983, expanded access in 1987, Fast-Track in 1988, priority review and Accelerated Approval in 1992, and breakthrough therapy designation in 2012. As a result, the proportion of cancer drugs being approved under the expedited approval program is increasing [14,15].
However, this might allow the approval of drugs based on less rigorous evidence, such as unvalidated surrogate outcomes or a single arm [16,17,18]. The improvement in the surrogate outcomes was frequently not verified to improve overall survival in the confirmatory trials or meta-analysis [19,20]. Moreover, 40% of oncology drugs with expedited review have not completed confirmatory trials or have not demonstrated their benefits, raising concern regarding their efficacy [14]. Of the cancer drugs that have received accelerated approval thus far, 17 have been either withdrawn by the FDA (e.g., bevacizumab for HER2-negative breast cancer) or voluntarily withdrawn by the manufacturer (e.g., nivolumab and pembrolizumab for small cell lung cancer (SCLC)) after failing to prove a clinical benefit in the post-approval trial [21,22].
Since clinical evidence of cancer drugs is often generated based on small, single-arm studies with surrogate outcomes, conducting economic evaluation for those cancer drugs is challenging [23]. With their high prices and uncertain (marginal) clinical value, cancer drugs are frequently rejected by health technology assessment (HTA) bodies. As a result, countries such as the United Kingdom and Canada have adopted separate processes, such as cancer drug funds (CDF), risk-sharing schemes, and pan-Canadian Oncology Drug Review (pCODR), to provide extra access for expensive cancer drugs that failed to meet the cost effectiveness threshold [24].
Then, do the “special” paths help cancer patients’ health, encourage the development of innovative drugs, and maximize population health, besides their high price? Maybe not. If cancer drugs without rigorous clinical evidence can be approved and reimbursed despite their high price, then patients may take cancer drugs with no clear benefit or that even cause potential risks, which may hurt patients’ health. Drugs reimbursed based on special pathways, such as CDF, have a poor cost effectiveness ratio and uncertain clinical value. Therefore, the need for those separate processes has been questioned in terms of value, fairness, and transparency [25,26]. With their prices being so high, easy access also harms the sustainability of the health care budget [2].
Moreover, it signals to pharmaceutical industries that less innovative drugs (second in class) with uncertain clinical value can still bring financial success [27]. The recent trend shows that the proportion of drugs with new mechanisms of action has decreased [28], suggesting newly developed drugs may not address unmet clinical needs while their prices have soared. Yet, cancer drugs have comprised the highest revenue of pharmaceuticals over the last decade [27], with global spending expected to reach USD 269 billion by 2025 [1]. Although accessing drugs with uncertain clinical and economic value may allow patients to have early access to drugs, it also creates several problems that we should carefully consider.
2. Proposals
Is it worth it to have a separate process just for cancer? Each society may have different preferences, yet a “special process” should be cautiously adopted only after an active discussion on the pros and cons. Having early access to drugs, although the drugs have uncertain clinical value, might be the only option for some patients without any alternatives. However, it hurts not only equity, transparency, innovation, and sustainability but also potentially patients’ health since the risk is not rigorously reviewed [29].
Thus, a special process should be applied only in limited conditions (Does it address unmet clinical needs? Is it innovative?), and if expedited review is applied to certain drugs, then their clinical and economic evidence should be routinely reviewed and appropriate action should be taken. For example, confirmatory clinical trials should be conducted for all cancer drugs approved under expedited review, and an immediate change of label should be followed if the promised outcome was not validated in the confirmatory trial. The regulatory agencies should be more active and even aggressive in compelling confirmatory trials within a certain time frame and promptly change labels based on the trials. In addition, although challenging, economic evaluation of cancer drugs should be conducted as much as possible, and the value of the cancer drugs should be re-evaluated as real-world evidence emerges, thus giving the industry a signal that cancer drugs with uncertain values will be eventually evaluated as such. Having a separate fund for cancer, such as CDF, is not sustainable, lacks justification, and is not fair. Instead of waiving economic evaluation for cancer drugs, flexible application of the threshold for cancer drugs, within the national health system, should be implemented (Figure 1).
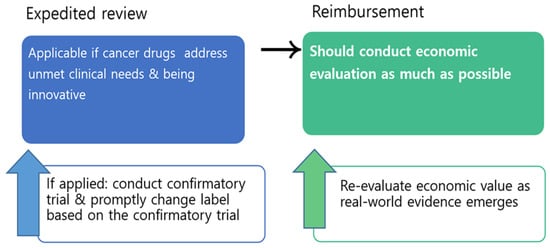
Figure 1.
Market access process proposal for cancer drugs.
Along with the aging population, the burden of cancer will escalate, and the challenges related to expensive cancer drugs will intensify. In the face of soaring cancer drug expenditure, we need to clarify boundaries and send clear signals to industries before it is too late.
Author Contributions
Conceptualization, H.-Y.K. and S.B.; investigation, G.S.; writing—original draft preparation, G.S.; writing—review and editing, S.B.; visualization, H.-Y.K., G.S. and S.B.; supervision, S.B.; project administration, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation NRF-2021R1F1A1050281.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
SeungJin Bae is currently serving the Economic Subcommittee of the Pharmaceutical Benefit Coverage Advisory Committee.
References
- IQVIA. Global Oncology Trends. 2021. Available online: https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncology-trends-2021 (accessed on 30 December 2021).
- DeMartino, P.C.; Miljković, M.D.; Prasad, V. Potential Cost Implications for All US Food and Drug Administration Oncology Drug Approvals in 2018. JAMA Intern. Med. 2021, 181, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Patel, N.; Lu, Y.-C.; Walker, R.; Younis, M. The Financial Burden of Cancer on Families in the United States. Int. J. Environ. Res. Public Health 2021, 18, 3790. [Google Scholar] [CrossRef] [PubMed]
- Vogler, S. Can we achieve affordable cancer medicine prices? Developing a pathway for change. Expert Rev. Pharm. Outcomes Res. 2021, 21, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Gandjour, A. The price of curing cancer. BMC Health Serv. Res. 2021, 21, 1328. [Google Scholar] [CrossRef]
- Moye-Holz, D.; Vogler, S. Comparison of Prices and Affordability of Cancer Medicines in 16 Countries in Europe and Latin America. Appl. Health Econ. Health Policy 2022, 20, 67–77. [Google Scholar] [CrossRef]
- Pace, J.; Ghinea, N.; Pearson, S.-A.; Kerridge, I.; Lipworth, W. Consumer perspectives of accelerated access to medicines: A qualitative study. J. Health Organ. Manag. 2021, 35, 987–1007. [Google Scholar] [CrossRef]
- Salas-Vega, S.; Iliopoulos, O.; Mossialos, E. Assessment of Overall Survival, Quality of Life, and Safety Benefits Associated with New Cancer Medicines. JAMA Oncol. 2017, 3, 382–390. [Google Scholar] [CrossRef]
- Salas-Vega, S.; Shearer, E.; Mossialos, E. Relationship between costs and clinical benefits of new cancer medicines in Australia, France, the UK, and the US. Soc. Sci. Med. 2020, 258, 113042. [Google Scholar] [CrossRef]
- Saluja, R.; Arciero, V.S.; Cheng, S.; McDonald, E.; Wong, W.W.L.; Cheung, M.C.; Chan, K.K.W. Examining Trends in Cost and Clinical Benefit of Novel Anticancer Drugs Over Time. J. Oncol. Pract. 2018, 14, e280–e294. [Google Scholar] [CrossRef]
- Vivot, A.; Jacot, J.; Zeitoun, J.D.; Ravaud, P.; Crequit, P.; Porcher, R. Clinical benefit, price and approval characteristics of FDA-approved new drugs for treating advanced solid cancer, 2000–2015. Ann. Oncol. 2017, 28, 1111–1116. [Google Scholar] [CrossRef]
- Vokinger, K.N.; Hwang, T.J.; Grischott, T.; Reichert, S.; Tibau, A.; Rosemann, T.; Kesselheim, A.S. Prices and clinical benefit of cancer drugs in the USA and Europe: A cost–benefit analysis. Lancet Oncol. 2020, 21, 664–670. [Google Scholar] [CrossRef]
- Kepplinger, E.E. FDA’s expedited approval mechanisms for new drug products. Biotechnol. Law Rep. 2015, 34, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Beaver, J.A.; Howie, L.J.; Pelosof, L.; Kim, T.; Liu, J.; Goldberg, K.B.; Sridhara, R.; Blumenthal, G.M.; Farrell, A.T.; Keegan, P.; et al. A 25-Year Experience of US Food and Drug Administration Accelerated Approval of Malignant Hematology and Oncology Drugs and Biologics: A Review. JAMA Oncol. 2018, 4, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Darrow, J.J.; Avorn, J.; Kesselheim, A.S. FDA Approval and Regulation of Pharmaceuticals, 1983–2018. JAMA 2020, 323, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Naci, H.; Gurpinar, E.; Poplavska, E.; Pinto, A.; Aggarwal, A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: Retrospective cohort study of drug approvals 2009–13. BMJ 2017, 359, j4530. [Google Scholar] [CrossRef]
- Fojo, T.; Mailankody, S.; Lo, A. Unintended Consequences of Expensive Cancer Therapeutics—The Pursuit of Marginal Indications and a Me-Too Mentality That Stifles Innovation and Creativity: The John Conley Lecture. JAMA Otolaryngol.–Head Neck Surg. 2014, 140, 1225–1236. [Google Scholar] [CrossRef]
- Kesselheim, A.S.; Wang, B.; Franklin, J.M.; Darrow, J.J. Trends in utilization of FDA expedited drug development and approval programs, 1987–2014: Cohort study. BMJ 2015, 351, h4633. [Google Scholar] [CrossRef]
- Naci, H.; Smalley, K.R.; Kesselheim, A.S. Characteristics of Preapproval and Postapproval Studies for Drugs Granted Accelerated Approval by the US Food and Drug Administration. JAMA 2017, 318, 626–636. [Google Scholar] [CrossRef]
- Gyawali, B.; Hey, S.P.; Kesselheim, A.S. Assessment of the Clinical Benefit of Cancer Drugs Receiving Accelerated Approval. JAMA Intern. Med. 2019, 179, 906–913. [Google Scholar] [CrossRef]
- FDA. Withdrawn | Cancer Accelerated Approvals. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/withdrawn-cancer-accelerated-approvals (accessed on 21 March 2022).
- Gyawali, B.; Rome, B.N.; Kesselheim, A.S. Regulatory and clinical consequences of negative confirmatory trials of accelerated approval cancer drugs: Retrospective observational study. BMJ 2021, 374, n1959. [Google Scholar] [CrossRef]
- Dawoud, D.; Naci, H.; Ciani, O.; Bujkiewicz, S. Raising the bar for using surrogate endpoints in drug regulation and health technology assessment. BMJ 2021, 374, n2191. [Google Scholar] [CrossRef] [PubMed]
- Morrell, L.; Wordsworth, S.; Schuh, A.; Middleton, M.R.; Rees, S.; Barker, R.W. Will the reformed Cancer Drugs Fund address the most common types of uncertainty? An analysis of NICE cancer drug appraisals. BMC Health Serv. Res. 2018, 18, 393. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Fojo, T.; Chamberlain, C.; Davis, C.; Sullivan, R. Do patient access schemes for high-cost cancer drugs deliver value to society?—lessons from the NHS Cancer Drugs Fund. Ann. Oncol. 2017, 28, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.; Chamberlain, C.; Hollingworth, W. Did It Matter That the Cancer Drugs Fund Was Not NICE? A Retrospective Review. Value Health 2016, 19, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Light, D.W.; Lexchin, J. Why do cancer drugs get such an easy ride? BMJ 2015, 350, h2068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olivier, T.; Haslam, A.; Prasad, V. Anticancer Drugs Approved by the US Food and Drug Administration From 2009 to 2020 According to Their Mechanism of Action. JAMA Netw. Open 2021, 4, e2138793. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Mailankody, S. The UK Cancer Drugs Fund Experiment and the US Cancer Drug Cost Problem: Bearing the Cost of Cancer Drugs Until It Is Unbearable. Mayo Clin. Proc. 2016, 91, 707–712. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).