Development and Feasibility of an Inpatient Cancer-Related Sarcopenia Pathway at a Major Cancer Centre
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Participants
2.4. Phase I: Development of the Sarc-Pathway
2.4.1. Adherence to Best Practice Audit
2.4.2. Developing Key Components of the Sarc-Pathway Model
- For the overall sarc-pathway model, small changes were made as part of an iterative and continuous improvement cycle early in the pilot period and to enable compliance with local COVID-19 restrictions, i.e., with no group exercise classes available. Fortnightly project team meetings provided an avenue for decisions or changes to be made relating to the sarc-pathway over time. Supplementary File S1 describes the sarc-pathway model applied into clinical practice from approximately 3 weeks into the pilot until pilot end.
- The adherence to best practice audit helped identify local strengths and opportunities for improvement of the current clinical pathway.
- A clinical care pathway format was utilised to enable clear designation of actions at specific time-points [14,15] and to enable a comprehensive design; this was overlaid with the AACTT framework to help define behaviours and components of care to help assess uptake and/or adherence [19]. The component behaviours of action (behaviour), actor (who does the action), context (the setting in which the behaviour occurs), target (whom the actor performs the action on) and time (the time period or duration) were defined [19] (Table 1).
- Project team and wider multidisciplinary clinician input was sought and incorporated into the development of the sarc-pathway prior to Phase II piloting. The inclusion of valid and reliable screening tools, assessment and outcome measures into the sarc-pathway was considered essential. A pragmatic approach in the choice of tools and measures was taken, specifically in regard to equipment that was available to clinicians, the complexity and therefore the training required to conduct the measure and the appropriateness for the acute ward context. A two-page fact sheet specific to cancer-related sarcopenia and the sarc-pathway model was developed in consultation with patients and incorporated as part of the sarc-pathway screening process.
- The definition of sarcopenia was critical to defining the clinical criteria required for a sarcopenia diagnosis and the EWGSOP2 definition was chosen for the sarc-pathway [2]. The EWGSOP2 defines probable sarcopenia as low muscle strength and additionally, a sarcopenia diagnosis is confirmed if low muscle quantity (mass) is found [2]. All cut-points applied for each clinical measure are specified in Supplementary File S1.
- Strong consideration was given to incorporation of the sarc-pathway into existing ward clinical processes, specifically in regard to risk screening and referrals to multidisciplinary allied health clinicians. AHA-NA were considered well placed to conduct screening in a ward setting, whilst the dietitians and physiotherapists were identified as the core expert clinical disciplines to provide nutrition and exercise assessments and interventions, respectively (as a multimodal package), to address cancer-related sarcopenia.
2.4.3. Key Implementation Strategies Prior to Pathway Piloting
- Engaged project team: The multidisciplinary and expert project team members met fortnightly throughout Phases I-II and applied a continuous quality feedback cycle to make real-time modifications to the sarc-pathway before and during the pilot.
- Staff training and competency packages: Tailored education and training was developed by members of the project team and provided to all multidisciplinary allied health clinicians providing care on the sarc-pathway. Competency packages for new tools or skills included in the sarc-pathway, not already used within usual clinical practices, were developed and completed by clinicians, specifically in regard to sarcopenia screening for the AHA-NA, body composition measurement via bioimpedance spectroscopy (BIS) for the dietitians and AHA-NA’s and five-time chair stand test (5-CST) for the physiotherapists and allied health assistant-physiotherapist (AHA-PT’s). Staff training and competency packages took 1–2 h to complete and were completed by staff within one month of commencing the pilot.
- Embedding the sarc-pathway within digital technology: Outcome measures were captured into discrete and reportable data fields and template documentation for all allied health clinician encounters for consistent reporting within the recruitment sites’ existing electronic medical record (EMR).
- Communication: Sharing of important milestones and key project updates occurred within allied health and with multidisciplinary nursing and medical colleagues approximately monthly throughout the project.
2.5. Phase II: Pilot Test the Feasibility of the Sarc-Pathway
2.5.1. Reach
2.5.2. Intervention Fidelity (Intervention Delivery and Adherence)
- Number and proportion of participants screened as being ‘at risk/probable’ for sarcopenia who were referred to the dietitian and physiotherapist for assessment, based on the sarcopenia screening tool and calf circumference [combined strength, assistance in walking, rise from chair, climb stairs and falls (SARC-F) and calf circumference, SARC-CalF] [22] and handgrip strength (HGS) [23]
- Number and proportion of participants screened as being ‘at risk/probable’ for sarcopenia who were referred to the dietitian and physiotherapist and were assessed and received treatment, as indicated in the sarc-pathway
- Number and proportion of participants who had clinical assessment measures performed as per the sarc-pathway for nutritional status, muscle mass, muscle strength and/or physical function. These included:
- ○
- Nutritional status using the Patient-Generated Subjective Global Assessment (PG-SGA) [24,25] (performed by dietitian): this includes a subjective assessment of weight-loss, nutritional symptoms, food intake and activity levels and an objective assessment of body composition (fat, muscle stores and fluid status, scored as “0” = no deficit, “1” = mild deficit, “2” = moderate and “3” = severe). Each component of the PG-SGA is scored between 0 and 4 to provide an overall score (typical scores range from 0 to 35) and category of nutritional status (A = well-nourished, B = moderately/suspected malnutrition and C = severe malnutrition);
- ○
- Muscle mass using (i) BIS for estimated appendicular lean mass (ALM) [2] (performed by dietitian): segmental analysis on the Impedimed SOZOTM estimates ALM equating skeletal muscle mass of each arm and leg [26,27]; (ii) Body Mass Index (BMI)-adjusted calf circumference as a proxy measure of muscle mass (performed by AHA-NA) [2,28].
- ○
- Muscle strength using (i) the 5-times chair stand test (5-CST) [2,29] (performed by physiotherapist) measures the time a participant takes to stand up and sit down 5 times, without using arms, from a standard height chair; and (ii) hand grip strength (performed by AHA-NA), utilising the Jamar dynamometer with clinicians recording 3 measurements on each side from the participant and using the maximum result [2,23].
- ○
- Performance status using the Australia-modified Karnofsky Performance Status scale (AKPS) [30] (performed by physiotherapist): utilised to measure overall performance status, whereby the clinician observes the participant’s ability to perform common tasks relating to activity, work and self-care. It is assessed on an 11-point scale with a higher score equating to a better level of function, ranging from 0 (dead) to 100 (normal and no complaints; no evidence of disease).
2.5.3. Participant and Multidisciplinary Clinician Acceptability
2.5.4. Exploratory Outcomes
2.5.5. Resource Utilisation Associated with Implementation of the Sarc-Pathway
2.6. Data Management and Analysis
3. Results
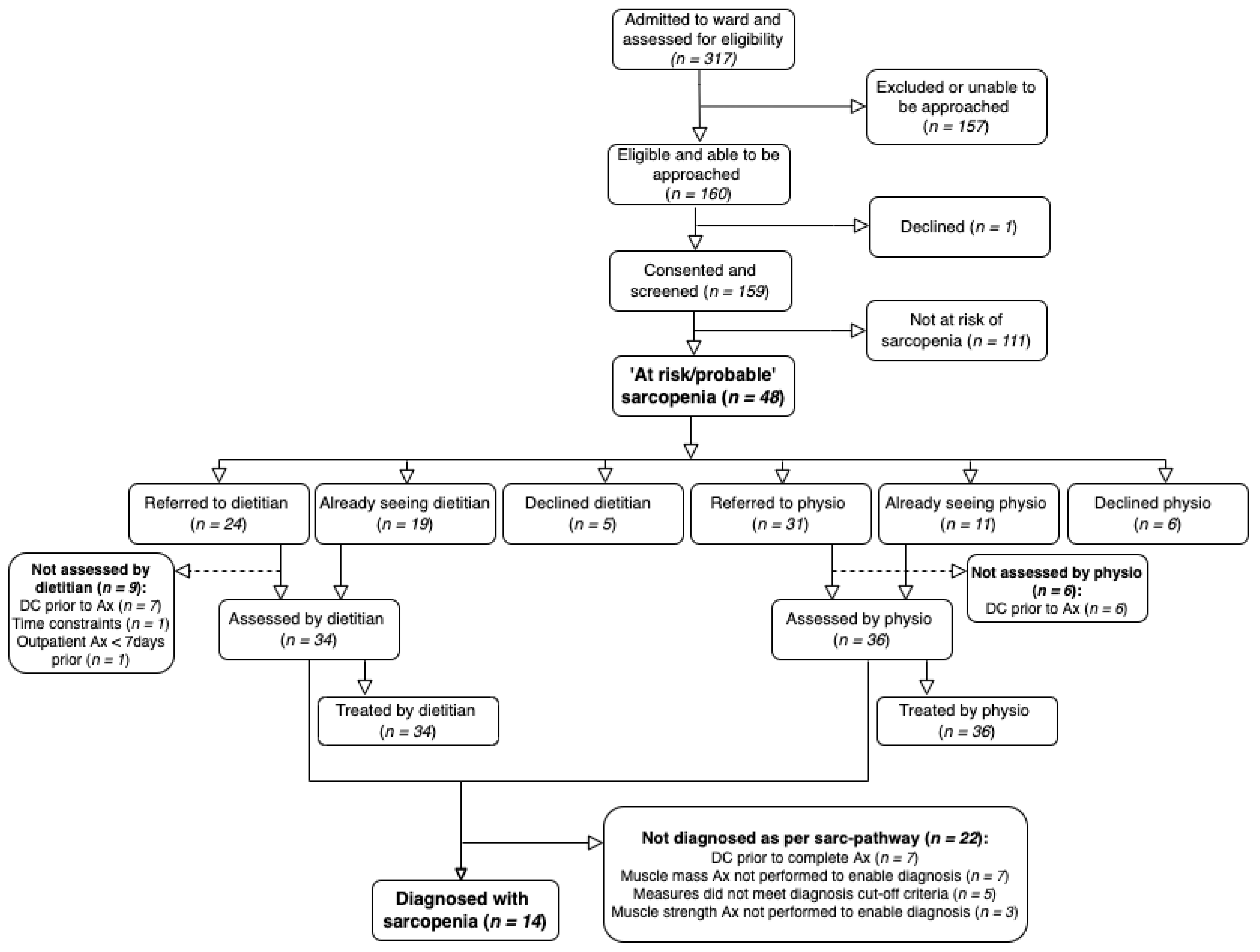
3.1. Characteristics of Participants and Reach
3.2. Intervention Fidelity (Intervention Delivery and Adherence)
3.2.1. Intervention Delivery
3.2.2. Clinical Assessment Measures
3.2.3. Pre-and Post-Adherence to Best Practice Audit
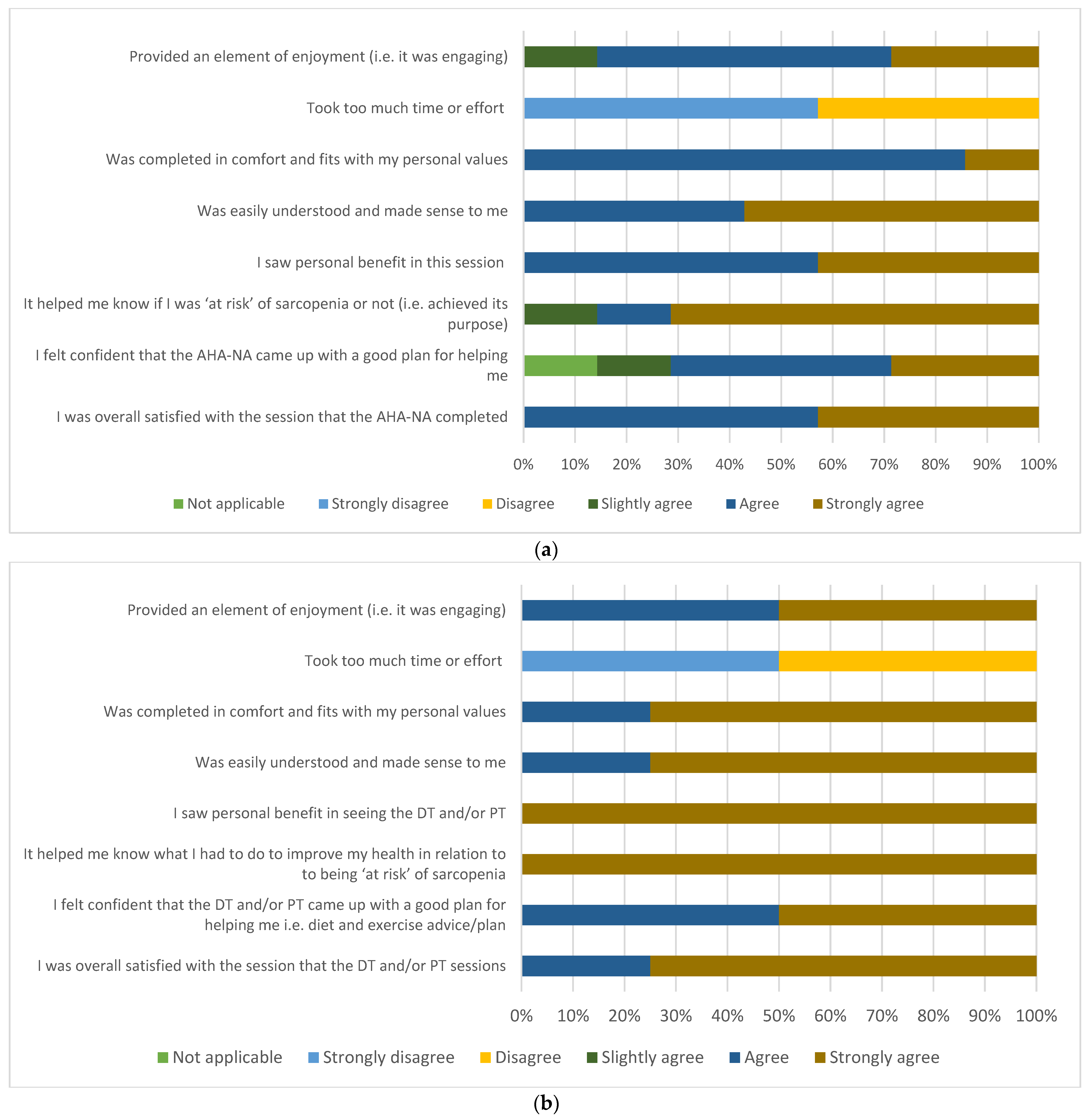
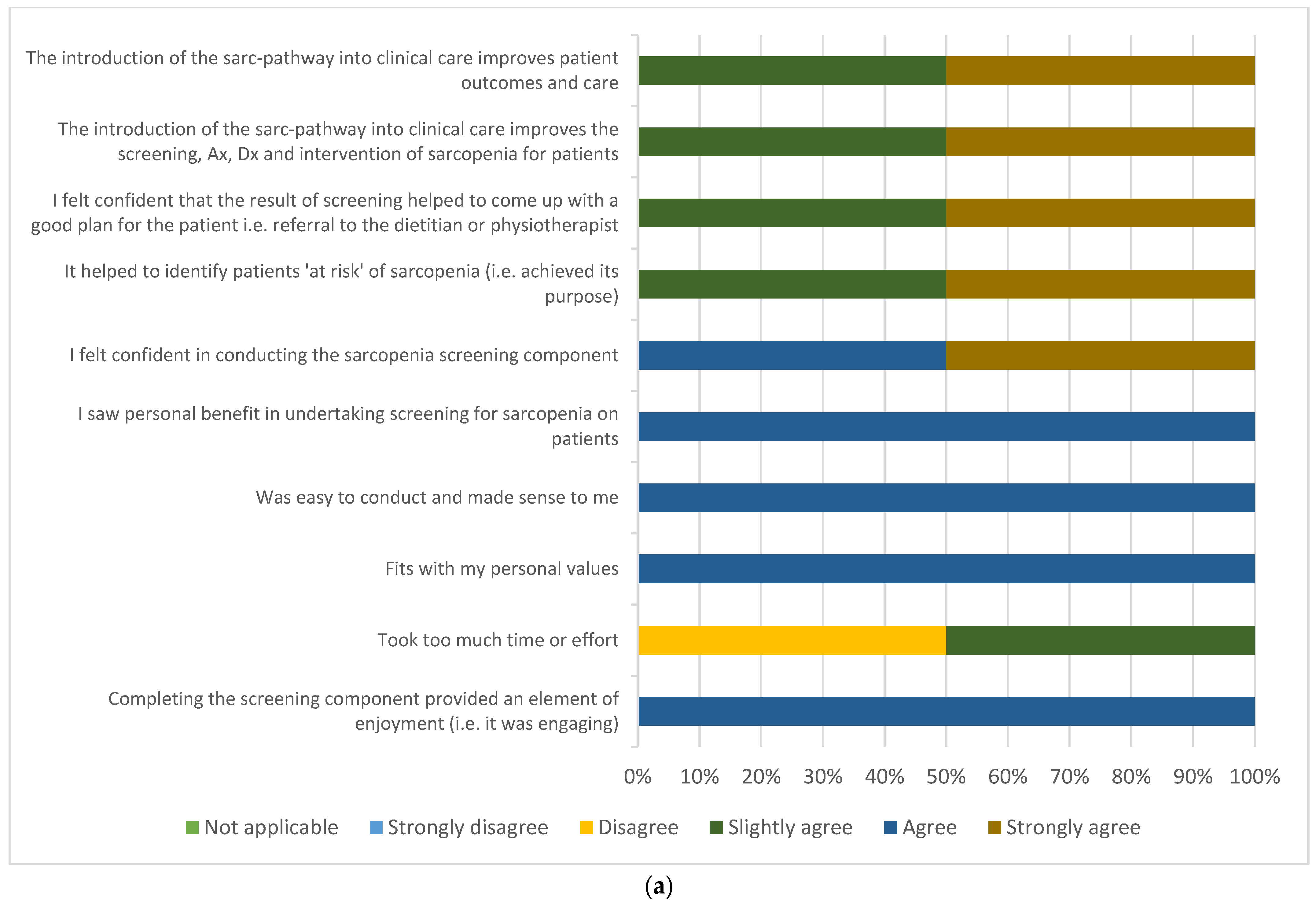
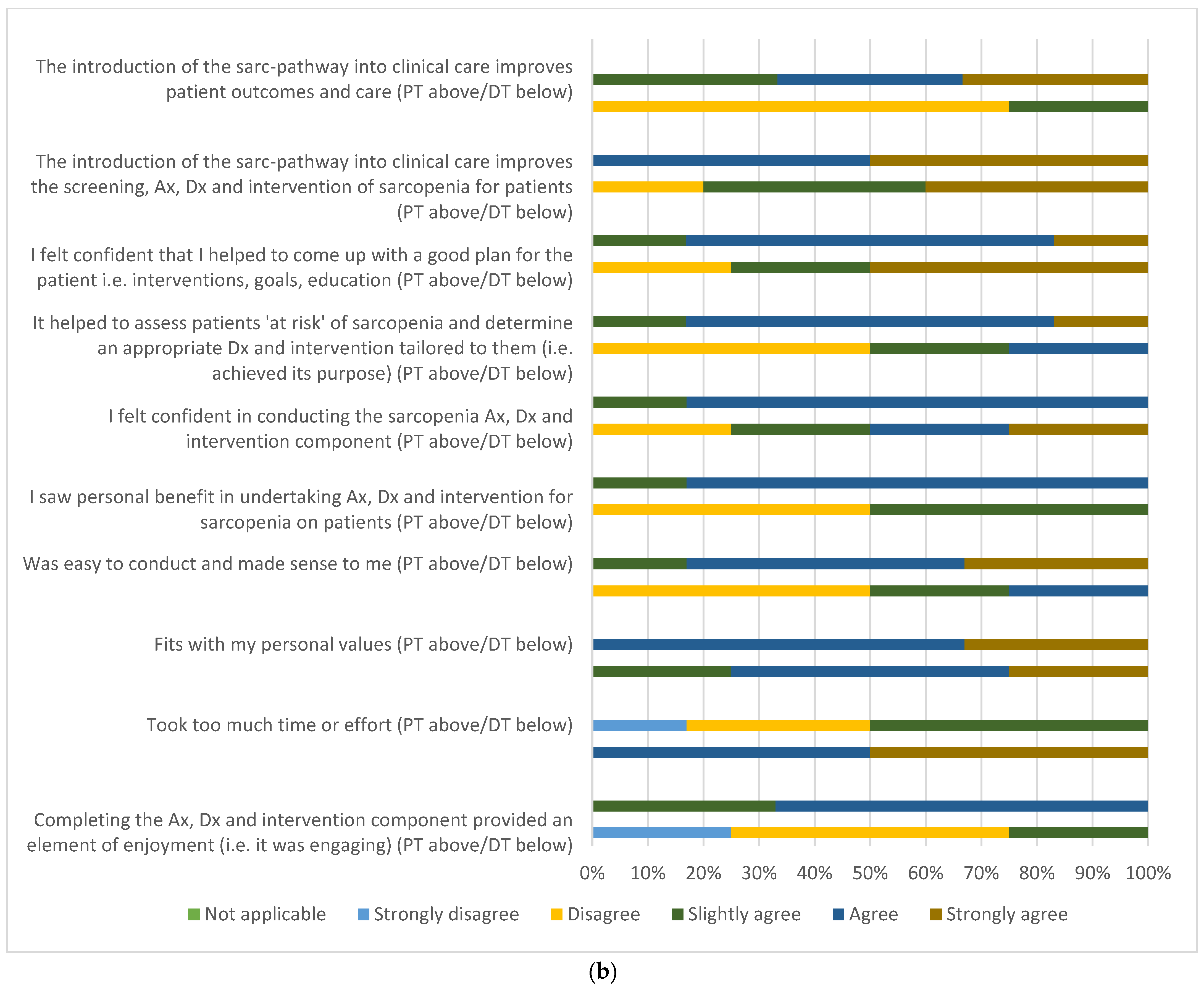
3.3. Participant and Multidisciplinary Clinician Acceptability
3.3.1. Participant Acceptability Survey
3.3.2. Clinician Acceptability Survey
3.4. Exploratory Outcomes
3.5. Resource Utilisation with Implementation of the Sarc-Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; de van der Schueren, M.A.; den Braver, N.R.; Berkhof, J.; Langius, J.A.; Verheul, H.M. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef] [Green Version]
- Kiss, N.; Loeliger, J.; Findlay, M.; Isenring, E.; Baguley, B.J.; Boltong, A.; Butler, A.; Deftereos, I.; Eisenhuth, M.; Fraser, S.F.; et al. Clinical Oncology Society of Australia: Position statement on cancer-related malnutrition and sarcopenia. Nutr. Diet. 2020, 77, 416–425. [Google Scholar] [CrossRef]
- Prado, C.M.; Laviano, A.; Gillis, C.; Sung, A.D.; Gardner, M.; Yalcin, S.; Dixon, S.; Newman, S.M.; Bastasch, M.D.; Sauer, A.C.; et al. Examining guidelines and new evidence in oncology nutrition: A position paper on gaps and opportunities in multimodal approaches to improve patient care. Support. Care Cancer 2022, 30, 3073–3083. [Google Scholar] [CrossRef]
- Meza-Valderrama, D.; Marco, E.; Davalos-Yerovi, V.; Muns, M.D.; Tejero-Sanchez, M.; Duarte, E.; Sanchez-Rodriguez, D. Sarcopenia, Malnutrition and Cachexia: Adapting Definitions and Terminology of Nutritional Disorders in Older People with Cancer. Nutrients 2021, 13, 761. [Google Scholar] [CrossRef]
- Findlay, M.; Bauer, J.; Brown, T.; Davidson, W.; Isenring, E.; Kiss, N.; Kurmis, R.; Loeliger, J.; Sandison, A.; Talwar, B.; et al. Evidence-Based Practice Guidelines for the Nutritional Management of Adult Patients with Head and Neck Cancer. 2011. Available online: https://wiki.cancer.org.au/australia/COSA:Head_and_neck_cancer_nutrition_guidelines (accessed on 26 February 2022).
- Isenring, E.; Zabel, R.; Bannister, M.; Brown, T.; Findlay, M.; Kiss, N.; Loeliger, J.; Johnstone, C.; Camilleri, B.; Davidson, W.; et al. Updated evidence-based practice guidelines for the nutritional management of patients receiving radiation therapy and/or chemotherapy. Nutr. Diet. 2013, 70, 312–324. [Google Scholar] [CrossRef]
- Thompson, K.L.; Elliott, L.; Fuchs-Tarlovsky, V.; Levin, R.M.; Voss, A.C.; Piemonte, T. Oncology Evidence-Based Nutrition Practice Guideline for Adults. J. Acad. Nutr. Diet. 2017, 117, 297–310.e247. [Google Scholar] [CrossRef]
- Marshall, K.M.; Loeliger, J.; Nolte, L.; Kelaart, A.; Kiss, N.K. Prevalence of malnutrition and impact on clinical outcomes in cancer services: A comparison of two time points. Clin. Nutr. 2019, 38, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Department of Health & Human Services. Supervision and Delegation Framework for Allied Health Assistants; Victoria, D.O.H., Ed.; Department of Health & Human Services: Melbourne, Australia, 2013. Available online: https://www.health.vic.gov.au/publications/supervision-and-delegation-framework-for-allied-health-assistants (accessed on 26 February 2022).
- Dewar, S.L.; Porter, J. The Effect of Evidence-Based Nutrition Clinical Care Pathways on Nutrition Outcomes in Adult Patients Receiving Non-Surgical Cancer Treatment: A Systematic Review. Nutr. Cancer 2018, 70, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Lykins, T.C. Nutrition support clinical pathways. Nutr. Clin. Pract. 1996, 11, 16–20. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Presseau, J.; McCleary, N.; Lorencatto, F.; Patey, A.M.; Grimshaw, J.M.; Francis, J.J. Action, actor, context, target, time (AACTT): A framework for specifying behaviour. Implement. Sci. 2019, 14, 102. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Borrelli, B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J. Public Health Dent. 2011, 71 (Suppl. 1), S52–S63. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Tian, Z.; Thapa, S.; Sun, H.; Wen, S.; Xiong, H.; Yu, S. Comparing SARC-F with SARC-CalF for screening sarcopenia in advanced cancer patients. Clin. Nutr. 2020, 39, 3337–3345. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Ottery, F.D. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996, 12, S15–S19. [Google Scholar] [CrossRef]
- Bauer, J.; Capra, S.; Ferguson, M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 2002, 56, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Kuhlmann, M.K.; Kaysen, G.A.; Sarkar, S.; Kaitwatcharachai, C.; Khilnani, R.; Stevens, L.; Leonard, E.F.; Wang, J.; Heymsfield, S.; et al. Segment-specific resistivity improves body fluid volume estimates from bioimpedance spectroscopy in hemodialysis patients. J. Appl. Physiol. 2006, 100, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, K.L.; Earthman, C.P. Update on body composition tools in clinical settings: Computed tomography, ultrasound and bioimpedance applications for assessment and monitoring. Eur. J. Clin. Nutr. 2019, 73, 73–187. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.C.; Mehrnezhad, A.; Razaviarab, N.; Barbosa-Silva, T.G.; Heymsfield, S.B. Calf circumference: Cutoff values from the NHANES 1999–2006. Am. J. Clin. Nutr. 2021, 113, 1679–1687. [Google Scholar] [CrossRef]
- Munoz-Bermejo, L.; Adsuar, J.C.; Mendoza-Munoz, M.; Barrios-Fernandez, S.; Garcia-Gordillo, M.A.; Perez-Gomez, J.; Carlos-Vivas, J. Test-Retest Reliability of Five Times Sit to Stand Test (FTSST) in Adults: A Systematic Review and Meta-Analysis. Biology 2021, 10, 510. [Google Scholar] [CrossRef]
- Abernethy, A.P.; Shelby-James, T.; Fazekas, B.S.; Woods, D.; Currow, D.C. The Australia-modified Karnofsky Performance Status (AKPS) scale: A revised scale for contemporary palliative care clinical practice [ISRCTN81117481]. BMC Palliat. Care 2005, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Sekhon, M.; Cartwright, M.; Francis, J.J. Acceptability of healthcare interventions: An overview of reviews and development of a theoretical framework. BMC Health Serv. Res. 2017, 17, 88. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef]
- Orkin, A.M.; Gill, P.J.; Ghersi, D.; Campbell, L.; Sugarman, J.; Emsley, R.; Steg, P.G.; Weijer, C.; Simes, J.; Rombey, T.; et al. Guidelines for Reporting Trial Protocols and Completed Trials Modified Due to the COVID-19 Pandemic and Other Extenuating Circumstances: The CONSERVE 2021 Statement. JAMA 2021, 326, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.; Greig, C.; Majid, Z.; Masud, T.; Moorey, H.; Pinkney, T.; Jackson, T. The feasibility of conducting acute sarcopenia research in hospitalised older patients: A prospective cohort study. Eur. Geriatr. Med. 2021, 1–11. Available online: https://doi.org/10.1007/s41999-021-00565-6 (accessed on 26 February 2022). [CrossRef] [PubMed]
- Bauer, J.; Morley, J.E.; Schols, A.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Sousa, I.M.; Gonzalez, M.C.; Bielemann, R.M.; Rocha, I.M.G.; Barbalho, E.R.; Carvalho, A.L.M.; Medeiros, G.O.C.; Silva, F.M.; Fayh, A.P.T. Agreement between muscle mass assessments by computed tomography and calf circumference in patients with cancer: A cross-sectional study. Clin. Nutr. ESPEN 2022, 47, 183–188. [Google Scholar] [CrossRef]

| Action: | Conduct sarcopenia screening (and re-screening) i.e., SARC-CalF, HGS | Provide written information to all participants screened (both at risk and at low risk of sarcopenia) | Refer participants at risk of sarcopenia to the dietitian and physiotherapist | Complete full individualised assessment with participants | Complete clinical assessment measures (for dietitian and physiotherapist assessments and diagnosis of sarcopenia, i.e., PG-SGA, BIS, 5-CST, AKPS) | Deliver individualised interventions | Where indicated, deliver outpatient care and/or refer to external services |
| Actor: | AHA-NA | AHA-NA | AHA-NA | Dietitian and physiotherapist | AHA-NA, dietitian, physiotherapist (may be delegated to AHA-PT) | Dietitian, physiotherapist (may be delegated to AHA-PT) | Dietitian and physiotherapist |
| Context: | Acute cancer inpatient ward | Acute cancer inpatient ward | Acute cancer inpatient ward—referral via EMR | Acute cancer inpatient ward—participants’ room and/or ward | Acute cancer inpatient ward—participants’ room, ward and/or gym | Acute cancer inpatient ward—participants’ room, ward and/or gym | Clinic room, gym, via telehealth and/or via external provider |
| Target: | Eligible participants admitted to the ward (and those screened as low risk of sarcopenia on admission and still an inpatient at day 7) | All participants screened (both at risk and at low risk of sarcopenia) | Participants considered at risk of sarcopenia after screening | Participants considered at risk of sarcopenia after screening | Participants considered at risk of sarcopenia after screening and undertaking assessment by the dietitian and physiotherapist | Participants considered at risk of sarcopenia after screening and/or diagnosed with sarcopenia | Participants considered at risk of sarcopenia after screening and/or diagnosed with sarcopenia requiring ongoing intervention post discharge |
| Time *: | Within 2 days of admission for initial screen (day 6–8 for rescreen) | Within 2 days of admission | Within 2 days of admission | Within 1 day of referral being placed via the EMR | Baseline measures—AHA-NA: within 2 days of admission; dietitian/ physiotherapist within 1 day of referral; pre-discharge measures—1–2 days prior to hospital discharge by dietitian/physiotherapist | Within 1 day of referral from NA and then as specified by dietitian/physiotherapist | Following discharge from hospital |
| Characteristics | Participants on the Sarc-Pathway (n = 159) |
|---|---|
| Age (years), median (IQR) | 61 (49, 70) |
| Gender (male) | 89 (56.0) |
| Cancer diagnosis: | |
| Sarcoma | 27 (17.0) |
| Lung | 26 (16.4) |
| Lower gastrointestinal | 19 (11.9) |
| Skin/melanoma | 18 (11.3) |
| Upper gastrointestinal | 14 (8.8) |
| Genitourinary | 13 (8.2) |
| Head and neck | 11 (6.9) |
| Haematological | 10 (6.3) |
| Cervical/ovarian | 10 (6.3) |
| Breast | 9 (5.7) |
| Brain and spine | 2 (1.3) |
| Length of hospital stay (days), median (IQR) | 5 (3, 7) |
| Component of the Sarc-Pathway | n = 159 |
|---|---|
| Screening within sarc-pathway timeframes (2 days of admission): | |
| Completed | 118 (74.2) |
| Not completed: | 41 (25.8) |
| Missed by AHA-NA | 19 (11.9) |
| Weekend (no AHA-NA screening) | 15 (9.4) |
| Transferred from other ward—delay | 2 (1.3) |
| No AHA-NA staffing available | 2 (1.3) |
| COVID-19 restrictions, i.e., isolation requirements for participant | 2 (1.3) |
| Participant medically unstable | 1 (0.6) |
| Referral to dietitian (n = 24) | |
| Referral to dietitian completed within sarc-pathway timeframes (1 day of screening): | |
| Completed | 24 (100) |
| Not completed | 0 (0) |
| Referral to physiotherapist (n = 31) | |
| Referral to physiotherapist completed within sarc-pathway timeframes (1 day of screening): | |
| Completed | 31 (100) |
| Not completed | 0 (0) |
| Assessment and treatment by dietitian (n = 34), i.e., those referred + already being seen | |
| Assessment and treatment by dietitian completed within sarc-pathway timeframes (1 day of referral): | |
| Completed | 34 (100) |
| Not completed | 0 (0) |
| Assessment and treatment by physiotherapist (n = 36), i.e., those referred + already being seen | |
| Assessment and treatment by physiotherapist completed within sarc-pathway timeframes (1 day of referral): | |
| Completed | 33 (91.7) |
| Not completed: | 3 (8.3) |
| COVID-19 precautions, i.e., isolation requirements for participant | 1 (2.8) |
| Time delays due to competing priorities | 1 (2.8) |
| Known already to clinician and clinical measures not collected as per sarc-pathway | 1 (2.8) |
| Clinical Assessment Measures | Participants | Score/Outcome |
|---|---|---|
| Screening (n = 159) | ||
| Hand Grip Strength (Maximum), kg | ||
| Completed | 159 (100.0) | 28 (20, 37) |
| Not completed: | 0 (0.0) | |
| SARC-F Score | ||
| Completed | 159 (100.0) | 2 (1, 3) |
| Not completed: | 0 (0.0) | |
| Calf Circumference (Maximum), cm | ||
| Completed | 159 (100.0) | 36.8 (5.8) |
| Not completed: | 0 (0.0) | |
| SARC-CalF Score | ||
| Completed | 159 (100.00) | 3.0 (1, 10) |
| Not completed: | 0 (0.0) | |
| Assessment by dietitian (n = 34) | ||
| ALM via BIS, kg | ||
| Completed | 7 (20.6) | 14.6 (2.2) |
| Not completed: | 27 (79.4) | |
| Patient declined but otherwise able | 8 (23.5) | |
| Patient unable due to medical/ physical limitations | 6 (17.6) | |
| Discharged before completed | 6 (17.6) | |
| Not attempted | 2 (5.9) | |
| Change in patient medical condition | 2 (5.9) | |
| Equipment issue | 1 (2.9) | |
| COVID-19 precautions | 1 (2.9) | |
| Patient became fatigued | 1 (2.9) | |
| PG-SGA Score | ||
| Completed | 26 (76.5) | 12.7 (4.9) PG-SGA A, n (%) = 6 (23.1) PG-SGA B, n (%) = 16 (61.5) PG-SGA C, n (%) = 4 (15.4) |
| Not completed: | 8 (23.5) | |
| Missed by clinician | 4 (11.8) | |
| Discharged before completion | 3 (8.8) | |
| COVID-19 precautions | 1 (2.9) | |
| Assessment by physiotherapist (n = 36) | ||
| 5-CST, seconds | ||
| Completed | 18 (50.0) | 17.5 (12.7, 23.3) |
| Not completed: | 18 (50.0) | |
| Patient unable due to medical/ physical limitations | 6 (16.7) | |
| Not attempted | 5 (13.9) | |
| Discharged before completion | 3 (8.3) | |
| COVID-19 precautions | 1 (2.8) | |
| Stopped mid-test | 1 (2.8) | |
| Change in patient medical condition | 1 (2.8) | |
| Missed | 1 (2.8) | |
| AKPS Score | ||
| Completed | 26 (72.2) | 100, n (%) = 0 (0) 90, n (%) = 1 (3.8) 80, n (%) = 3 (11.5) 70, n (%) =3 (11.5) 60, n (%) = 11 (42.3) 50, n (%) = 3 (11.5) 40, n (%) = 4 (15.4) 30, n (%) = 1 (3.8) ≤20, n (%) = 0 (0) |
| Not completed: | 10 (27.8) | |
| Missed | 10 (27.8) |
| Clinical Characteristics | Participants on the Sarc-Pathway |
|---|---|
| Sarcopenia risk (n = 159): | |
| At risk or probable sarcopenia * | 48 (30.2) |
| Not at risk | 111 (69.8) |
| Sarcopenia diagnosis * (n = 26): | |
| Yes | 14 (8.8) |
| No | 22 (13.8) |
| Malnutrition risk (n = 159): | |
| At risk (MST ≥ 2) | 62 (39.0) |
| Not at risk (MST < 2) | 97 (61.0) |
| Malnutrition diagnosis (n = 26): | |
| Yes (PG-SGA category B or C) | 20 (12.6) |
| No (PG-SGA category A) | 6 (3.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loeliger, J.; Edbrooke, L.; Daly, R.M.; Stewart, J.; Bucci, L.; Puskas, C.; Fitzgerald, M.; Baguley, B.J.; Kiss, N. Development and Feasibility of an Inpatient Cancer-Related Sarcopenia Pathway at a Major Cancer Centre. Int. J. Environ. Res. Public Health 2022, 19, 4038. https://doi.org/10.3390/ijerph19074038
Loeliger J, Edbrooke L, Daly RM, Stewart J, Bucci L, Puskas C, Fitzgerald M, Baguley BJ, Kiss N. Development and Feasibility of an Inpatient Cancer-Related Sarcopenia Pathway at a Major Cancer Centre. International Journal of Environmental Research and Public Health. 2022; 19(7):4038. https://doi.org/10.3390/ijerph19074038
Chicago/Turabian StyleLoeliger, Jenelle, Lara Edbrooke, Robin M. Daly, Jane Stewart, Lucy Bucci, Carmen Puskas, Marnie Fitzgerald, Brenton J. Baguley, and Nicole Kiss. 2022. "Development and Feasibility of an Inpatient Cancer-Related Sarcopenia Pathway at a Major Cancer Centre" International Journal of Environmental Research and Public Health 19, no. 7: 4038. https://doi.org/10.3390/ijerph19074038






