Incidence of Peri-Implantitis and Relationship with Different Conditions: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Oral Evaluation
- Probing depth (PD) (in mm): to assess the depth of probing for each implant with a North Carolina periodontal probe applying a pressure of 0.15 Ncm [11].
- Modified Sulcular Bleeding Index (mBI): to assess bleeding or non-bleeding of each implant, registered from 0 to 3, according to the criteria of Mombelli et al. (BOP) [12].
- Modified plaque index (mPI): measure the amount of bacterial plaque on implants registered according to the O’Leary index, which represents the percentage of dental surfaces affected by bacterial plaque [13].
- Mucosal recession (REC): quantify the recession in each implant defined as the distance (mm) from the implant platform as a stable mark and mucosal margin [13].
- Suppuration (SUP): register the suppuration or not around implants according to the dichotomous scale (0/1), using the University of North Carolina probe 15, applying a force of 0.15 Ncm [11].
2.3. General Data
- Sex: male, female.
- Smoking habits: No, Yes < 10/day, Yes > 10/day
- Alcohol habits: No, moderate consumers (<0.5 L/day), heavy consumers (>0.5 L/day).
- Systemic diseases: arterial hypertension (AHT), diabetes mellitus (DM), osteoporosis, cardiovascular diseases.
- Periodontal disease: Non-, mild (level of clinical attachment—CAL 1–2 mm), moderate (CAL 3–4 mm), Advanced (>5 mm) [12].
- Maintenance: No, every 4 months, every 6 months, every 12 months.
- Reasons for tooth loss.
- Date of implant placement.
2.4. Implants Data
- Diameter and length of each implant.
- Type of prosthetic connection: 1: bone level, 2: tissue level, 3: external connection [14].
2.5. Diagnosis of Peri-implantitis
- Evidence of visual inflammatory changes in the soft tissues of the peri-implant combined with bleeding on probing and suppuration [15].
- Increasing the depth of the probing pocket compared to the measurements obtained at the placement of the prothesis.
- Progressive bone loss in relation to the radiographic evaluation of the bone level assessment one year after implant prosthetic rehabilitation placement.
2.6. Radiographic Assessment
2.7. Satisfaction Questionnaire
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esposito, M.; Grusovin, M.G.; Willings, M.; Coulthard, P.; Worthington, H.V. The effectiveness of immediate, early, and conventional loading of dental implants: A Cochrane systematic review of randomized controlled clinical trials. Int. J. Oral Maxillofac. Implants 2007, 22, 893–904. [Google Scholar] [PubMed]
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 197–212. [Google Scholar] [CrossRef] [PubMed]
- Hansson, H.A.; Albrektsson, T.; Brånemark, P.I. Structural aspects of the interface between tissue and titanium implants. J. Prosthet. Dent. 1983, 50, 108–113. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), S267–S290. [Google Scholar] [CrossRef] [PubMed]
- Fransson, C.; Lekholm, U.; Jemt, T.; Berglundh, T. Prevalence of subjects with progressive bone loss at implants. Clin. Oral Implants Res. 2005, 16, 440–446. [Google Scholar] [CrossRef]
- Roos-Jansaker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine- to fourteen-year follow-up of implant treatment. Part II: Presence of peri-implant lesions. J. Clin. Periodontol. 2006, 33, 290–295. [Google Scholar] [CrossRef]
- Rodrigo, D.; Sánz-Sánchez, I.; Figuero, E.; Llodrá, J.C.; Bravo, M.; Caffesse, R.G.; Vallcorba, N.; Guerrero, A.; Herrera, D. Prevalence and Risk Indicators of Peri-implant Diseases in Spain. J. Clin. Periodontol. 2018, 45, 1510–1520. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. S1), S304–S312. [Google Scholar] [CrossRef]
- Astolfi, V.; Gómez-Menchero, A.; Ríos-Santos, J.V.; Bullón, P.; Galeote, F.; Ríos-Carrasco, B.; Bullón de la Fuente, B.; Herrero-Climent, M. Influence of Removing or Leaving the Prosthesis after Regenerative Surgery in Peri-Implant Defects: Retrospective Study: 32 Clinical Cases with 2 to 8 Years of Follow-Up. Int. J. Environ. Res. Public Health 2021, 18, 645. [Google Scholar] [CrossRef]
- The World Medical Association. Wma Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Subjects. In Proceedings of the 64th WMA General Assembly, Fortaleza, Brazil, October 2013; Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 26 January 2022).
- Monje, A.; Pons, R.; Roccuzzo, A.; Salvi, G.E.; Nart, J. Reconstructive therapy for the management of peri-implantitis via submerged guided bone regeneration: A prospective case series. Clin. Implant Dent. Relat. Res. 2020, 22, 342–350. [Google Scholar] [CrossRef]
- Mombelli, A.; Van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef] [PubMed]
- InternationalWorkshop for a Classification of Periodontal Diseases and Conditions. Papers. Oak Brook. Ilinnois. 30 October–2 November 1999. Ann. Periodontol. 1999, 4, 1–112. [Google Scholar]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1–review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar] [PubMed]
- Mombelli, A.; Lang, N.P. The diagnosis and treatment of periimplantitis. Periodontol. 2000 1998, 17, 63–76. [Google Scholar] [CrossRef]
- Karoussis, I.K.; Salvi, G.E.; Heitz-Mayfield, L.J.; Bragger, U.; Hammerle, C.H.; Lang, N.P. Long term implant prognosis in patients with and without a history of chronic periodontitis: A 10-year prospective cohort study of the ITI dental implant system. Clin. Oral Implant. Res. 2003, 14, 329–339. [Google Scholar] [CrossRef]
- Koldsland, O.C.; Scheie, A.A.; Aass, A.M. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone oss. J. Periodontol. 2010, 81, 231–238. [Google Scholar] [CrossRef]
- Casado, P.L.; Pereira, M.C.; Duarte, M.E.; Granjeiro, J.M. History of chronic periodontitis is a high risk indicator for peri-implant disease. Braz. Dent. J. 2013, 24, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Dalago, H.R.; Schuldt, F.G.; Rodrigues, M.A.; Renvert, S.; Bianchini, M.A. Risk indicators for periimplantitis. A cross-sectional study with 916 implants. Clin. Oral Implant. Res. 2017, 28, 144–150. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Fauri, M.; Avila-Ortiz, G.; Fernández-Barbero, J.E.; Cabrera-León, A.; Sánchez-Fernández, E. Influence of alcohol and tobacco habits on peri-implant marginal bone loss: A prospective study. Clin. Oral Implant. Res. 2005, 16, 579–586. [Google Scholar] [CrossRef]
- Wu, X.; Al-Abedalla, K.; Eimar, H.; Madathil, S.A.; Abi-Nader, S.; Daniel, N.G.; Nicolau, B.; Tamimi, F. Antihypertensive medications and the survival rate of osseointegrated dental implants: A cohort study. Clin. Implant. Dent. Relat. Res. 2016, 18, 1171–1182. [Google Scholar] [CrossRef]
- Rokn, A.; Aslroosta, H.; Akbari, S.; Najafi, H.; Zayeri, F.; Hashemi, K. Prevalence of periimplantitis in patients not participating in well-designed supportive periodontal treatments: A cross-Sectional study. Clin. Oral Implant. Res. 2017, 28, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Papi, P.; Letizia, C.; Pilloni, A.; Petramala, L.; Saracino, V.; Rosella, D.; Pompa, G. Peri-implant diseases and metabolic syndrome components: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 866–875. [Google Scholar] [PubMed]
- Monje, A.; Catena, A.; Borgnakke, W.S. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: Systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, H. The role of systemic diseases and local conditions as risk factors. Periodontol. 2000 2022, 88, 36–51. [Google Scholar] [CrossRef]
- Renvert, S.; Aghazadeh, A.; Hallström, H.; Persson, G.R. Factors related to peri-implantitis–A retrospective study. Clin. Oral Implant. Res. 2014, 25, 522–529. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, Y.K.; Park, J.Y.; Kim, S.G.; Kim, M.J.; Yun, P.Y. Short–term clinical retrospective study of implants in geriatric patients older than 70 years. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 442–446. [Google Scholar] [CrossRef]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Roccuzzo, M.; Bonino, F.; Aglietta, M.; Dalmasso, P. Ten-year results of a three arms prospective cohort study on implants in periodontally compromised patients. Part 2: Clinical results. Clin. Oral Implant. Res. 2012, 23, 389–395. [Google Scholar] [CrossRef]
- Schwarz, F.; Becker, K.; Sahm, N.; Horstkemper, T.; Rousi, K.; Becker, J. The prevalence of peri-implant diseases for two-piece implants with an internal tubein- tube connection: A cross-sectional analysis of 512 implants. Clin. Oral Implant. Res. 2017, 28, 24–28. [Google Scholar] [CrossRef]
- Marrone, A.; Lasserre, J.; Bercy, P.; Brecx, M.C. Prevalence and risk factors for periimplant disease in belgian adults. Clin. Oral Implant. Res. 2013, 24, 934–940. [Google Scholar] [CrossRef]
- Costa, F.O.; Takenaka-Martinez, S.; Cota, L.O.; Ferreira, S.D.; Silva, G.L.; Costa, J.E. Periimplant disease in subjects with and without preventive maintenance: A 5-year follow-up. J. Clin. Periodontol. 2012, 39, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Roos-Jansaker, A.M.; Renvert, H.; Lindahl, C.; Renvert, S. Nine- to fourteen-year follow-up of implant treatment. Part III: Factors associated with periimplant lesions. J. Clin. Periodontol. 2006, 33, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, G.; Arnhart, C.; Heuberer, S.; Huber, C.D.; Watzek, G.; Gruber, R. Periimplantitis and late implant failures in postmenopausal women: A cross sectional study. J. Clin. Periodontol. 2011, 38, 950–955. [Google Scholar] [CrossRef] [PubMed]


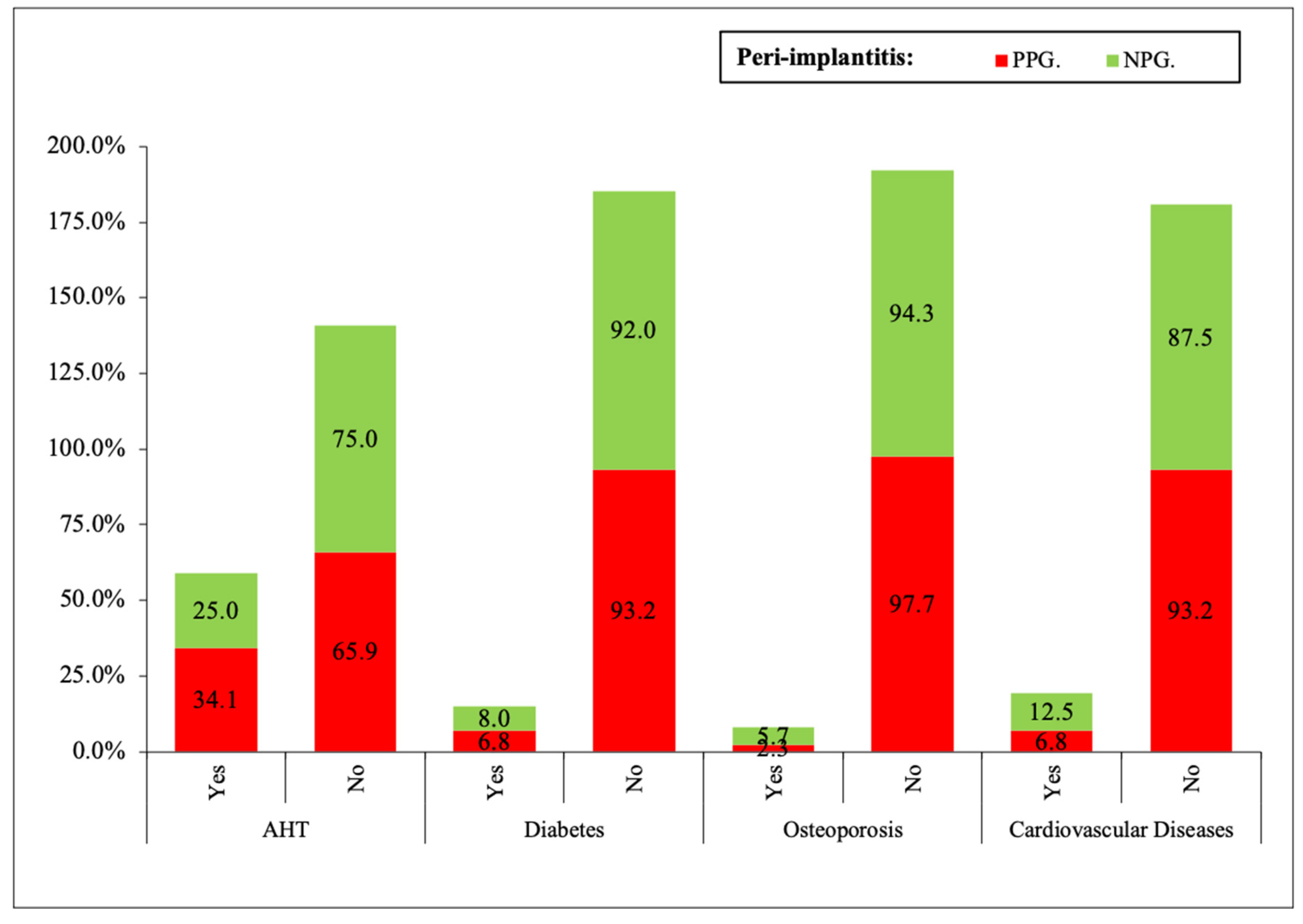


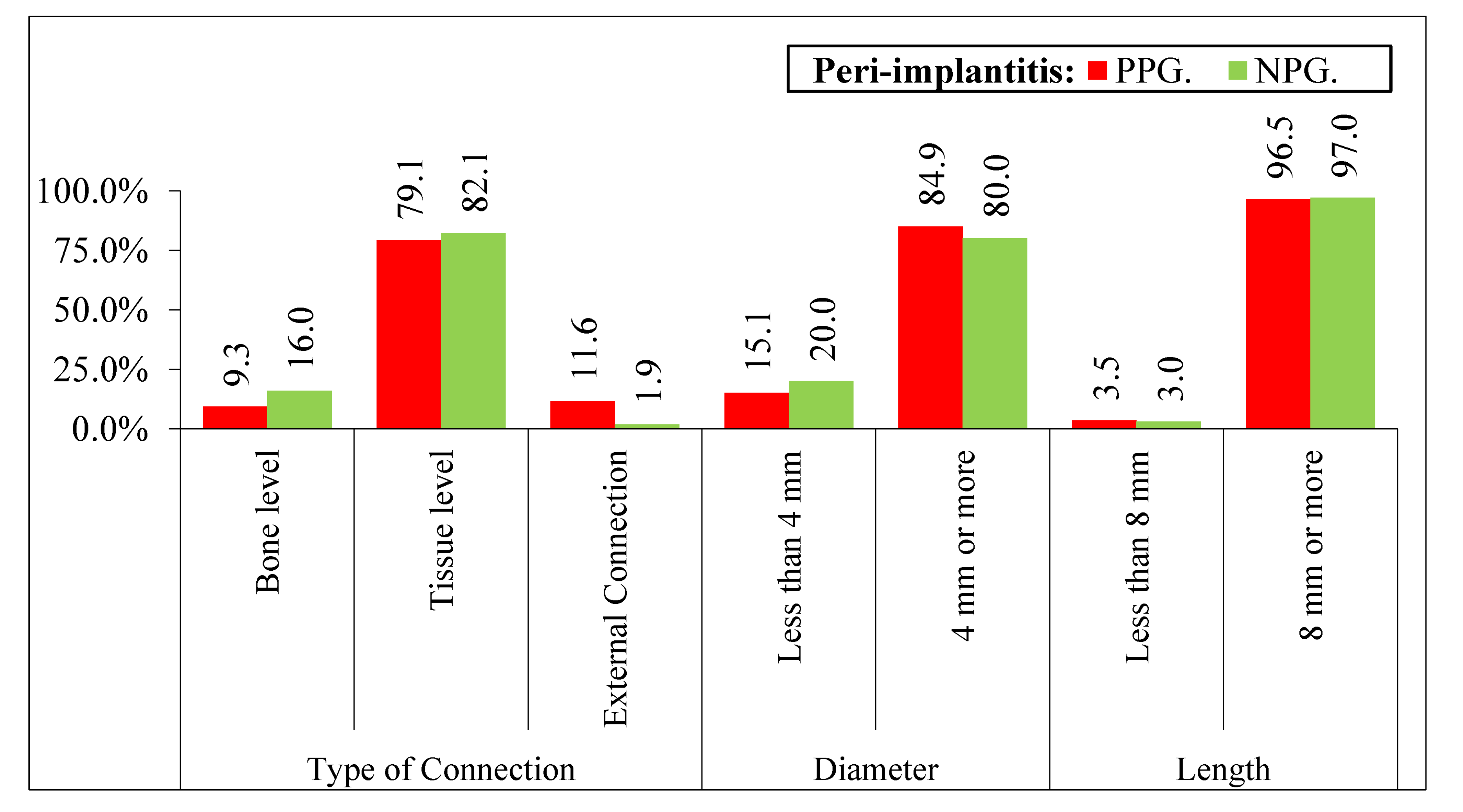
| Variable | Categories | Yes | No | Signification |
|---|---|---|---|---|
| Mastication | Good | 67.4 | 75.9 | <0.05 |
| Acceptable | 12.8 | 13.9 | ||
| Bad | 19.8 * 1 | 10.2 * 1 | ||
| Phonetic | Good | 72.1 | 67.6 | <0.05 |
| Acceptable | 17.4 * 1 | 27.7 * 1 | ||
| Bad | 10.5 * 1 | 4.7 * 1 | ||
| Aesthetic | Good | 40.7 * 4 | 64.8 * 4 | <0.001 |
| Acceptable | 44.2 * 3 | 26.9 * 3 | ||
| Bad | 15.1 * 2 | 8.3 * 2 | ||
| Hygiene | Good | 58.1 * 2 | 72.9 * 2 | <0.0001 |
| Acceptable | 19.8 | 20.9 | ||
| Bad | 22.1 * 5 | 6.2 * 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astolfi, V.; Ríos-Carrasco, B.; Gil-Mur, F.J.; Ríos-Santos, J.V.; Bullón, B.; Herrero-Climent, M.; Bullón, P. Incidence of Peri-Implantitis and Relationship with Different Conditions: A Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 4147. https://doi.org/10.3390/ijerph19074147
Astolfi V, Ríos-Carrasco B, Gil-Mur FJ, Ríos-Santos JV, Bullón B, Herrero-Climent M, Bullón P. Incidence of Peri-Implantitis and Relationship with Different Conditions: A Retrospective Study. International Journal of Environmental Research and Public Health. 2022; 19(7):4147. https://doi.org/10.3390/ijerph19074147
Chicago/Turabian StyleAstolfi, Víctor, Blanca Ríos-Carrasco, Francisco Javier Gil-Mur, José Vicente Ríos-Santos, Beatriz Bullón, Mariano Herrero-Climent, and Pedro Bullón. 2022. "Incidence of Peri-Implantitis and Relationship with Different Conditions: A Retrospective Study" International Journal of Environmental Research and Public Health 19, no. 7: 4147. https://doi.org/10.3390/ijerph19074147
APA StyleAstolfi, V., Ríos-Carrasco, B., Gil-Mur, F. J., Ríos-Santos, J. V., Bullón, B., Herrero-Climent, M., & Bullón, P. (2022). Incidence of Peri-Implantitis and Relationship with Different Conditions: A Retrospective Study. International Journal of Environmental Research and Public Health, 19(7), 4147. https://doi.org/10.3390/ijerph19074147










