Interventions to Improve Treatment Outcomes among Adolescents on Antiretroviral Therapy with Unsuppressed Viral Loads: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Selection and Data Extraction
2.2. Quality Assessment and Risk of Bias
2.3. Data Synthesis and Analysis
3. Results
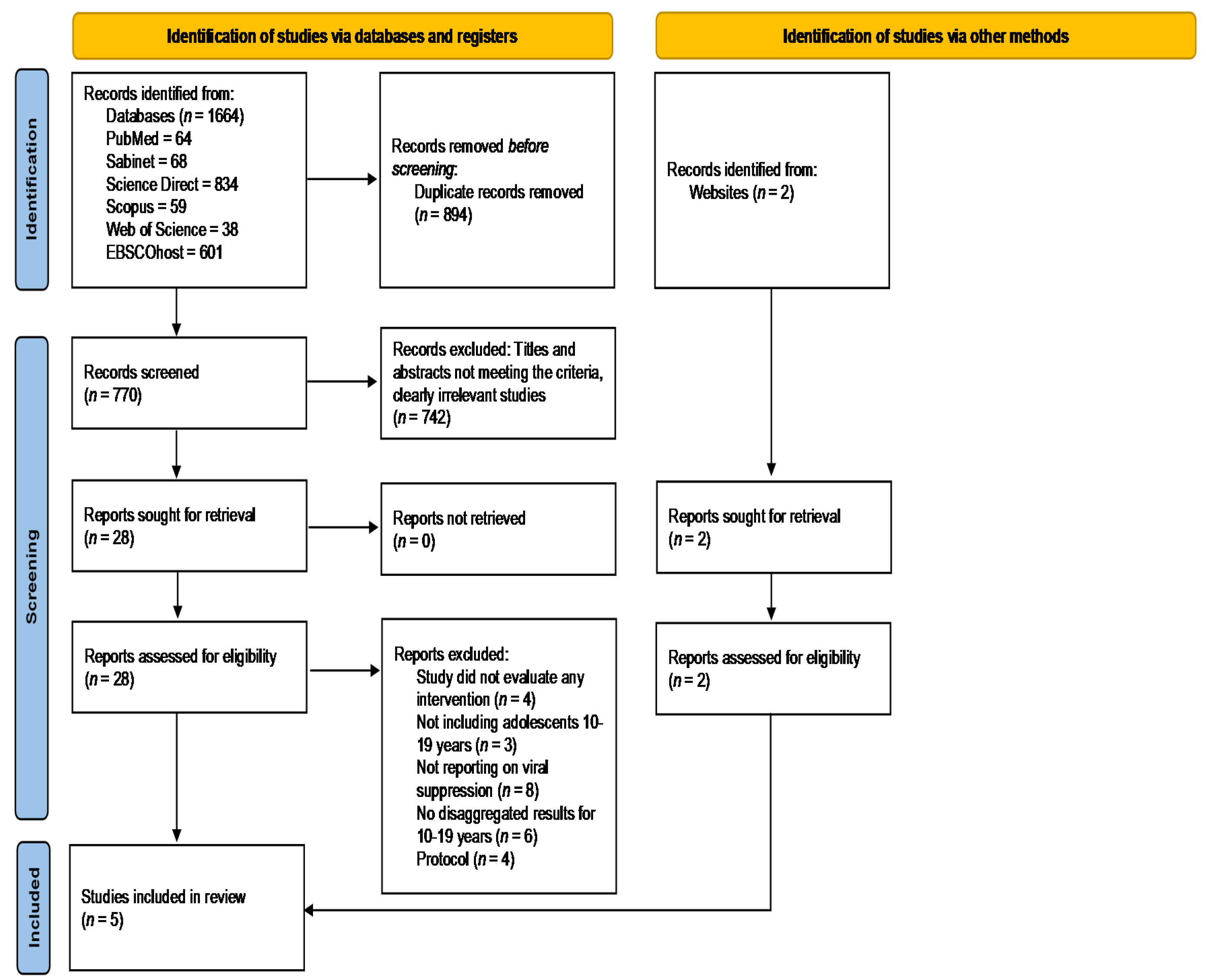
3.1. Identification of Relevant Studies
3.2. Study Characteristics
3.3. Description of Interventions
3.4. Primary Outcomes of Interventions
3.5. Secondary Outcomes
3.6. Quality Assessement of Studies
3.7. GRADE Recommendations
4. Discussion
4.1. Intensified Adherence Counselling
4.2. Peer-Led Multicomponent Differentiated Service Delivery
4.3. Family-Based Economic Empowerment
4.4. Conditional Economic Incentives and Motivational Interviewing
4.5. Strengths and Limitations of the Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNICEF. Adolescent HIV Prevention—UNICEF DATA. Available online: https://data.unicef.org/topic/hivaids/adolescents-young-people/ (accessed on 10 September 2020).
- UNAIDS. UNAIDS Data. Available online: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf (accessed on 6 May 2020).
- WHO. WHO|Definition of Key Terms. Available online: http://www.who.int/hiv/pub/guidelines/arv2013/intro/keyterms/en/ (accessed on 11 October 2016).
- Bekker, L.G.; Siberry, G.K.; Hirnschall, G. Ensuring Children and Adolescents Are Not Left Behind. Available online: https://journals.lww.com/jaids (accessed on 6 May 2020).
- UNICEF. HIV and AIDS in Adolescents—UNICEF Data. Available online: https://data.unicef.org/topic/adolescents/hiv-aids/ (accessed on 11 December 2020).
- Sherr, L.; Cluver, L.D.; Toska, E.; He, E. Differing psychological vulnerabilities among behaviourally and perinatally HIV infected adolescents in South Africa–implications for targeted health service provision. AIDS Care 2018, 30, 92–101. [Google Scholar] [CrossRef]
- UNAIDS. Ending the AIDS Epidemic for Adolescents, with Adolescents: A Practical Guide to Meaningfully Engage Adolescents in the AIDS Response; UNAIDS: Geneva, Switzerland, 2016. [Google Scholar]
- UNAIDS. Global HIV & AIDS Statistics—2020 Fact Sheet|UNAIDS. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 20 December 2012).
- Ssali, L.; Kalibala, S.; Birungi, J.; Egessa, A.; Wangisi, J.; Okullu, J.L.; Bakanda, C.; Okoboi, S.; Obare, F. Retention of Adolescents Living with HIV in Care, Treatment, and Support Programs in Uganda. Available online: http://www.hivcore.org/Pubs/Uganda_AdolHAART_Rprt.pdf (accessed on 27 February 2018).
- WHO. Adolescent-Friendly Health Services for Adolescents Living with HIV: From Theory to Practice Technical Brief Peer Driven Adolescent HIV Models of Care. Available online: http://apps.who.int/bookorders (accessed on 10 January 2022).
- Zanoni, B.C.; Sibaya, T.; Cairns, C.; Haberer, J.E. Barriers to Retention in Care are Overcome by Adolescent-Friendly Services for Adolescents Living with HIV in South Africa: A Qualitative Analysis. AIDS Behav. 2018, 23, 957–965. [Google Scholar] [CrossRef]
- Grimsrud, A.T.; Pike, C.; Bekker, L.G. The power of peers and community in the continuum of HIV care. Lancet Glob. Health 2020, 8, e167–e168. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Gerver, S.M.; Fidler, S.; Ward, H. Adherence to antiretroviral therapy in adolescents living with HIV: Systematic review and meta-analysis. AIDS 2014, 28, 1945–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nachega, J.B.; Mills, E.J.; Schechter, M. Antiretroviral therapy adherence and retention in care in middle-income and low-income countries: Current status of knowledge and research priorities. Curr. Opin. HIV AIDS 2010, 5, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Ridgeway, K.; Dulli, L.S.; Murray, K.R.; Silverstein, H.; Dal Santo, L.; Olsen, P.; Darrow de Mora, D.; McCarraher, D.R. Interventions to improve antiretroviral therapy adherence among adolescents in low- and middle-income countries: A systematic review of the literature. PLoS ONE 2018, 13, e0189770. [Google Scholar] [CrossRef] [Green Version]
- Casale, M.; Carlqvist, A.; Cluver, L. Recent Interventions to Improve Retention in HIV Care and Adherence to Antiretroviral Treatment among Adolescents and Youth: A Systematic Review. AIDS Patient Care STDS 2019, 33, 237–252. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, P.; Munthali, C.; Ferguson, J.; Armstrong, A.; Kranzer, K.; Ferrand, R.A.; Ross, D.A. Service delivery interventions to improve adolescents’ linkage, retention and adherence to antiretroviral therapy and HIVcare. Trop. Med. Int. Health 2015, 20, 1015–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reif, L.K.; Abrams, E.J.; Arpadi, S.; Elul, B.; McNairy, M.L.; Fitzgerald, D.W.; Kuhn, L. Interventions to Improve Antiretroviral Therapy Adherence Among Adolescents and Youth in Low- and Middle-Income Countries: A Systematic Review 2015–2019. AIDS Behav. 2020, 24, 2797–2810. [Google Scholar] [CrossRef] [Green Version]
- Munyayi, F.K.; van Wyk, B. Interventions for improving treatment outcomes in adolescents on antiretroviral therapy with unsuppressed viral loads: A systematic review protocol. BMJ Open 2021, 11, e049452. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst. Rev. 2015, 4, 148–160. [Google Scholar] [CrossRef] [Green Version]
- Price, A. Mendeley and More for Systematic Reviews. Available online: http://www.ithinkwell.org/mendeley-and-more-for-systematic-reviews/ (accessed on 3 November 2020).
- Egger, M.; Smith, G.D. Principles of and Procedures for Systematic Reviews. In Systematic Reviews in Health Care: Meta-Analysis in Context, 2nd ed.; Egger, M., Smith, G.D., Altman, D.G., Eds.; BMJ Publishing Group: London, UK, 2008; pp. 23–42. [Google Scholar]
- Haghighat, R.; Steinert, J.; Cluver, L. The effects of decentralising antiretroviral therapy care delivery on health outcomes for adolescents and young adults in low- and middle-income countries: A systematic review. Glob. Health Action 2019, 12, 1668596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mearns, H.; Otiku, P.K.; Shelton, M.; Kredo, T.; Kagina, B.M.; Schmidt, B.-M. Screening strategies for adults with type 2 diabetes mellitus: A systematic review protocol. Syst. Rev. 2020, 9, 156. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Izcovich, A.; Rochwerg, B.; Florez, I.D.; Hazlewood, G.; Alhazanni, W.; Yepes-Nuñez, J.; Santesso, N.; Guyatt, G.H.; Schünemann, H.J. GRADE approach to drawing conclusions from a network meta-analysis using a partially contextualised framework. BMJ 2020, 371, m3907. [Google Scholar] [CrossRef] [PubMed]
- Ekwunife, O.I.; Ofomata, C.J.; Okafor, C.E.; Anetoh, M.U.; Kalu, S.O.; Ele, P.U.; Eleje, G.U. Cost-effectiveness and feasibility of conditional economic incentives and motivational interviewing to improve HIV health outcomes of adolescents living with HIV in Anambra State, Nigeria. BMC Health Serv. Res. 2021, 21, 685. [Google Scholar] [CrossRef]
- Ndhlovu, C.E.; Kouamou, V.; Nyamayaro, P.; Dougherty, L.; Willis, N.; Ojikutu, B.O.; Makadzange, A.T. The transient effect of a peer support intervention to improve adherence among adolescents and young adults failing antiretroviral therapy in Harare, Zimbabwe: A randomized control trial. AIDS Res. Ther. 2021, 18, 32. [Google Scholar] [CrossRef]
- Mavhu, W.; Willis, N.; Mufuka, J.; Bernays, S.; Tshuma, M.; Mangenah, C.; Maheswaran, H.; Mangezi, W.; Apollo, T.; Araya, R.; et al. Effect of a differentiated service delivery model on virological failure in adolescents with HIV in Zimbabwe (Zvandiri): A cluster-randomised controlled trial. Lancet Glob. Health 2020, 8, e264–e275. [Google Scholar] [CrossRef] [Green Version]
- Nasuuna, E.; Kigozi, J.; Babirye, L.; Muganzi, A.; Sewankambo, N.K.; Nakanjako, D. Low HIV viral suppression rates following the intensive adherence counseling (IAC) program for children and adolescents with viral failure in public health facilities in Uganda. BMC Public Health 2018, 18, 1048. [Google Scholar] [CrossRef]
- Ssewamala, F.M.; Dvalishvili, D.; Mellins, C.A.; Geng, E.H.; Makumbi, F.; Neilands, T.B.; McKay, M.; Damulira, C.; Nabunya, P.; Bahar, O.S.; et al. The long-term effects of a family based economic empowerment intervention (Suubi+Adherence) on suppression of HIV viral loads among adolescents living with HIV in southern Uganda: Findings from 5-year cluster randomized trial. PLoS ONE 2020, 15, e0228370. [Google Scholar] [CrossRef] [Green Version]
- Ekwunife, O.I.; Anetoh, M.U.; Kalu, S.O.; Ele, P.U.; Eleje, G.U. Conditional economic incentives and motivational interviewing to improve adolescents’ retention in HIV care and adherence to antiretroviral therapy in Southeast Nigeria: Study protocol for a cluster randomised trial. Trials 2018, 19, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobanputra, K.; Parker, L.A.; Azih, C.; Okello, V.; Maphalala, G.; Kershberger, B.; Khogali, M.; Lujan, J.; Antierens, A.; Teck, R.; et al. Factors Associated with Virological Failure and Suppression after Enhanced Adherence Counselling, in Children, Adolescents and Adults on Antiretroviral Therapy for HIV in Swaziland. PLoS ONE 2015, 10, e0116144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernays, S.; Tshuma, M.; Willis, N.; Mvududu, K.; Chikeya, A.; Mufuka, J.; Cowan, F.; Mavhu, W. Scaling up peer-led community-based differentiated support for adolescents living with HIV: Keeping the needs of youth peer supporters in mind to sustain success. J. Int. AIDS Soc. 2020, 23, e25570. [Google Scholar] [CrossRef]
- Arnold, E.M.; Swendeman, D.; Harris, D.; Fournier, J.; Kozina, L.; Abdalian, S.; Rotheram, M.J.; Adolescent Medicine Trials Network CARES Team. The Stepped Care Intervention to Suppress Viral Load in Youth Living with HIV: Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2019, 8, e10791. [Google Scholar] [CrossRef] [PubMed]
- Crowley, T.; Rohwer, A. Self-management interventions for adolescents living with HIV: A systematic review. BMC Infect. Dis. 2021, 21, 431. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Hrapcak, S.; Ameyan, W.; Lovich, R.; Ronan, A.; Schmitz, K.; Hatane, L. Peer Support for Adolescents and Young People Living with HIV in Sub-Saharan Africa: Emerging Insights and a Methodological Agenda. Curr. HIV/AIDS Rep. 2019, 16, 467–474. [Google Scholar] [CrossRef]
- Technical Brief about Differentiated Service Delivery for Adolescents and Young Adults Living with HIV in South Africa 2019. Available online: http://teampata.org/wp-content/uploads/2019/02/DSD_Policy-Brief_2019.pdf (accessed on 25 January 2022).
- Lejone, T.I.; Kopo, M.; Bachmann, N.; Brown, J.A.; Glass, T.R.; Muhairwe, J.; Matsela, T.; Scherrer, R.; Chere, L.; Namane, T.; et al. PEBRA trial–effect of a peer-educator coordinated preference-based ART service delivery model on viral suppression among adolescents and young adults living with HIV: Protocol of a cluster-randomized clinical trial in rural Lesotho. BMC Public Health 2020, 20, 425. [Google Scholar] [CrossRef] [Green Version]
- Abelman, R.; Alons, C.; Stockman, J.; Teri, I.; Grimsrud, A.; Ombija, M.; Makwindi, C.; Odionyi, J.; Tumbare, E.; Longwe, B.; et al. Implementation of differentiated service delivery for paediatric HIV care and treatment: Opportunities, challenges and experience from seven sub-Saharan African countries. Fam. Med. Community Health 2020, 8, e000393. [Google Scholar] [CrossRef]
- Grimsrud, A.; Bygrave, H.; Wilkinson, L. The Case for Family-Centered Differentiated Service Delivery for HIV. JAIDS J. Acquir. Immune Defic. Syndr. 2018, 78, S124–S127. [Google Scholar] [CrossRef]
- Tozan, Y.; Sun, S.; Capasso, A.; Wang, J.S.-H.; Neilands, T.B.; Bahar, O.S.; Damulira, C.; Ssewamala, F. Evaluation of a savings-led family-based economic empowerment intervention for AIDS-affected adolescents in Uganda: A four-year follow-up on efficacy and cost-effectiveness. PLoS ONE 2019, 14, e0226809. [Google Scholar] [CrossRef]
- Bhana, A.; Mellins, C.A.; Petersen, I.; Alicea, S.; Myeza, N.; Holst, H.; Abrams, E.; John, S.; Chhagan, M.; Nestadt, D.F.; et al. The VUKA family program: Piloting a family-based psychosocial intervention to promote health and mental health among HIV infected early adolescents in South Africa. AIDS Care 2013, 26, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Damulira, C.; Mukasa, M.N.; Byansi, W.; Nabunya, P.; Kivumbi, A.; Namatovu, P.; Namuwonge, F.; Dvalishvili, D.; Bahar, O.S.; Ssewamala, F. Examining the relationship of social support and family cohesion on ART adherence among HIV-positive adolescents in southern Uganda: Baseline findings. Vulnerable Child. Youth Stud. 2019, 14, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Galárraga, O.; Kuo, C.; Mtukushe, B.; Maughan-Brown, B.; Harrison, A.; Hoare, J. iSAY (incentives for South African youth): Stated preferences of young people living with HIV. Soc. Sci. Med. 2020, 265, 113333. [Google Scholar] [CrossRef] [PubMed]
- Nshimyumuremyi, J.N.; Mukesharurema, G.; Uwamariya, J.; Mutunge, E.; Goodman, A.S.; Ndahimana, J.D.; Barnhart, D.A. Implementation and Adaptation of a Combined Economic Empowerment and Peer Support Program among Youth Living with HIV in Rural Rwanda. J. Int. Assoc. Provid. AIDS Care (JIAPAC) 2022, 21, 232595822110640. [Google Scholar] [CrossRef] [PubMed]
- Okonji, E.F.; Mukumbang, F.C.; Orth, Z.; Vickerman-Delport, S.A.; Van Wyk, B. Psychosocial support interventions for improved adherence and retention in ART care for young people living with HIV (10–24 years): A scoping review. BMC Public Health 2020, 20, 1841. [Google Scholar] [CrossRef] [PubMed]
- Arayasirikul, S.; Turner, C.; Trujillo, D.; Le, V.; Beltran, T.; Wilson, E.C. Does the Use of Motivational Interviewing Skills Promote Change Talk Among Young People Living with HIV in a Digital HIV Care Navigation Text Messaging Intervention? Health Promot. Pract. 2020, 21, 738–743. [Google Scholar] [CrossRef]
| First Author, Year | Study Country, Settings | Study Design | Study Population, Sample Size | Intervention | Follow-Up Period | Outcome(s) Measured | Results |
|---|---|---|---|---|---|---|---|
| Nasuuma, 2018 | Uganda, 15 Public health facilities | Retrospective Cohort | 9 months–19 years, N = 449; n = 192 adolescents (10–19 years) | Intensified Adherence Counselling:
| 19 months | Viral Suppression (<1000 copies/mL) after 3 monthly sessions | Overall viral suppression (10–19 years): 29% |
| Mavhu, 2020 | Zimbabwe, 16 PHC clinics | Cluster RCT | 13–19 years, N = 496 | Peer-led multicomponent DSD intervention (Zvandiri): Enhanced HIV care support for unsuppressed adolescents VL ≥ 1000 copies/mL through:
Standard care (adherence counselling for unsuppressed clients). | 96 weeks | Viral Suppression (<1000 copies/mL) | Risk Ratio = 1.17 (95% CI 1.04–1.32) |
| Retention in Care | Adjusted prevalence ratio of discontinuation of ART for ≥3 months = 0.68 (95% CI 0.23–1.99, p = 0.45) | ||||||
| Adherence | Adjusted prevalence ratio of attendance <80% of scheduled visits = 0.80 (95% CI 0.32–2.02, p = 0.62) | ||||||
| Ssewamala, 2020 | Uganda, 39 Healthcare clinics, 5 districts | Cluster RCT | 10–16 years, N = 702; n = 288 (adolescents with detectable VL at baseline) | Family-based economic empowerment intervention. Intervention group received:
Standard of care (SOC), medical and psychosocial support (ART and adherence information leaflets, adherence sessions facilitated by lay counsellors and expert clients who are living with HIV) | 5 years | Viral Suppression (<40 copies/mL) | Incidence Rate Ratio = 1.468 (95% CI 1.064–2.038, p = 0.008). |
| Ndhlovu, 2021 | Zimbabwe, 1 Referral Hospital, Family Care Clinic | RCT | 10–24 years, N = 212; n = 134 (63%) adolescents aged 10–19 years | Community-based peer support intervention (Zvandiri): Enhanced HIV care support for unsuppressed adolescents VL ≥ 400 copies/mL through:
Standard of care (adherence counselling for unsuppressed clients). | 36 weeks | Viral Suppression (<1000 copies/mL) | Adjusted OR = 1.14 (95% CI 0.82–1.59), p = 0.439, at week 36 |
| Self-reported Adherence (≥95%) | Intervention arm = 66.0% SOC arm = 68.9% p = 0.655, at week 36 | ||||||
| Ekwunife, (Pre-print) | Nigeria, 12 Hospitals | Cluster RCT | Adolescents (10–19 years), N = 246 | Conditional Economic Incentives and Motivational Interviewing Intervention group received:
Standard of care,
| 2 years | Viral Suppression VL < 20 copies/mL | The difference in viral suppression between intervention and control group = 11.7% |
| ROB Domain | Nasuuma et al., 2018 |
|---|---|
| Bias due to confounding | Low |
| Bias in selection of participants into the study | Low |
| Bias in classification of interventions | Low |
| Bias due to deviations from intended interventions | Moderate * |
| Bias due to missing data | Moderate * |
| Bias in measurement of outcomes | Low |
| Bias in selection of the reported result | Low |
| Overall Risk of Bias | MODERATE |
| Mavhu et al., 2020 | Ssewamala et al., 2020 | Ndhlovu et al., 2021 | Ekwunife et al., Pre-Print, 2021 | |
|---|---|---|---|---|
| Section A: Is the basic study design valid for an RCT? | ||||
| 1. Did the study address a clearly focused research question? | √ | √ | √ | √ |
| 2. Was the assignment of participants to interventions randomized? | √ | √ | √ | √ |
| 3. Were all participants who entered the study accounted for at its conclusion? | √ | √ | √ | Cannot tell |
| Section B: Was the study methodologically sound? | ||||
| 4. Were participants, investigators, assessors/analyzers “blinded”? | X | X | X | X |
| 5. Were the study groups similar at the start of the RCT? | √ | √ | √ | √ |
| 6. Apart from experimental intervention, did each group receive same level care? | √ | √ | √ | X |
| Section C: What are the results? | ||||
| 7. Were the effects of intervention reported comprehensively? | √ | √ | √ | Cannot tell |
| 8. Was the precision of the estimate of the intervention/treatment effect reported? | √ | √ | √ | X |
| 9. Do the benefits of the experimental intervention outweigh the harms/costs? | √ | √ | √ | √ |
| Section D: Will the results help locally? | ||||
| 10. Can the results be applied to your local population/in your context? | √ | √ | √ | √ |
| 11. Would the experimental intervention provide greater value to people in your care? | √ | √ | √ | √ |
| Domain | Nasuuma et al., 2018 | Mavhu et al., 2020 | Ssewamala et al., 2020 | Ndhlovu et al., 2021 | Ekwunife et al., Pre-Print, 2021 |
|---|---|---|---|---|---|
| Risk of Bias | Moderate | Low | Low | Low | High |
| Consistency | NA | NA | NA | NA | NA |
| Directness | High | High | High | High | Low |
| Imprecision | NA | Low | Moderate | Moderate | NA |
| Publication Bias | NA | NA | NA | NA | NA |
| Final Quality of Evidence | LOW d | HIGH | MODERATE a | MODERATE e | LOW o |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munyayi, F.K.; van Wyk, B.; Mayman, Y. Interventions to Improve Treatment Outcomes among Adolescents on Antiretroviral Therapy with Unsuppressed Viral Loads: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 3940. https://doi.org/10.3390/ijerph19073940
Munyayi FK, van Wyk B, Mayman Y. Interventions to Improve Treatment Outcomes among Adolescents on Antiretroviral Therapy with Unsuppressed Viral Loads: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(7):3940. https://doi.org/10.3390/ijerph19073940
Chicago/Turabian StyleMunyayi, Farai Kevin, Brian van Wyk, and Yolanda Mayman. 2022. "Interventions to Improve Treatment Outcomes among Adolescents on Antiretroviral Therapy with Unsuppressed Viral Loads: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 7: 3940. https://doi.org/10.3390/ijerph19073940
APA StyleMunyayi, F. K., van Wyk, B., & Mayman, Y. (2022). Interventions to Improve Treatment Outcomes among Adolescents on Antiretroviral Therapy with Unsuppressed Viral Loads: A Systematic Review. International Journal of Environmental Research and Public Health, 19(7), 3940. https://doi.org/10.3390/ijerph19073940







