Abstract
Carcinogenic effects of tobacco smoke may affect breast tumorigenesis. To assess whether cigarette smoking is associated with breast cancer characteristics, we investigated the relationships between smoking, pathological characteristics, and outcomes in 2153 women diagnosed with breast cancer 2001–2016. Patients were classified as never, former, or current smokers at the time of diagnosis. Logistic regression and multivariable Cox proportional hazards analysis were performed to determine whether smoking was associated with tumor characteristics. Multivariable Cox proportional hazards analysis was conducted to compare former or current smokers to never smokers in survival with adjustment for the potential confounders. The majority of women (61.8%) never smoked, followed by former smokers (26.2%) and current smokers (12.0%). After adjustment for demographic variables, body mass index, and comorbidities, tumor characteristics were not significantly associated with smoking status or pack-years smoked. Ten-year overall survival was significantly lower for former and current smokers compared to never smokers (p = 0.0105). However, breast cancer specific survival did not differ significantly between groups (p = 0.1606). Although cigarette smoking did not alter the underlying biology of breast tumors or breast cancer-specific survival, overall survival was significantly worse in smokers, highlighting the importance of smoking cessation in the recently diagnosed breast cancer patient.
1. Introduction
Cigarette smoking is the leading cause of preventable morbidity and mortality in the US, with approximately 480,000 deaths in the US each year attributable to cigarette smoking (Health Effects of Cigarette Smoking|CDC; accessed on 29 September 2021). Cigarette smoking has been associated with a number of health conditions, including cardiovascular and pulmonary disease, cancer, adverse reproductive outcomes, and other chronic health conditions [1]. Smoking-related illnesses are estimated to cost the United States $225 billion in direct medical costs and $156 billion in lost productivity each year (Economic Trends in Tobacco|Smoking and Tobacco Use|CDC, accessed on 29 September 2021). In 2019, there were an estimated 34.1 million cigarette smokers in the United States (US), with 15.3% of adult males and 12.7% of adult females being current smokers [2].
While a number of studies suggest that smoking is a moderate risk factor for breast cancer, whether smoking influences tumor characteristics or survival is less clear. Identification of tumor phenotypes enriched in smokers may evince the underlying effects on tumor development. For example, the enrichment of TP53 mutations in breast tumors from smokers [3] may promote the basal-like subtype, which is characterized by high rates (80%) of TP53 mutations [4], while cigarette smoking has been reported to have anti-estrogenic effects [5], which may reduce the risk of estrogen receptor (ER) positive tumors. In conjunction, nicotine, which promotes the proliferation of human breast cancer cell lines [6], may result in larger tumor sizes in smokers compared to non-smokers. Thus, in this study, we investigated whether smoking status was associated with tumor characteristics and survival among female breast cancer patients.
2. Materials and Methods
Patients were enrolled in the Clinical Breast Care Project (CBCP) from breast clinics at the Murtha Cancer Center/Walter Reed National Military Medical Center (MCC/WRNMMC), Bethesda, MD, Anne Arundel Medical Center (AAMC), Annapolis, MD or Joyce Murtha Cancer Center (JMBCC), Windber, PA. Enrollment criteria included being an adult over the age of 18 years, mentally competent and willing to provide informed consent. Patients were provided with consent forms that included permission to gather demographic, pathological, and survival data and that described the primary research uses of the samples. All patients included in this study were diagnosed with invasive breast cancer between 2001 and 2016.
All data were extracted from the CBCP database. Smoking status was based on patient-reported past and current cigarette smoking. Smokers were defined as those who had smoked at least 100 cigarettes in their lifetime before breast cancer diagnosis. Former smokers were those who quit smoking ≥1 year prior to diagnosis. Pack-years were calculated as the product of the number of years smoked and number of packs smoked per day. Comorbidities were included in the analyses using the Charlson comorbidity index (CCI) calculated before breast cancer diagnosis. Additional demographic data included age at diagnosis, self-described race/ethnicity, body mass index (BMI), education levels, marital status, and site of patient enrollment. Pathological data included histological type, anatomic tumor stage, size, and lymph node status [7], and grade [8,9]. Biomarkers included in the analyses included estrogen receptor (ER), progesterone receptor (PR), and HER2, with positivity assigned according to ASCO/CAP guidelines [10,11]. Information on vital status and date at death was collected through annual review of electronic health records.
We first described the distributions of demographic characteristics, site of enrollment, BMI, and CCI between never smokers, former smokers, and current smokers using Chi-square test between never, former, and current smokers. We then compared the groups by smoking status in pathologic variables (type, stage, size, grade, ER status, PR status, HER2 status, and node status of tumor). Subsequently, we compared 25–49 or ≥50 pack-years to pack-years <25 in pathologic features. In these analyses, multivariable logistic regression analysis was conducted for each of the pathologic variables controlling for demographic variables, site of enrollment, BMI, and CCI. Patients with unknown demographic or pathological data were excluded. In survival analysis, we used Kaplan–Meier curve to compare the groups divided by smoking status in 10-year overall or breast cancer-specific survival. If a patient died during the 10-year period after diagnosis, the follow up time was calculated as the time from diagnosis to death. If death was not observed during the period, follow-up time was censored at the end of the tenth year. Patients who were not dead through the end of the study without a full ten-year follow-up time were censored on the study ending 31 December 2020. We then used multivariable Cox proportional hazards model to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for former and current smokers compared to never-smokers. In the Cox models, we adjusted for age, race, marital status, education, tumor type, tumor stage, tumor size, tumor grade, node status, tumor hormone receptor statuses (HER2, ER and PR), BMI, CCI, and site of enrollment. The same approach was used for the comparison of survival between the groups defined by pack-year.
3. Results
3.1. Study Characteristics
Between 2001 and 2016, 2153 women with invasive breast cancer enrolled in the CBCP had complete smoking information, 34 women were excluded for not having sufficient data to determine smoking status at the time of diagnosis. The majority of women (n = 1330, 61.8%) never smoked, followed by former smokers (n = 564, 26.2%) and current smokers (n = 259, 12.0%). Age at diagnosis, race/ethnicity, education levels, CCI and site of enrollment differed significantly by smoking status (Table 1). The average ages at diagnosis were 57.4 years, 61.2 years, and 55.5 years in never, former, and current smokers, respectively. Former smokers were less likely to be Non-Hispanic Black (NHB) or Asian or to have a college education and were more likely to have comorbidities than never smokers. Current smokers were less likely to be NHB, to have a college education or be married than never smokers.

Table 1.
Demographic characteristics of 2153 women with breast cancer enrolled in the CBCP 2001–2016. p-values reflect differences between never, former, and current smokers.
3.2. Tumor Pathology
Tumor stage, size, and lymph node status differed significantly by smoking status, with the former smokers being more likely to have stage I, tumor size T1, and negative lymph nodes (Table 2). After multivariable analyses, adjusting for age at diagnosis, race, marital status, education levels, site of enrollment, BMI and CCI only lymph node status was associated with smoking status, with former smokers having lower risk (OR 0.76, 95% CI, 0.60–0.97) compared to never smokers (Table 3). Evaluation of the data by pack-year also found no significant differences in tumor characteristics between women who smoked 25–49 years or ≥50 years and those who smoked <25 years (data not shown).

Table 2.
Clinicopathological characteristics for 2153 women with breast cancer enrolled in the CBCP 2001–2016.

Table 3.
Logistic regression model adjusted for age, race, marital status, education levels, site of enrollment, BMI, and CCI for women diagnosed with invasive breast cancer 2001–2016.
3.3. Survival
BCSS did not differ significantly by smoking status (Figure 1) after adjustment for age, race, marital status, education, tumor stage, tumor characteristics (grade, type, size), node status, tumor hormone receptor statuses (HER2, ER, and PR), BMI, CCI, and site of enrollment, the HRs were 1.40 (95% CI, 1.04–1.88) and 1.59 (95% CI, 1.06–2.37) for former and current smokers for overall survival, and 1.44 (95% CI, 0.92–2.24) and 1.37 (95% CI, 0.79–2.40) for former and current smokers for BCSS, compared with non-smokers. Further analysis shows no significant differences in either overall survival or BCSS by pack-year: HRs were 0.97 (95% CI, 0.59–1.60) and 1.18 (95% CI, 0.67–2.08) for women with 25–49 and ≥50 pack-years for overall survival, compared with women who smoked <25 pack-years. The corresponding HRs were 0.89 (95% CI, 0.42–1.90) and 1.03 (95% CI, 0.40–2.67) for BCSS.
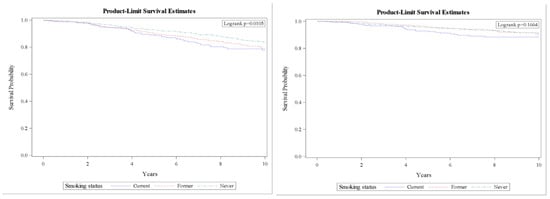
Figure 1.
10-year survival curves for overall (left) and breast cancer specific survival (right). Controlled for age, race, BMI, CCI, marital status, education, treatment site, stage, tumor characteristics (grade, type, size), node status, and hormone receptor (HER2, ER, and PR).
4. Discussion
Nicotine and its metabolites have been detected in breast fluids from nonlactating smokers, and many of the carcinogens in tobacco smoke cause mammary tumors in animal models [12,13], suggesting that smoking-derived carcinogens may be present in the breast and increase the risk of breast cancer. Data from this study suggest that while current smokers have worse overall survival, cigarette smoking is not associated with an enrichment of any tumor characteristics or breast cancer-specific survival.
Differences in tumor stage, size, and lymph node status, which reflect the time at which a tumor is resected, may be attributable not to smoking-derived carcinogen exposure in the breast but rather to other factors, such as decreased adherence to mammographic screening. In a study of 89,058 women enrolled in the Women’s Health Initiative, current smokers were significantly less likely to undergo mammographic screening with concomitant higher stage breast cancer diagnoses [14]. In contrast, former smokers were significantly more likely to undergo mammography. This higher use of regular screening may explain the lower rates of metastatic lymph nodes in former smokers detected in our study.
The association of smoking with breast cancer biomarkers has been mixed. In our study, no significant association was detected between smoking status and ER, PR, or HER2 status. Similarly, ER status did not differ significantly by smoking status in studies from the Cancer Prevention Study II study [15] or Multiethnic Cohort [16]. Data from the EPIC study, however, showed an association between current smoking and ER+ tumors (HR = 1.23, 95% CI 1.04–1.45) [17]. When evaluating tumor subtypes, smoking >20 years was associated with luminal (OR 1.51, 95% CI 1.19–1.93) but not basal-like (OR 0.90 95% CI 0.57–1.43) breast cancer [18]. In contrast, evaluation of smoking and subtype using data from the Surveillance, Epidemiology, and End Results (SEER) database found no difference in breast cancer subtypes by smoking status [19]. Despite these mixed results, these data suggest that the hypothesized antiestrogenic effects of cigarette smoking do not reduce the risk of ER+ tumors.
As with tumor characteristics, the association between cigarette smoking and BCSS is controversial. In our study, smoking status was associated with overall survival, but not BCSS. While some studies have found no association between smoking and BCSS [20,21,22,23,24], a number of other studies have found significant associations [25,26,27,28,29,30,31]. The study from Braithwaite et al. included a similar number of patients as our study (2265 and 2153, receptively) [27]. The Braithwhite et al. study, however, found a two-fold higher BCSS rate in current smokers compared to never smokers (HR = 2.01, 95% CI 1.27–3.18), while our study did not detect any differences in BCSS in current compared to never smokers (HR = 1.37; 95% CI, 0.79–2.40). This difference may be attributable to the length of follow-up. The average length of follow-up in the study by Braithwhite et al. was 12.3 years (11.9 years, 12.2 years, and 12.4 years for current, former, and never smokers). The average length of follow-up in our study was 8.1 years (7.42 years, 9.33 years, and 8.32 years for current, former, and never smokers, respectively). Additional studies that found an association between BCSS and current smoking had larger sample sizes and follow-up times of >10 years [29,30,31]. Data from the Carolina Breast Cancer Study evaluated BCSS in 1808 women and did not detect an association between smoking and BCSS at five years, however, 13-year conditional BCSS was elevated amongst current smokers [32]. Meta-analysis found that while all-cause mortality was significantly associated with smoking across all time periods, smoking was associated with BCSS only for those with follow-up >10 years [26]. Thus, we do not exclude the possibility that there is a long-term effect of smoking on breast cancer-specific survival if such effects occur after 10 years. In contrast, overall survival, despite adjusting for comorbidities, was significantly lower for both former and current compared to never smokers. These results suggest that smoking has general, rather than breast-specific, effects on health status.
In addition to follow-up <10 years, this study has limitations. For example, this study was a hospital rather than a population-based study. This cohort may not, therefore, be reflective of the general US population. In addition, our study did evaluate smoking status at diagnosis and did not include data on smoking cessation after diagnosis. Smoking cessation at or around the time of diagnosis has been recognized as a critical component in increasing the survival of lung cancer patients [33]. Smoking cessation has also demonstrated improved survival in breast cancer patients, with women who quit smoking at diagnosis having a 33% lower risk of death than those who continue to smoke [34,35,36]. The lack of significant differences in BCSS by smoking status in our study, therefore, may be impacted by the rate of smoking cessation. Finally, data regarding exposure to second-hand smoke was not collected in the CBCP questionnaires. In a study of 1755 women, Strumylaite et al. found that exposure to passive smoke was associated with an increased risk of breast cancer [37]. Risk increased with longer exposure, however, no significant association was detected for hormone receptor status. A second study 1508 women found no association between either overall or breast cancer-specific survival and exposure to second-hand smoke [38]. Thus, although the burden of breast cancer attributable to exposure to passive smoking could not be addressed in our study, previous studies do not suggest passive smoking is associated with breast cancer characteristics or survival
5. Conclusions
Cigarette smoking did not significantly alter tumor pathological characteristics of breast tumors. Although breast cancer-specific survival did not differ significantly, overall survival was significantly worse in smokers, and smoking cessation should be encouraged in the recently diagnosed breast cancer patient.
Author Contributions
Conceptualization, R.E.E.; methodology, K.Z.; analysis, S.D., A.P.; resources, C.D.S.; data curation, L.A.L.; writing—original draft preparation, R.E.E.; writing—review and editing, S.D., A.P., L.A.L., C.D.S., K.Z.; funding acquisition, C.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a cooperative agreement from the Uniformed Services University of the Health Sciences HU0001-16-2-0004 through the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Walter Reed National Military Medical Center (protocol #20704 approved 3 March 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data provided upon request.
Acknowledgments
The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions or policies of Uniformed Services University of the Health Sciences (USUHS), The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the Department of Defense (DoD) or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
Conflicts of Interest
The authors declare no conflict of interest.
References
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2014.
- Cornelius, M.E.; Wang, T.W.; Jamal, A.; Loretan, C.G.; Neff, L.J. Tobacco Product Use Among Adults—United States, 2019. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.; Edmiston, S.N.; Cui, L.; Drouin, S.S.; Pang, J.; He, M.; Tse, C.-K.; Geradts, J.; Dressler, L.; Liu, E.T.; et al. Prevalence and spectrum of p53 mutations associated with smoking in breast cancer. Cancer Res. 2002, 62, 1987–1995. [Google Scholar] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Baron, J.A.; La Vecchia, C.; Levi, F. The antiestrogenic effect of cigarette smoking in women. Am. J. Obstet. Gynecol. 1990, 162, 502–514. [Google Scholar] [CrossRef]
- Dasgupta, P.; Rizwani, W.; Pillai, S.; Kinkade, R.; Kovacs, M.; Rastogi, S.; Banerjee, S.; Carless, M.; Kim, E.; Coppola, D.; et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int. J. Cancer 2009, 124, 36–45. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Edge, S.B.; Giuliano, A. New and Important Changes in the TNM Staging System for Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 457–467. [Google Scholar] [CrossRef]
- Bloom, H.J.; Richardson, W.W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br. J. Cancer 1957, 11, 359–377. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Hecht, S.S. Tobacco smoke carcinogens and breast cancer. Environ. Mol. Mutagen. 2002, 39, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, N.L.; Gruenke, L.D.; Beelen, T.C.; Castagnoli, N., Jr.; Craig, J.C. Nicotine in breast fluid of nonlactating women. Science 1978, 199, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Eng, V.A.; David, S.P.; Li, S.; Ally, M.S.; Stefanick, M.; Tang, J.Y. The association between cigarette smoking, cancer screening, and cancer stage: A prospective study of the women’s health initiative observational cohort. BMJ Open 2020, 10, e037945. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, M.M.; Gapstur, S.M.; Sun, J.; Diver, W.R.; Hannan, L.M.; Thun, M.J. Active smoking and breast cancer risk: Original cohort data and meta-analysis. J. Natl. Cancer Inst. 2013, 105, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Gram, I.T.; Park, S.Y.; Maskarinec, G.; Wilkens, L.R.; Haiman, C.A.; Le Marchand, L. Smoking and breast cancer risk by race/ethnicity and oestrogen and progesterone receptor status: The Multiethnic Cohort (MEC) study. Int. J. Epidemiol. 2019, 48, 501–511. [Google Scholar] [CrossRef]
- Dossus, L.; Boutron-Ruault, M.C.; Kaaks, R.; Gram, I.T.; Vilier, A.; Fervers, B.; Manjer, J.; Tjonneland, A.; Olsen, A.; Overva, K.; et al. Active and passive cigarette smoking and breast cancer risk: Results from the EPIC cohort. Int. J. Cancer 2014, 134, 1871–1888. [Google Scholar] [CrossRef]
- Butler, E.N.; Tse, C.K.; Bell, M.E.; Conway, K.; Olshan, A.F.; Troester, M.A. Active smoking and risk of Luminal and Basal-like breast cancer subtypes in the Carolina Breast Cancer Study. Cancer Causes Control 2016, 27, 775–786. [Google Scholar] [CrossRef]
- Baglia, M.L.; Cook, L.S.; Mei-Tzu, C.; Wiggins, C.; Hill, D.; Porter, P.; Li, C.I. Alcohol, smoking, and risk of Her2-overexpressing and triple-negative breast cancer relative to estrogen receptor-positive breast cancer. Int. J. Cancer 2018, 143, 1849–1857. [Google Scholar] [CrossRef]
- Goldvaser, H.; Gal, O.; Rizel, S.; Hendler, D.; Neiman, V.; Shochat, T.; Sulkes, A.; Brenner, B.; Yerushalmi, R. The association between smoking and breast cancer characteristics and outcome. BMC Cancer 2017, 17, 624. [Google Scholar] [CrossRef]
- Holmes, M.D.; Murin, S.; Chen, W.Y.; Kroenke, C.H.; Spiegelman, D.; Colditz, G.A. Smoking and survival after breast cancer diagnosis. Int. J. Cancer 2007, 120, 2672–2677. [Google Scholar] [CrossRef]
- Kakugawa, Y.; Kawai, M.; Nishino, Y.; Fukamachi, K.; Ishida, T.; Ohuchi, N.; Minami, Y. Smoking and survival after breast cancer diagnosis in Japanese women: A prospective cohort study. Cancer Sci. 2015, 106, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Simonsson, M.; Markkula, A.; Rose, C.; Ingvar, C.; Jernstrom, H. Impacts of smoking on endocrine treatment response in a prospective breast cancer cohort. Br. J. Cancer 2016, 115, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Gaudet, M.M.; Eng, S.M.; Abrahamson, P.E.; Shantakumar, S.; Teitelbaum, S.L.; Britton, J.A.; Bell, P.; Thomas, J.A.; Neugut, A.I.; et al. Active and passive cigarette smoke and breast cancer survival. Ann. Epidemiol. 2007, 17, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, F.; Zhang, X.; Li, Z.; Li, H. Smoking increases risks of all-cause and breast cancer specific mortality in breast cancer individuals: A dose-response meta-analysis of prospective cohort studies involving 39725 breast cancer cases. Oncotarget 2016, 7, 83134–83147. [Google Scholar] [CrossRef]
- Duan, W.; Li, S.; Meng, X.; Sun, Y.; Jia, C. Smoking and survival of breast cancer patients: A meta-analysis of cohort studies. Breast 2017, 33, 117–124. [Google Scholar] [CrossRef]
- Braithwaite, D.; Izano, M.; Moore, D.H.; Kwan, M.L.; Tammemagi, M.C.; Hiatt, R.A.; Kerlikowske, K.; Kroenke, C.H.; Sweeney, C.; Habel, L.; et al. Smoking and survival after breast cancer diagnosis: A prospective observational study and systematic review. Breast Cancer Res. Treat. 2012, 136, 521–533. [Google Scholar] [CrossRef]
- Sollie, M.; Bille, C. Smoking and mortality in women diagnosed with breast cancer-a systematic review with meta-analysis based on 400,944 breast cancer cases. Gland Surg. 2017, 6, 385–393. [Google Scholar] [CrossRef]
- Pierce, J.P.; Patterson, R.E.; Senger, C.M.; Flatt, S.W.; Caan, B.; Natarajan, L.; Nechuta, S.; Poole, E.M.; Shu, X.-O.; Chen, W.Y. Lifetime cigarette smoking and breast cancer prognosis in the After Breast Cancer Pooling Project. J. Natl. Cancer Inst. 2014, 106, djt359. [Google Scholar] [CrossRef]
- Passarelli, M.N.; Newcomb, P.A.; Hampton, J.M.; Trentham-Dietz, A.; Titus, L.J.; Egan, K.M.; Baron, J.A.; Willett, W.C. Cigarette Smoking before and after Breast Cancer Diagnosis: Mortality from Breast Cancer and Smoking-Related Diseases. J. Clin. Oncol. 2016, 34, 1315–1322. [Google Scholar] [CrossRef]
- Ordóñez-Mena, J.M.; Schöttker, B.; Mons, U.; Jenab, M.; Freisling, H.; Bueno-De-Mesquita, B.; O’Doherty, M.G.; Scott, A.; Kee, F.; Stricker, B.H.; et al. Quantification of the smoking-associated cancer risk with rate advancement periods: Meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med. 2016, 14, 62. [Google Scholar] [CrossRef]
- Parada, H., Jr.; Sun, X.; Tse, C.K.; Olshan, A.F.; Troester, M.A.; Conway, K. Active smoking and survival following breast cancer among African American and non-African American women in the Carolina Breast Cancer Study. Cancer Causes Control 2017, 28, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Del Riccio, M.; Vettori, V.; Scotti, V.; Martinoli, C.; Raimondi, S.; Cammarata, G.; Palli, D.; Banini, M.; Masala, G.; et al. Quitting smoking at or around diagnosis improves the overall survival of lung cancer patients: A systematic review and meta-analysis. J. Thorac. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mizota, Y.I.T.; Ohashi, Y.; Mukai, H.; Yamamoto, S. The effects of smoking and smoking cessation on postmenopausal breast cancer recurrence—Results from the Cohort 05 of the Rainbow of KIBOU (ROK) study: A prospective breast cancer survivor cohort in Japan. Cancer Res. 2020, 80, nr P3-08-36. [Google Scholar]
- Passarelli, M.N.; Newcomb, P.A. Survival Benefits of Smoking Cessation after Breast Cancer Diagnosis. JNCI Cancer Spectr. 2017, 1, pkx005. [Google Scholar] [CrossRef] [PubMed]
- Parada, H., Jr.; Bradshaw, P.T.; Steck, S.E.; Engel, L.S.; Conway, K.; Teitelbaum, S.L.; Neugut, A.I.; Santella, R.M.; Gammon, M.D. Postdiagnosis Changes in Cigarette Smoking and Survival Following Breast Cancer. JNCI Cancer Spectr. 2017, 1, pkx001. [Google Scholar] [CrossRef] [PubMed]
- Strumylaite, L.; Kregzdyte, R.; Poskiene, L.; Bogusevicius, A.; Pranys, D.; Norkute, R. Association between lifetime exposure to passive smoking and risk of breast cancer subtypes defined by hormone receptor status among non-smoking Caucasian women. PLoS ONE 2017, 12, e0171198. [Google Scholar] [CrossRef]
- Parada, H., Jr.; Bradshaw, P.T.; Engel, L.S.; Conway, K.; Steck, S.E.; Teitelbaum, S.L.; Neugut, A.I.; Santella, R.M.; Gammon, M.D. Environmental Tobacco Smoke Exposure and Survival Following Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 278–280. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).