Comparison of Bioelectrical Impedance Analysis with DXA in Adolescents with Cystic Fibrosis before and after a Resistance Training Intervention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Anthropometric Measurements
2.3. Body Composition Assessments
2.3.1. Bioelectrical Impedance Analysis
2.3.2. Dual Energy X-ray Absorptiometry
2.4. Resistance Exercise Training Intervention
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Body Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scotet, V.; L’Hostis, C.; Férec, C. The changing epidemiology of cystic fibrosis: Incidence, survival and impact of the cftr gene discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Ode, K.L.; Moran, A. New insights into cystic fibrosis-related diabetes in children. Lancet Diabetes Endocrinol. 2013, 1, 52–58. [Google Scholar] [CrossRef]
- O’Riordan, S.M.P.; Dattani, M.T.; Hindmarsh, P.C. Cystic Fibrosis-Related Diabetes in Childhood. Horm. Res. Paediatr. 2010, 73, 15–24. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, S.M.; Robinson, P.D.; Donaghue, K.C.; Moran, A. Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr. Diabetes 2009, 10, 43–50. [Google Scholar] [CrossRef]
- Hameed, S.; Morton, J.R.; Jaffé, A.; Field, P.I.; Belessis, Y.; Yoong, T.; Katz, T.; Verge, C.F. Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care 2010, 33, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Limoli, D.H.; Yang, J.; Khansaheb, M.K.; Helfman, B.; Peng, L.; Stecenko, A.A.; Goldberg, J.B. Staphylococcus aureus and pseudomonas aeruginosa co-infection is associated with cystic fibrosis-related diabetes and poor clinical outcomes. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 947–953. [Google Scholar] [CrossRef]
- Shoemaker, M.J.; Hurt, H.; Arndt, L. The evidence regarding exercise training in the management of cystic fibrosis: A systematic review. Cardiopulm. Phys. Ther. J. 2008, 19, 75–83. [Google Scholar] [CrossRef]
- Holmes, C.J.; Granados, A. Resistance Training Recommendations for Children and Adolescents With Cystic Fibrosis–Related Diabetes. Strength Cond. J. 2021, 44, 111–118. [Google Scholar] [CrossRef]
- Waller, M. Strength and conditioning for the person with cystic fibrosis. Strength Cond. J. 2001, 23, 37. [Google Scholar] [CrossRef]
- Yang, Z.; Scott, C.A.; Mao, C.; Tang, J.; Farmer, A.J. Resistance exercise versus aerobic exercise for type 2 diabetes: A systematic review and meta-analysis. Sports Med. 2014, 44, 487–499. [Google Scholar] [CrossRef]
- Ishiguro, H.; Kodama, S.; Horikawa, C.; Fujihara, K.; Hirose, A.S.; Hirasawa, R.; Yachi, Y.; Ohara, N.; Shimano, H.; Hanyu, O.; et al. In search of the ideal resistance training program to improve glycemic control and its indication for patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Sports Med. 2016, 46, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.H.; Goncalves, R.L.S.; Madiraju, A.K.; Strasser, B.; Sparks, L.M. Resistance training to improve type 2 diabetes: Working toward a prescription for the future. Nutr. Metab. 2017, 14, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, N.; Bunout, D.; Hirsch, S.; Barrera, G.; Leiva, L.; Henríquez, S.; De la Maza, M.P. Premature loss of muscle mass and function in type 2 diabetes. Diabetes Res. Clin. Pract. 2016, 117, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Julian, V.; Blondel, R.; Pereira, B.; Thivel, D.; Boirie, Y.; Duclos, M. Body composition is altered in pre-diabetic patients with impaired fasting glucose tolerance: Results from the nhanes survey. J. Clin. Med. Res. 2017, 9, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Srikanthan, P.; Karlamangla, A.S. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef] [Green Version]
- Stallings, V.A.; Stark, L.J.; Robinson, K.A.; Feranchak, A.P.; Quinton, H. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: Results of a systematic review. J. Am. Diet. Assoc. 2008, 108, 832–839. [Google Scholar] [CrossRef]
- Enright, S.; Chatham, K.; Ionescu, A.A.; Unnithan, V.B.; Shale, D.J. The influence of body composition on respiratory muscle, lung function and diaphragm thickness in adults with cystic fibrosis. J. Cyst. Fibros. 2007, 6, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Calella, P.; Valerio, G.; Brodlie, M.; Donini, L.M.; Siervo, M. Cystic fibrosis, body composition, and health outcomes: A systematic review. Nutrition 2018, 55–56, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Holmes, C.J.; Racette, S.B. The utility of body composition assessment in nutrition and clinical practice: An overview of current methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Pietrobelli, A.; Formica, C.; Wang, Z.; Heymsfield, S.B. Dual-energy X-ray absorptiometry body composition model: Review of physical concepts. Am. J. Physiol. 1996, 271 Pt 1, E941–E951. [Google Scholar] [CrossRef]
- Liao, Y.-S.; Li, H.-C.; Lu, H.-K.; Lai, C.-L.; Wang, Y.-S.; Hsieh, K.-C. Comparison of bioelectrical impedance analysis and dual energy X-ray absorptiometry for total and segmental bone mineral content with a three-compartment model. Int. J. Environ. Res. Public Health 2020, 17, 2595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.R. Body composition in athletes and sports nutrition: An examination of the bioimpedance analysis technique. Eur. J. Clin. Nutr. 2013, 67 (Suppl. S1), S54–S59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forslund, A.H.; Johansson, A.G.; Sjödin, A.; Bryding, G.; Ljunghall, S.; Hambraeus, L. Evaluation of modified multicompartment models to calculate body composition in healthy males. Am. J. Clin. Nutr. 1996, 63, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Desimone, M.E.; Sherwood, J.; Soltman, S.C.; Moran, A. Telemedicine in cystic fibrosis. J. Clin. Transl. Endocrinol. 2021, 26, 100270. [Google Scholar] [CrossRef]
- CDC. Radiation in Healthcare: Bone Density (DEXA Scan). Available online: https://www.cdc.gov/nceh/radiation/dexa-scan.html (accessed on 11 March 2022).
- RadiologyInfo.org. Bone Density Scan (DEXA or DXA). Available online: https://www.radiologyinfo.org/en/info/dexa#4e5fc56c0c574037b4a97daae263b622 (accessed on 11 March 2022).
- Chula de Castro, J.A.; Lima, T.R.; Silva, D.A.S. Body composition estimation in children and adolescents by bioelectrical impedance analysis: A systematic review. J. Bodyw. Mov. Ther. 2018, 22, 134–146. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gomez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Gába, A.; Kapuš, O.; Cuberek, R.; Botek, M. Comparison of multi- and single-frequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of body composition in post-menopausal women: Effects of body mass index and accelerometer-determined physical activity. J. Hum. Nutr. Diet. 2015, 28, 390–400. [Google Scholar] [CrossRef]
- Chen, K.T.; Chen, Y.Y.; Wang, C.W.; Chuang, C.L.; Chiang, L.M.; Lai, C.L.; Lu, H.K.; Dwyer, G.B.; Chao, S.P.; Shih, M.K.; et al. Comparison of standing posture bioelectrical impedance analysis with dxa for body composition in a large, healthy chinese population. PLoS ONE 2016, 11, e0160105. [Google Scholar] [CrossRef] [Green Version]
- Thomson, R.; Brinkworth, G.D.; Buckley, J.D.; Noakes, M.; Clifton, P.M. Good agreement between bioelectrical impedance and dual-energy X-ray absorptiometry for estimating changes in body composition during weight loss in overweight young women. Clin. Nutr. 2007, 26, 771–777. [Google Scholar] [CrossRef]
- Yamada, Y.; Watanabe, Y.; Ikenaga, M.; Yokoyama, K.; Yoshida, T.; Morimoto, T.; Kimura, M. Comparison of single- or multifrequency bioelectrical impedance analysis and spectroscopy for assessment of appendicular skeletal muscle in the elderly. J. Appl. Physiol. 2013, 115, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Charatsi, A.M.; Dusser, P.; Freund, R.; Maruani, G.; Rossin, H.; Boulier, A.; Le Bourgeois, M.; Chedevergne, F.; de Blic, J.; Letourneur, A.; et al. Bioelectrical impedance in young patients with cystic fibrosis: Validation of a specific equation and clinical relevance. J. Cyst. Fibros. 2016, 15, 825–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, S.; Wilson, J.; Kotsimbos, T.; Bailey, M.; Nyulasi, I. Body composition assessment in adults with cystic fibrosis: Comparison of dual-energy X-ray absorptiometry with skinfolds and bioelectrical impedance analysis. Nutrition 2005, 21, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Ziai, S.; Coriati, A.; Chabot, K.; Mailhot, M.; Richter, M.V.; Rabasa-Lhoret, R. Agreement of bioelectric impedance analysis and dual-energy X-ray absorptiometry for body composition evaluation in adults with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 585–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, C.J.; Racette, S.B.; Symonds, L.; Arbeláez, A.M.; Cao, C.; Granados, A. Feasibility and Efficacy of Telehealth-Based Resistance Exercise Training in Adolescents with Cystic Fibrosis and Glucose Intolerance. Int. J. Environ. Res. Public Health 2022, 19, 3297. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-W.; Liao, Y.-S.; Lu, H.-K.; Hsiao, P.-L.; Chen, Y.-Y.; Chi, C.-C.; Hsieh, K.-C. Validation of two portable bioelectrical impedance analyses for the assessment of body composition in school age children. PLoS ONE 2017, 12, e0171568. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hui, S.S.-C. Validity of Four Commercial Bioelectrical Impedance Scales in Measuring Body Fat among Chinese Children and Adolescents. BioMed Res. Int. 2015, 201, 614858. [Google Scholar] [CrossRef] [Green Version]
- Brantlov, S.; Jødal, L.; Lange, A.; Rittig, S.; Ward, L.C. Standardisation of bioelectrical impedance analysis for the estimation of body composition in healthy paediatric populations: A systematic review. J. Med. Eng. Technol. 2017, 41, 460–479. [Google Scholar] [CrossRef]
- Heyward, V.H.; Wagner, D.R. Applied Body Composition Assessment; Human Kinetics: Champaign, IL, USA, 2004. [Google Scholar]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef] [Green Version]
- Segal, K.R.; Van Loan, M.; Fitzgerald, P.I.; Hodgdon, J.A.; Van Itallie, T.B. Lean body mass estimation by bioelectrical impedance analysis: A four-site cross-validation study. Am. J. Clin. Nutr. 1988, 47, 7–14. [Google Scholar] [CrossRef]
- Lands, L.C.; Gordon, C.; Bar-Or, O.; Blimkie, C.J.; Hanning, R.M.; Jones, N.L.; Moss, L.A.; Webber, C.E.; Wilson, W.M.; Heigenhauser, G.J. Comparison of three techniques for body composition analysis in cystic fibrosis. J. Appl. Physiol. 1993, 75, 162–166. [Google Scholar] [CrossRef]
- Boneva-Asiova, Z.; Boyanov, M.A. Body composition analysis by leg-to-leg bioelectrical impedance and dual-energy X-ray absorptiometry in non-obese and obese individuals. Diabetes Obes. Metab. 2008, 10, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- de Castro, J.A.C.; de Lima, L.R.A.; Silva, D.A.S. Accuracy of octa-polar bioelectrical impedance analysis for the assessment of total and appendicular body composition in children and adolescents with HIV: Comparison with dual energy X-ray absorptiometry and air displacement plethysmography. J. Hum. Nutr. Diet. 2018, 31, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.N.; Krustrup, P.; Araújo Póvoas, S.C.; Castagna, C. Accuracy and reliability of the InBody 270 multi-frequency body composition analyser in 10-12-year-old children. PLoS ONE 2021, 16, e0247362. [Google Scholar] [CrossRef] [PubMed]
- Völgyi, E.; Tylavsky, F.A.; Lyytikäinen, A.; Suominen, H.; Alén, M.; Cheng, S. Assessing body composition with DXA and bioimpedance: Effects of obesity, physical activity, and age. Obesity 2008, 16, 700–705. [Google Scholar] [CrossRef]
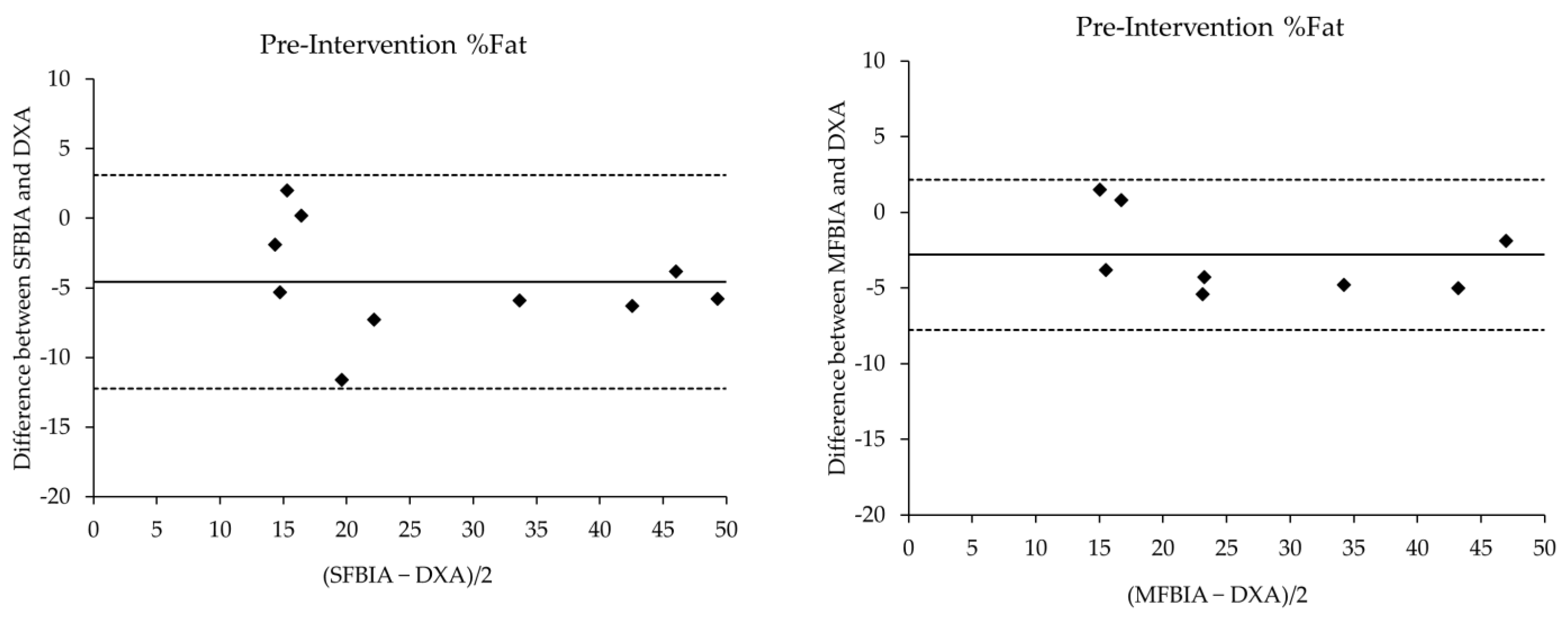
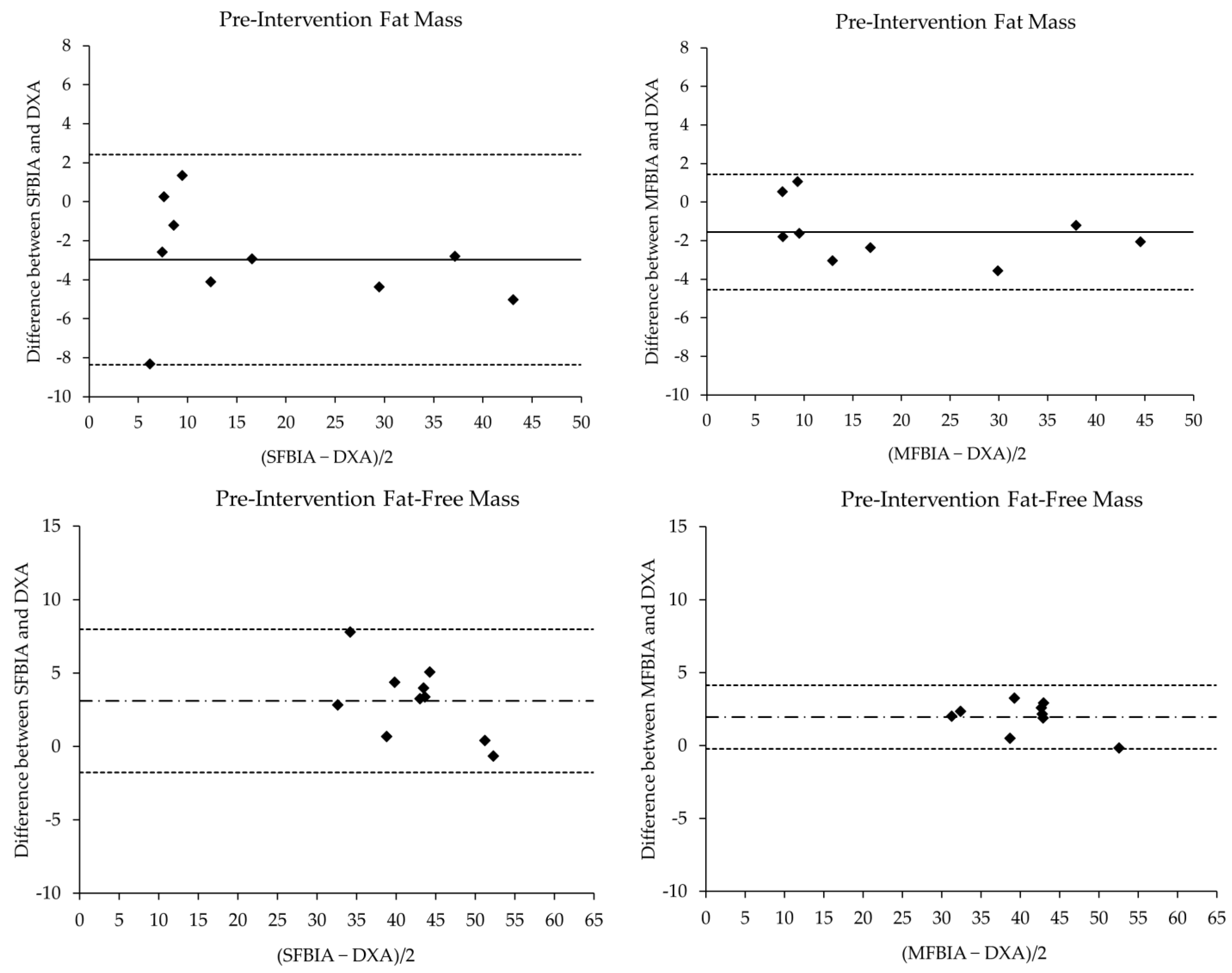
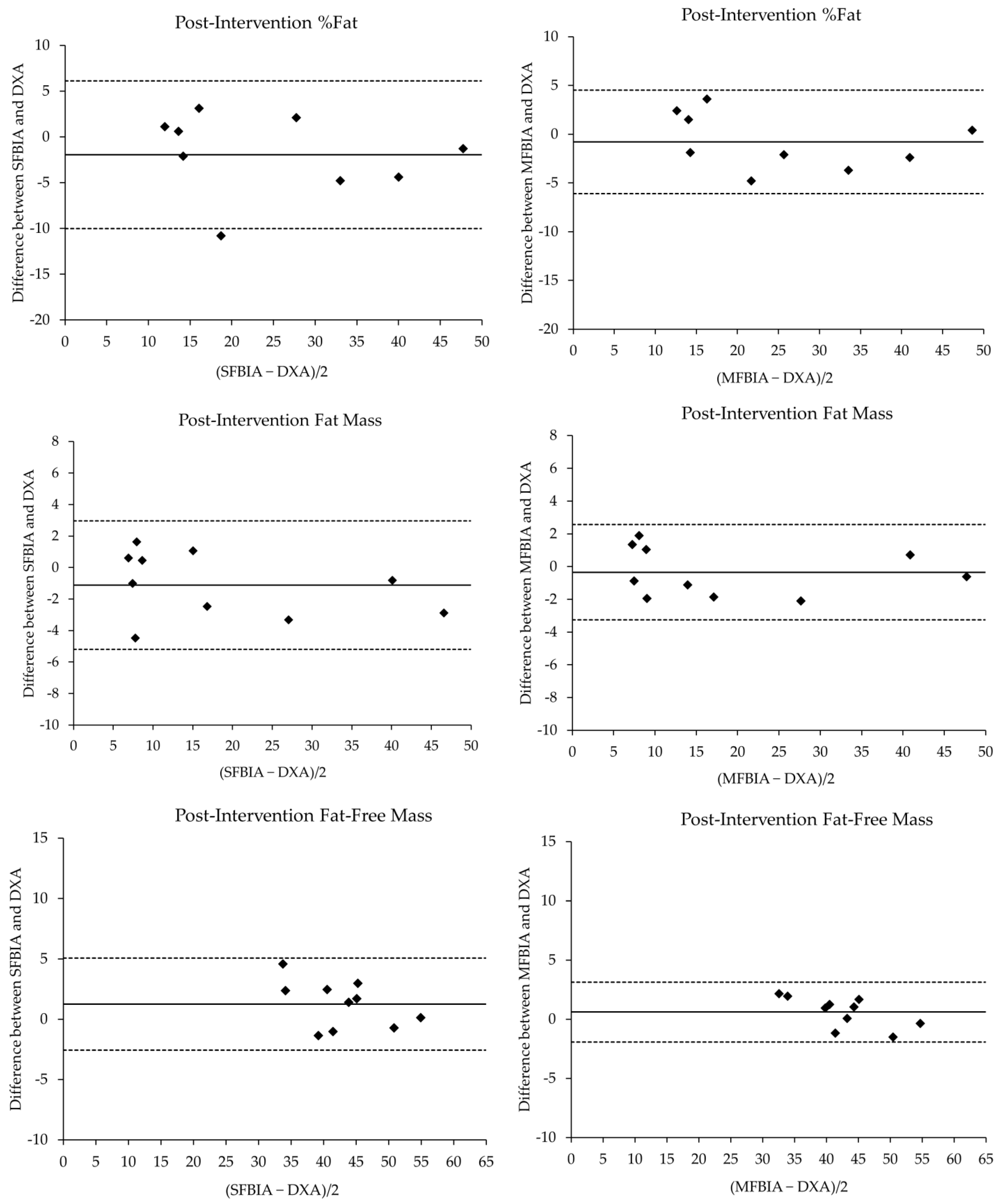
| Pre-Intervention | N | Mean ± SD | p-Value | Effect Size | r | SEE | CE ± 1.96SD | 95% LOA | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||||
| %Fat Total | ||||||||||
| DXA | 9 | 31.29 ± 14.66 | --- | --- | --- | --- | --- | --- | --- | --- |
| SFBIA | 9 | 26.42 ± 13.81 * | 0.01 | 0.34 | Moderate | 0.97 * | 3.81 | −4.57 ± 1.14 | −12.32 | 3.18 |
| MFBIA | 9 | 28.48 ± 13.93 * | 0.01 | 0.20 | Moderate | 0.99 * | 2.52 | −2.81 ± 0.74 | −7.80 | 2.18 |
| FM (kg) | ||||||||||
| DXA | 9 | 19.27 ± 14.08 | --- | --- | --- | --- | --- | --- | --- | --- |
| SFBIA | 9 | 16.30 ± 13.39 * | 0.01 | 0.21 | Moderate | 0.98 * | 2.74 | −2.97 ± 0.80 | −8.38 | 2.44 |
| MFBIA | 9 | 18.83 ± 13.90 * | 0.02 | 0.03 | Small | 1.00 * | 1.49 | −1.56 ± 0.44 | −4.54 | 1.42 |
| FFM (kg) | ||||||||||
| DXA | 9 | 40.77 ± 7.19 | --- | --- | --- | --- | --- | --- | --- | --- |
| SFBIA | 9 | 43.89 ± 5.70 * | <0.01 | 0.48 | Moderate | 0.95 * | 1.86 | 3.12 ± 0.73 | −1.86 | 8.10 |
| MFBIA | 9 | 41.59 ± 6.13 * | <0.01 | 0.12 | Small | 0.99 * | 1.03 | 1.96 ± 0.35 | −0.43 | 4.34 |
| Post-Intervention | ||||||||||
| %Fat Total | ||||||||||
| DXA | 10 | 28.35 ± 15.34 | --- | --- | --- | --- | --- | --- | --- | --- |
| SFBIA | 10 | 26.38 ± 14.42 | 0.16 | 0.13 | Small | 0.96 * | 4.08 | −1.97 ± 1.14 | −9.70 | 5.76 |
| MFBIA | 10 | 27.56 ± 14.56 | 0.38 | 0.05 | Small | 0.99 * | 2.68 | −0.79 ± 0.75 | −5.85 | 4.27 |
| FM (kg) | ||||||||||
| DXA | 10 | 18.99 ± 15.02 | --- | --- | --- | --- | --- | --- | --- | --- |
| SFBIA | 10 | 17.87 ± 14.28 | 0.12 | 0.08 | Small | 0.99 * | 2.01 | −1.12 ± 0.58 | −5.04 | 2.80 |
| MFBIA | 10 | 18.64 ± 14.80 | 0.48 | 0.02 | Small | 1.00 * | 1.54 | −0.35 ± 0.41 | −3.12 | 2.42 |
| FFM (kg) | ||||||||||
| DXA | 10 | 42.30 ± 7.18 | --- | --- | --- | --- | --- | --- | --- | --- |
| SFBIA | 10 | 43.55 ± 6.29 | 0.07 | 0.19 | Small | 0.97 * | 1.71 | 1.26 ± 0.54 | −2.44 | 4.95 |
| MFBIA | 10 | 42.90 ± 6.31 | 0.17 | 0.09 | Small | 0.99 * | 0.95 | 0.60 ± 0.36 | −1.82 | 3.03 |
| Mean ± SD | Pre-Post Differences | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Pre | Post | Mean Difference | Effect Size | SD | SEM | 95% CI Diff | p-Value | |||
| Lower | Upper | ||||||||||
| DXA | |||||||||||
| %Fat | 10 | 29.69 ± 14.72 | 28.35 ± 15.34 | −1.34 * | 0.09 | Small | 1.59 | 0.50 | 0.20 | 2.48 | 0.03 |
| FM (kg) | 10 | 19.27 ± 14.08 | 18.99 ± 15.02 | −0.29 | 0.02 | Small | 1.68 | 0.53 | −0.92 | 1.49 | 0.60 |
| FFM (kg) | 10 | 40.77 ± 7.19 | 42.30 ± 7.18 | 1.53 * | 0.21 | Moderate | 1.43 | 0.45 | −2.55 | −0.50 | 0.01 |
| SFBIA | |||||||||||
| %Fat | 10 | 25.12 ± 13.66 | 26.38 ± 14.42 | 1.26 | 0.09 | Small | 3.62 | 1.15 | −3.85 | 1.33 | 0.30 |
| FM (kg) | 10 | 16.30 ± 13.39 | 17.87 ± 14.28 | 1.57 | 0.11 | Small | 2.58 | 0.81 | −3.41 | 0.28 | 0.09 |
| FFM (kg) | 10 | 43.89 ± 5.70 | 43.55 ± 6.29 | −0.34 | 0.05 | Small | 2.76 | 0.87 | −1.63 | 2.32 | 0.71 |
| MFBIA | |||||||||||
| %Fat | 9 | 28.48 ± 13.93 | 29.09 ± 14.57 | 0.61 | 0.04 | Small | 1.96 | 0.65 | −2.12 | 0.90 | 0.38 |
| FM (kg) | 9 | 18.83 ± 13.90 | 19.83 ± 15.19 | 1.00 | 0.07 | Small | 1.91 | 0.64 | −2.47 | 0.48 | 0.16 |
| FFM (kg) | 9 | 41.59 ± 6.13 | 42.14 ± 6.20 | 0.55 | 0.09 | Small | 1.76 | 0.59 | −1.91 | 0.80 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmes, C.J.; Racette, S.B.; Symonds, L.; Arbeláez, A.M.; Cao, C.; Granados, A. Comparison of Bioelectrical Impedance Analysis with DXA in Adolescents with Cystic Fibrosis before and after a Resistance Training Intervention. Int. J. Environ. Res. Public Health 2022, 19, 4037. https://doi.org/10.3390/ijerph19074037
Holmes CJ, Racette SB, Symonds L, Arbeláez AM, Cao C, Granados A. Comparison of Bioelectrical Impedance Analysis with DXA in Adolescents with Cystic Fibrosis before and after a Resistance Training Intervention. International Journal of Environmental Research and Public Health. 2022; 19(7):4037. https://doi.org/10.3390/ijerph19074037
Chicago/Turabian StyleHolmes, Clifton J., Susan B. Racette, Leslie Symonds, Ana Maria Arbeláez, Chao Cao, and Andrea Granados. 2022. "Comparison of Bioelectrical Impedance Analysis with DXA in Adolescents with Cystic Fibrosis before and after a Resistance Training Intervention" International Journal of Environmental Research and Public Health 19, no. 7: 4037. https://doi.org/10.3390/ijerph19074037
APA StyleHolmes, C. J., Racette, S. B., Symonds, L., Arbeláez, A. M., Cao, C., & Granados, A. (2022). Comparison of Bioelectrical Impedance Analysis with DXA in Adolescents with Cystic Fibrosis before and after a Resistance Training Intervention. International Journal of Environmental Research and Public Health, 19(7), 4037. https://doi.org/10.3390/ijerph19074037







