Value-Based Pricing and Budget Impact Analysis for Multi-Indication Drugs: A Case Study of Immunotherapies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of the Multi-Indication Immunotherapies
2.2. Cost-Effectiveness Analysis and Scenarios for VBP
2.3. Budget Impact Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aitken, M.; Kleinrock, M.; Nass, D.; Simorellis, A. Global Oncology Trends 2019: Therapeutics, Clinical Development and Health System Implications; The IQVIA Institute for Human Data Science: Parsippany, NJ, USA, 2019. [Google Scholar]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.; Towse, A.; Zamora, B. Indication-Based Pricing (IBP) Consultation Report; Office of Health Economics: London, UK, 2020. [Google Scholar]
- Kudrin, A. Reimbursement challenges with cancer immunotherapeutics. Hum. Vaccines Immunother. 2012, 8, 1326–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, S.Y.; Kang, D.W.; Hwang, I.; Park, M.H. Analysis of the Expansion of the Reimbursement Coverage and Value-based Pricing for Anticancer Drugs in South Korea. Yakhak Hoeji 2021, 65, 150–157. [Google Scholar] [CrossRef]
- Bach, P.B. Indication-specific pricing for cancer drugs. JAMA 2014, 312, 1629–1630. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.; Towse, A.; Zamora, B. Indication-Based Pricing (IBP) Discussion Paper; Office of Health Economics: London, UK, 2019. [Google Scholar]
- Levaggi, R.; Pertile, P. Value-Based Pricing Alternatives for Personalised Drugs: Implications of Asymmetric Information and Competition. Appl. Health Econ. Health Policy 2020, 18, 357–362. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, H.; McCullagh, L.; Barry, M.; Walsh, C. The Interaction between Price Negotiations and Heterogeneity: Implications for Economic Evaluations. Med. Decis. Mak. 2020, 40, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Toumi, M. Value Added Medicines: Rethink, Reinvent & Optimize Medicines, Improving Patient Health & Access; Medicines for Europe: Brussels, Belgium, 2016. [Google Scholar]
- Wenzl, M.; Paris, V. Pharmaceutical Reimbursement and Prcing in Germany; OECD: Paris, France, 2018. [Google Scholar]
- Yeung, K.; Li, M.; Carlson, J.J. Using Performance-Based Risk-Sharing Arrangements to Address Uncertainty in Indication-Based Pricing. J. Manag. Care Spec. Pharm. 2017, 23, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 7 September 2020).
- Health Insurance Review & Assessment Service. Pharmaceutical Information. Available online: http://www.hira.or.kr/eng/main.do (accessed on 7 September 2020).
- Guyot, P.; Ades, A.E.; Ouwens, M.J.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Royston, P. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata J. 2017, 17, 786–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heslin, M.; Babalola, O.; Ibrahim, F.; Stringer, D.; Scott, D.; Patel, A. A Comparison of Different Approaches for Costing Medication Use in an Economic Evaluation. Value Health J. Int. Soc. Pharm. Outcomes Res. 2018, 21, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Registry, K.C.C. Annual Report of Cancer Statistics in Korea in 2017; National Cancer Center: Ilsan, South Korea, 2019.
- Jung, H.I.; Kang, D.W.; Ha, S.Y.; Lee, E.K.; Park, M.H. Value-Based Pricing for multi-indication drugs using Cost-effectiveness Analysis. In Proceedings of the 2021 Fall International Convention of The Pharmaceutical Society of Korea 2021, Jeonju, South Korea, 20–22 December 2021; p. 852. [Google Scholar]
- Mestre-Ferrandiz, J.; Zozaya, N.; Alcalá, B.; Hidalgo-Vega, Á. Multi-Indication Pricing: Nice in Theory but Can it Work in Practice? PharmacoEconomics 2018, 36, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Flume, M.; Bardou, M.; Capri, S.; Sola-Morales, O.; Cunningham, D.; Levin, L.A.; Touchot, N. Feasibility and attractiveness of indication value-based pricing in key EU countries. J. Mark. Access Health Policy 2016, 4, 30970. [Google Scholar] [CrossRef] [PubMed]
| Indication | Treatment | QALY | Cost (USD) | ICER (USD/QALY) | ||
|---|---|---|---|---|---|---|
| Expected Value | Difference | Expected Value | Difference | |||
| Atezolizumab | ||||||
| Second-line NSCLC (reimbursed indication) | Intervention | 1.23 | 0.29 | a | c | d |
| Comparator | 0.84 | b | ||||
| First-line metastatic NSCLC | Intervention | 1.36 | 0.29 | 2.3a | 2.9c | 2.8d |
| Comparator | 1.07 | 2b | ||||
| First-line locally advanced or metastatic TNBC | Intervention | 1.39 | 0.46 | 2a | 3.3c | 2.0d |
| Comparator | 0.93 | 1.5b | ||||
| Nivolumab | ||||||
| Second-line NSCLC (reimbursed indication) | Intervention | 1.09 | 0.37 | e | g | h |
| Comparator | 0.72 | f | ||||
| Second-line advanced RCC | Intervention | 2.40 | 0.44 | 1.8e | 0.8g | 0.7h |
| Comparator | 1.96 | 3.6f | ||||
| Second-line recurrent or metastatic HNSCC | Intervention | 0.93 | 0.45 | 0.5e | 0.2g | 0.2h |
| Comparator | 0.48 | 0.9f | ||||
| Pembrolizumab | ||||||
| Second-line NSCLC (reimbursed indication) | Intervention | 1.60 | 0.80 | i | k | l |
| Comparator | 0.80 | j | ||||
| First-line treatment of metastatic non-squamous NSCLC | Intervention | 1.59 | 0.42 | 1.0i | 0.8k | 1.4l |
| Comparator | 1.17 | 2.3j | ||||
| First-line treatment of metastatic squamous NSCLC | Intervention | 1.45 | 0.51 | 0.8i | 0.8k | 1.3l |
| Comparator | 0.94 | 0.9j | ||||
| First-line metastatic NSCLC (≥50% TPS) | Intervention | 2.36 | 1.09 | 1.5i | 1.7k | 1.2l |
| Comparator | 1.28 | 0.6j | ||||
| First-line treatment of metastatic or unresectable recurrent HNSCC as monotherapy | Intervention | 1.38 | 0.53 | 0.6i | 0.3k | 0.5l |
| Comparator | 0.84 | 2.1j | ||||
| First-line treatment of metastatic or unresectable recurrent HNSCC as combination therapy | Intervention | 1.19 | 0.36 | 0.5i | 0.3k | 0.7l |
| Comparator | 0.84 | 2.1j | ||||
| Second-line recurrent or metastatic HNSCC (≥50% TPS) | Intervention | 1.07 | 0.53 | 0.5i | 0.4k | 0.6l |
| Comparator | 0.54 | 1.1j | ||||
| First-line treatment of advanced renal cell carcinoma | Intervention | 3.29 | 0.32 | 1.8i | 1.0k | 2.4l |
| Comparator | 2.97 | 7.1j | ||||
| Indication | Current List Price (USD) | Value-Based Pricing | 4.75% Price Reduction, USD (%) | |||
|---|---|---|---|---|---|---|
| Scenario 1: IBP *, USD (%) | Scenario 2: IBP with Refund | Scenario 3: WAP, USD (%) | ||||
| Price (USD) | Refund Rate (%) | |||||
| Atezolizumab | ||||||
| Second-line NSCLC (reimbursed indication) | 2049 (reference) | 2049 (100.00%) | 2049 | 0.00% | 1398 (68.24%) | 1952 (95.25%) |
| First-line metastatic NSCLC | 585 (28.56%) | 71.44% | ||||
| First-line locally advanced or metastatic TNBC | 849 (41.42%) | 58.58% | ||||
| Nivolumab | ||||||
| Second-line NSCLC (reimbursed indication) | 1172 (reference) | 1172 (100.00%) | 1172 | 0.00% | 1450 (123.76%) | 1116 (95.25%) |
| Second-line advanced RCC | 1643 (140.24%) | 0.00% | ||||
| Second-line recurrent or metastatic HNSCC | 3853 (328.81%) | 0.00% | ||||
| Pembrolizumab | ||||||
| Second-line NSCLC (reimbursed indication) | 2527 (reference) | 2527 (100.00%) | 2527 | 0.00% | 2441 (96.61%) | 2407 (95.25%) |
| First-line treatment of metastatic non-squamous NSCLC | 1861 (73.63%) | 26.37% | ||||
| First-line treatment of metastatic squamous NSCLC | 1896 (75.03%) | 24.97% | ||||
| First-line metastatic NSCLC (≥50% TPS) | 2055 (81.31%) | 18.69% | ||||
| First-line treatment of metastatic or unresectable recurrent HNSCC as monotherapy | 4282 (169.45%) | 0.00% | ||||
| First-line treatment of metastatic or unresectable recurrent HNSCC as combination therapy | 3299 (130.53%) | 0.00% | ||||
| Second-line recurrent or metastatic HNSCC (≥50% TPS) | 4028 (159.38%) | 0.00% | ||||
| First-line treatment of advanced renal cell carcinoma | 1505 (59.55%) | 40.45% | ||||
| Value-Based Pricing | 4.75% Price Reduction | |||
|---|---|---|---|---|
| Scenario 1: IBP | Scenario 2: IBP with Refund | Scenario 3: WAP | ||
| Atezolizumab | ||||
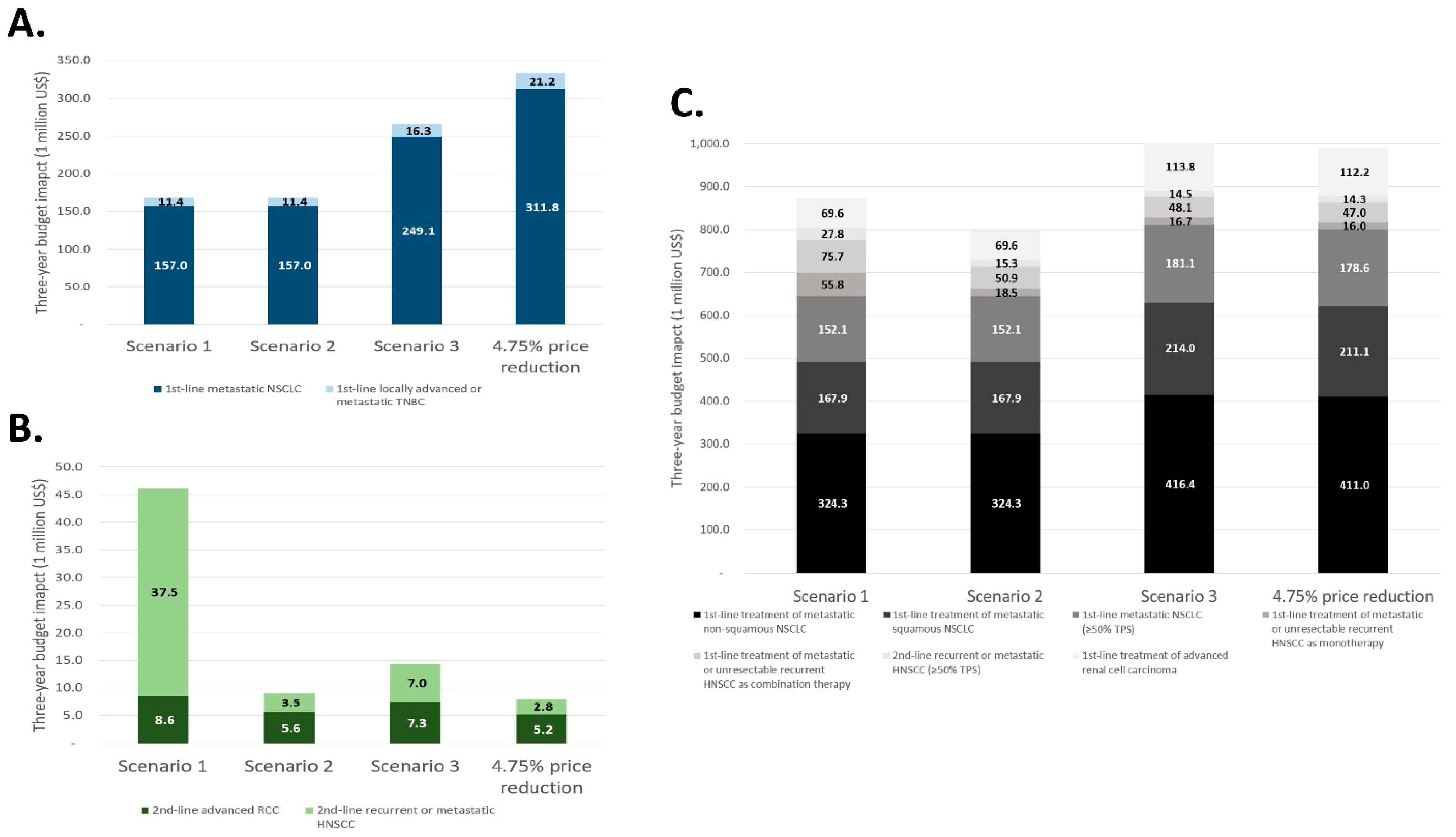
| Budget impact, USD | 168,448,872 | 168,448,872 | 265,447,233 | 333,057,487 |
| Increased rate | −49.42% | −49.42% | −20.30% | - |
| Nivolumab | ||||
| Budget impact, USD | 46,082,608 | 9,069,671 | 14,384,780 | 8,007,269 |
| Increased rate | +475.51% | +13.27% | +79.65% | - |
| Pembrolizumab | ||||
| Budget impact, USD | 873,195,849 | 798,495,489 | 1,004,647,710 | 989,960,348 |
| Increased rate | −11.79% | −19.34% | +1.48% | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, S.-Y.; Kang, D.-W.; Jung, H.-I.; Lee, E.-K.; Park, M.-H. Value-Based Pricing and Budget Impact Analysis for Multi-Indication Drugs: A Case Study of Immunotherapies. Int. J. Environ. Res. Public Health 2022, 19, 4105. https://doi.org/10.3390/ijerph19074105
Ha S-Y, Kang D-W, Jung H-I, Lee E-K, Park M-H. Value-Based Pricing and Budget Impact Analysis for Multi-Indication Drugs: A Case Study of Immunotherapies. International Journal of Environmental Research and Public Health. 2022; 19(7):4105. https://doi.org/10.3390/ijerph19074105
Chicago/Turabian StyleHa, So-Young, Dong-Won Kang, Hye-In Jung, Eui-Kyung Lee, and Mi-Hai Park. 2022. "Value-Based Pricing and Budget Impact Analysis for Multi-Indication Drugs: A Case Study of Immunotherapies" International Journal of Environmental Research and Public Health 19, no. 7: 4105. https://doi.org/10.3390/ijerph19074105
APA StyleHa, S.-Y., Kang, D.-W., Jung, H.-I., Lee, E.-K., & Park, M.-H. (2022). Value-Based Pricing and Budget Impact Analysis for Multi-Indication Drugs: A Case Study of Immunotherapies. International Journal of Environmental Research and Public Health, 19(7), 4105. https://doi.org/10.3390/ijerph19074105







