Gingival Phenotype Changes and the Prevalence of Mucogingival Deformities during the Early Transitional Dentition Phase—A Two-Year Longitudinal Study
Abstract
:1. Introduction
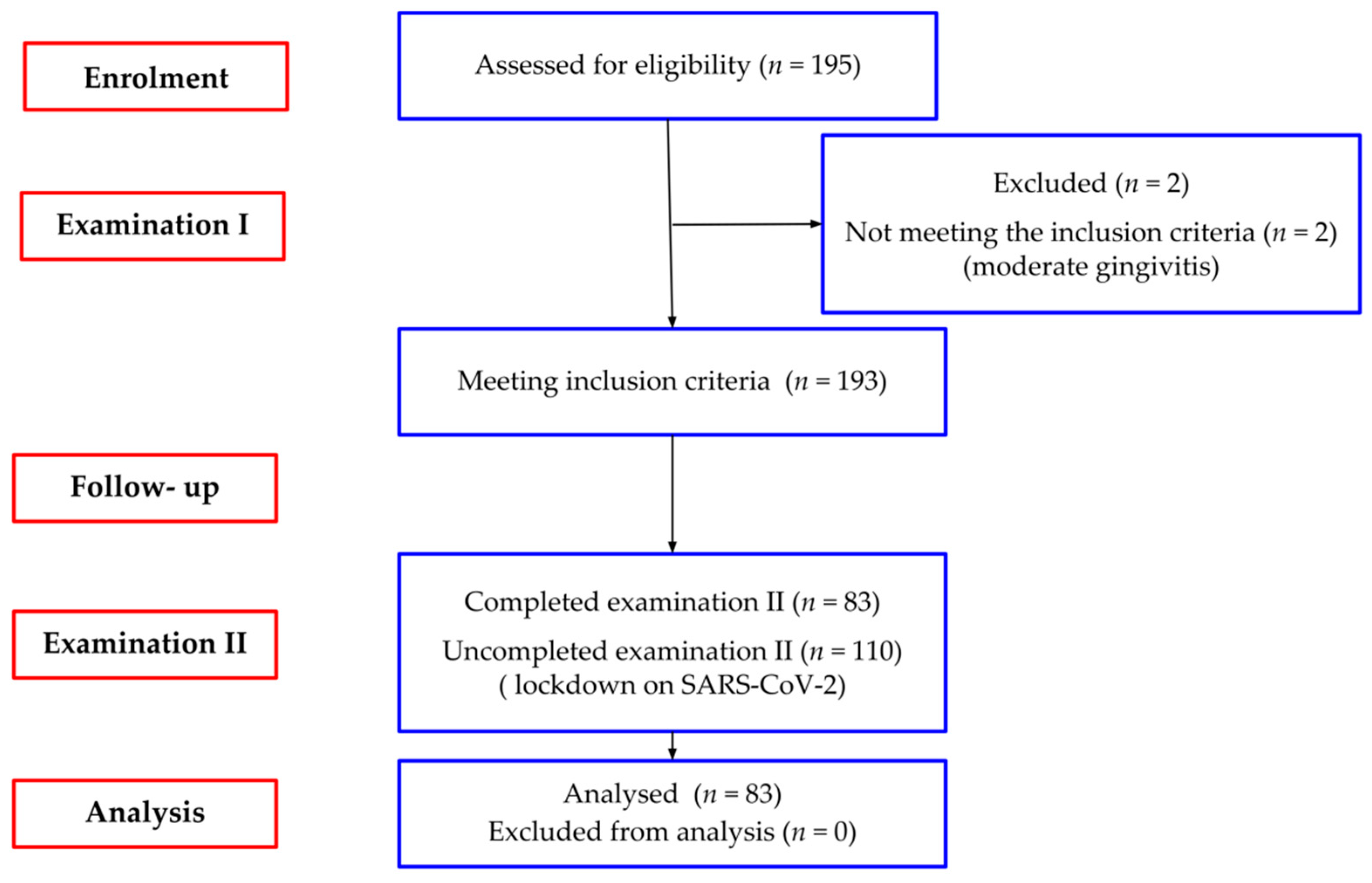
2. Materials and Methods
2.1. Study Population
2.2. Clinical Examination
- Plaque Index (Pl. I)—site specific evaluation of the presence of dental plaque according to the Silness i Löe criteria [25];
- Gingival Index (GI)—site specific assessment of the condition of the gingiva according to the Löe i Silness criteria [26];
- probing depth (PD)—at the mid-buccal aspect of the examined tooth from the gingival margin to the bottom of the gingival sulcus;
- keratinized tissue width (KTW)—at the mid-buccal aspect of the examined tooth from the gingival margin to the mucogingival junction using wrinkle technique [27];
- attached gingiva width (AGW)—subtracting the PD from the KTW;
- vestibule depth (VD—at the central lower incisor, from the gingival margin to the greatest concavity of the mucolabial fold reduced by the PD.
- thickness of attached gingiva (GT) was measured by PIROP ultrasonic biometer (Echo-Son, Puławy, Poland) with a fine transducer head (1.7 mm) (device frequency-20 MHz, ultrasonic impulse velocity-1540 m/s, accuracy up to 0.01 mm) [28]. The measurement at each point (halfway between muco-gingival junction and free gingival groove) was taken twice. The final recorded value was the arithmetic mean of 10 correctly performed measurements, automatically calculated by the device. In the case of a difference in the measurements greater than 0.05 mm, a third measurement was made. The mean value of the obtained measurements was subjected to analyses.
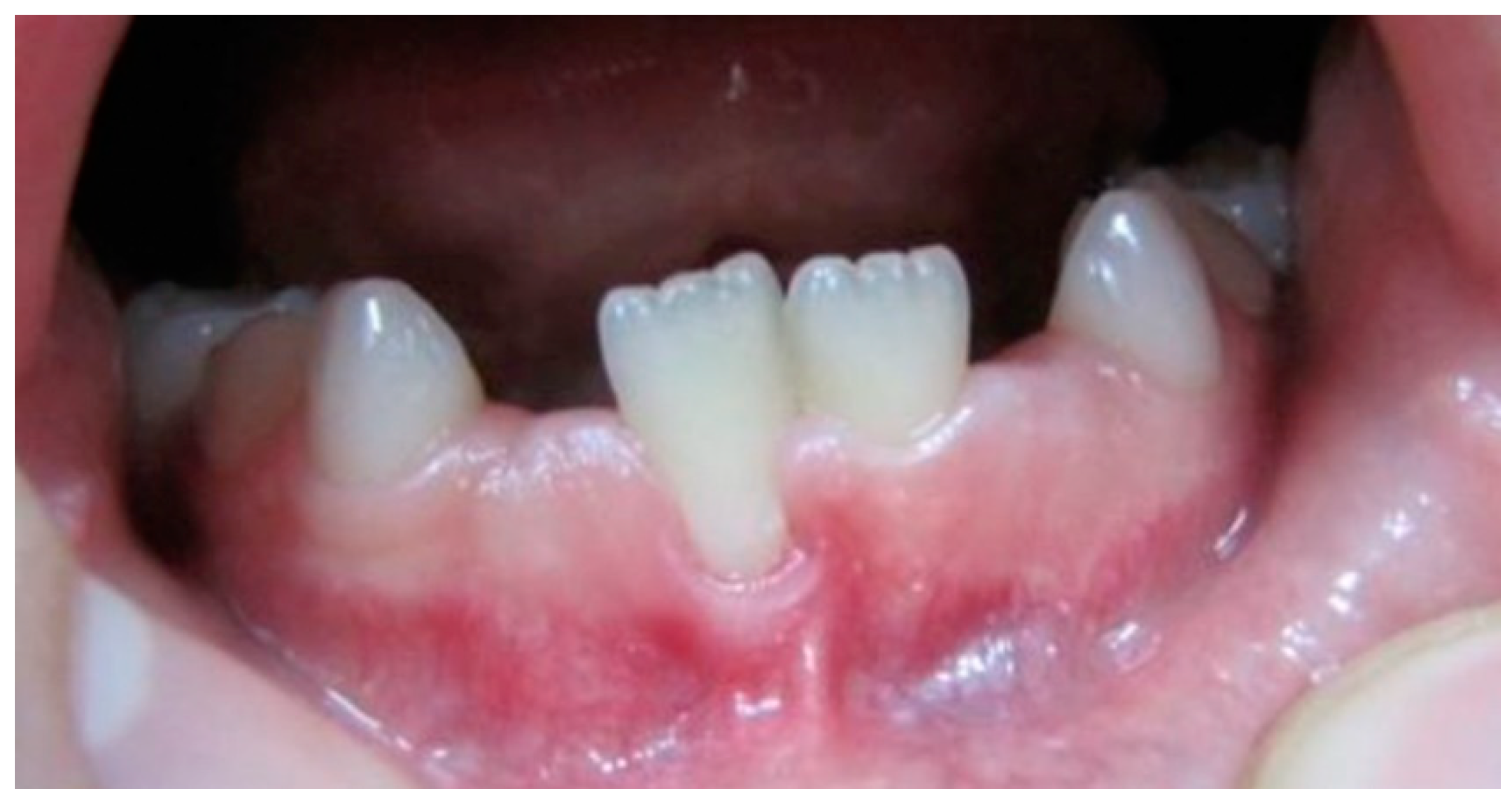
- presence of mucogingival deformities, such as gingival recessions, lack of keratinized gingiva, shallow vestibule, abnormal frenal attachment, gingival enlargement.
- mucosal—the frenal fibers attachment is positioned at the mucogingival junction;
- gingival—the frenal fibers attachment is inserted within the attached gingiva;
- papillary—the frenal attachment extends up to the interdental papilla;
- papilla penetrating—the frenal attachment crosses the alveolar process and extends up to the palatine papilla.
2.3. Statistical Analysis
3. Results
3.1. The Hygiene and the Gingiva
3.2. Probing Depth
3.3. Gingival Phenotype
3.3.1. Gingival Thickness
3.3.2. Width of Attached Gingiva
3.4. Mucogingival Deformities
3.4.1. Pseudo-Recessions Appearance
3.4.2. Vestibule Depth
3.4.3. Frenal Attachments
4. Discussion
5. Limitations
6. Conclusions
- thin gingival phenotype was defined in the majority of participants in the early transitional dentition phase,
- both parameters describing gingival phenotype, i.e., thickness and width of the attached gingiva declined from the deciduous to permanent dentition,
- gradual thickening of gingiva is initiated by a permanent tooth eruption,
- only single mild mucogingival deformities, such as pseudo-recession and shallow vestibule, were observed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, D.M.; Bassir, S.H.; Nguyen, T.T. Effect of gingival phenotype on the maintenance of periodontal health: An America Academy of Periodontology best evidence review. J. Periodontol. 2020, 91, 311–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, S.; Deepak, K.T.; Ambili, R.; Preeja, C.; Archana, V. Gingival biotype and its clinical significance—A review. Saudi J. Dent. Res. 2014, 5, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Zweers, J.; Thomas, R.Z.; Slot, D.E.; Weisgold, A.S.; Van der Weijden, F.G. Characteristics of periodontal biotype, its dimensions, associations and prevalence: A systematic review. J. Clin. Periodontol. 2014, 41, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Kao, R.T.; Pasquinelli, K. Thick vs. thin gingival tissue: A key determinant in tissue response to disease and restorative treatment. J. Calif. Dent. Assoc. 2002, 30, 521–526. [Google Scholar]
- Malhotra, R.; Grover, V.; Bhardwaj, A.; Mohindra, K. Analysis of the gingival biotype based on the measurement of the dentopapillary complex. J. Indian Soc. Periodontol. 2014, 18, 43–47. [Google Scholar] [CrossRef]
- Cortellini, P.; Bissada, N.F. Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. 1), S204–S213. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.; Sowmya, N.K.; Thomas, R.; Mehta, D.S. Periodontal biotype: Basics and clinical considerations. J. Interdiscip. Dent. 2016, 6, 44–49. [Google Scholar] [CrossRef]
- Shafizadeh, M.; Amid, R.; Tehranchi, A.; Motamedian, S.R. Evaluation of the association between gingival phenotype and alveolar bone thickness: A systematic review and meta-analysis. Arch. Oral. Biol. 2022, 133, 105287. [Google Scholar] [CrossRef]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), 237–248. [Google Scholar] [CrossRef]
- Wennström, J.L. The significance of the width and thickness of the gingiva in orthodontic treatment. Dtsch. Zahnarztl Z. 1990, 45, 136–141. [Google Scholar]
- Renkema, A.M.; Fudalej, P.S.; Renkema, A.; Bronkhorst, E.; Katsaros, C. Gingival recessions and the change of inclination of mandibular incisors during orthodontic treatment. Eur. J. Orthod. 2013, 35, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Wennström, J.L. Lack of association between width of attached gingiva and development of soft tissue recession. A 5-year longitudinal study. J. Clin. Periodontol. 1987, 14, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Joss-Vassalli, I.; Grebenstein, C.; Topouzelis, N.; Sculean, A.; Katsaros, C. Orthodontic therapy and gingival recession: A systematic review. Orthod. Craniofac. Res. 2010, 13, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Neiva, R. Periodontal soft tissue non-root coverage procedures: A systematic review from the AAP regeneration workshop. J. Periodontol. 2015, 86 (Suppl. 2), S56–S72. [Google Scholar] [CrossRef] [PubMed]
- Melsen, B.; Allais, D. Factors of importance for the development of dehiscences during labial movement of mandibular incisors: A retrospective study of adult orthodontic patients. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 552–561. [Google Scholar] [CrossRef]
- Kalina, E.; Zadurska, M.; Górski, B. Postorthodontic lower incisor and canine inclination and labial gingival recession in adult patients: A prospective study. J. Orofac. Orthop. 2021, 82, 246–256. [Google Scholar] [CrossRef]
- Bahar, B.S.K.B.; Alkhalidy, S.R.; Kaklamanos, E.G.; Athanasiou, A.E. Do orthodontic patients develop more gingival recession in anterior teeth compared to untreated individuals? A systematic review of controlled studies. Int. Orthod. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Wong, M.L.; Awang, C.F.; Ng, L.K.; Norlian, D.; Burhanudin, R.D.; Gere, M.J. Role of interceptive orthodontics in early mixed dentition. Singapore Dent. J. 2004, 26, 10–14. [Google Scholar]
- Vandana, K.L.; Savitha, B. Thickness of gingiva in association with age, gender and dental arch location. J. Clin. Periodontol. 2005, 32, 828–830. [Google Scholar] [CrossRef]
- Kolte, R.; Kolte, A.; Mahajan, A. Assessment of gingival thickness with regards to age, gender and arch location. J. Indian. Soc. Periodontol. 2014, 18, 478–481. [Google Scholar] [CrossRef]
- Maynard, J.G., Jr.; Ochsenbein, C. Mucogingival problems, prevalence and therapy in children. J. Periodontol. 1975, 46, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Kloukos, D.; Koukos, G.; Doulis, I.; Sculean, A.; Stavropoulos, A.; Katsaros, C. Gingival thickness assessment at the mandibular incisors with four methods: A cross-sectional study. J. Periodontol. 2018, 89, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Z.; Ong, M.M.A.; Yeo, A.B. Gingival profiles in a select Asian cohort: A pilot study. J. Investig. Clin. Dent. 2018, 9, e12269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascual, A.; Barallat, L.; Santos, A.; Levi, P., Jr.; Vicario, M.; Nart, J.; Medina, K.; Romanos, G.E. Comparison of Periodontal Biotypes between Maxillary and Mandibular Anterior Teeth: A Clinical and Radiographic Study. Int. J. Periodontics Restor. Dent. 2017, 37, 533–539. [Google Scholar] [CrossRef]
- Silness, J.; Löe, H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Löe, H.; Silness, J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Fischer, K.R.; Richter, T.; Kebschull, M.; Petersen, N.; Fickl, S. On the relationship between gingival biotypes and gingival thickness in young Caucasians. Clin. Oral Implants Res. 2015, 26, 865–869. [Google Scholar] [CrossRef]
- Gánti, B.; Bednarz, W.; Kőműves, K.; Vág, J. Reproducibility of the PIROP ultrasonic biometer for gingival thickness measurements. J. Esthet. Restor. Dent. 2019, 31, 263–267. [Google Scholar] [CrossRef]
- Placek, M.; Skach, M.; Mrklas, L. Significance of the labial frenum attachment in periodontal disease in man. Part I. Classification and epidemiology of the labial frenum attachment. J. Periodontol. 1974, 45, 891–894. [Google Scholar] [CrossRef]
- Dridi, S.M.; Chacun, D.; Joseph, C.; Dursun, E. Methodological Proposal to Assess Gingival Thickness in Children. Oral Health Prev. Dent. 2018, 16, 535–540. [Google Scholar] [CrossRef]
- Kus-Bartoszek, A.; Lipski, M.; Safranow, K.; Drozdzik, A. The attached gingiva thickness in the mandibular anterior region during the early transitional dentition phase. Quintessence Int. 2021, 52, 220–227. [Google Scholar] [CrossRef]
- Eghbali, A.; De Rouck, T.; De Bruyn, H.; Cosyn, J. The gingival biotype assessed by experienced and inexperienced clinicians. J. Clin. Periodontol. 2009, 36, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Cuny-Houchmand, M.; Renaudin, S.; Leroul, M.; Planche, L.; Guehennec, L.L.; Soueidan, A. Gingival biotype assessement: Visual inspection relevance and maxillary versus mandibular comparison. Open Dent. J. 2013, 7, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rouck, T.; Eghbali, R.; Collys, K.; De Bruyn, H.; Cosyn, J. The gingival biotype revisited: Transparency of the periodontal probe through the gingival margin as a method to discriminate thin from thick gingiva. J. Clin. Periodontol. 2009, 36, 428–433. [Google Scholar] [CrossRef]
- Ronay, V.; Sahrmann, P.; Bindl, A.; Attin, T.; Schmidlin, P.R. Current status and perspectives of mucogingival soft tissue measurement methods. J. Esthet. Restor. Dent. 2011, 23, 146–156. [Google Scholar] [CrossRef]
- Eger, T.; Müller, H.P.; Heinecke, A. Ultrasonic determination of gingival thickness. Subject variation and influence of tooth type and clinical features. J. Clin. Periodontol. 1996, 23, 839–845. [Google Scholar] [CrossRef]
- Müller, H.P.; Barrieshi-Nusair, K.M.; Könönen, E. Repeatability of ultrasonic determination of gingival thickness. Clin. Oral Investig. 2007, 11, 439–442. [Google Scholar] [CrossRef]
- Kan, J.Y.; Morimoto, T.; Rungcharassaeng, K.; Roe, P.; Smith, D.H. Gingival biotype assessment in the esthetic zone: Visual versus direct measurement. Int. J. Periodontics Restor. Dent. 2010, 30, 237–243. [Google Scholar]
- Aimetti, M.; Massei, G.; Morra, M.; Cardesi, E.; Romano, F. Correlation between gingival phenotype and Schneiderian membrane thickness. Int. J. Oral Maxillofac. Implants 2008, 23, 1128–1132. [Google Scholar]
- Kaya, Y.; Alkan, Ö.; Keskin, S. An evaluation of the gingival biotype and the width of keratinized gingiva in the mandibular anterior region of individuals with different dental malocclusion groups and levels of crowding. Korean J. Orthod. 2017, 47, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, V.; Sunny Mehrotra, N.; Vijay, V. Gingival biotype assessment: Variations in gingival thickness with regard to age, gender, and arch location. Indian J. Dent. Sci. 2017, 9, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Bosnjak, A.; Jorgić-Srdjak, K.; Maricević, T.; Plancak, D. The width of clinically-defined keratinized gingiva in the mixed dentition. ASDC J. Dent. Child. 2002, 69, 266–270. [Google Scholar] [PubMed]
- Andlin-Sobocki, A. Changes of facial gingival dimensions in children. A 2-year longitudinal study. J. Clin. Periodontol. 1993, 20, 212–218. [Google Scholar] [CrossRef]
- Bimstein, E.; Eidelman, E. Morphological changes in the attached and keratinized gingiva and gingival sulcus in the mixed dentition period. A 5-year longitudinal study. J. Clin. Periodontol. 1988, 15, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, M.R.; Akbarzadeh, A. Attached gingival width and gingival sulcus depth in the three dentition systems. J. Dent. School 2013, 31, 216–223. [Google Scholar]
- Kim, J.Y.; Jung, D.W.; Park, K.T. A clinical study of the width of attached gingiva in deciduous, mixed and permanent dentitions. J. Korean Acad. Pediatr. Dent. 2006, 33, 678–685. [Google Scholar]
- Singh, S.; Vandana, K.L. Assessment of width of attached gingiva in primary, mixed and permanent dentition: Part-2. SRM J. Res. Dent. Sci. 2017, 8, 157–161. [Google Scholar]
- Bhatia, G.; Kumar, A.; Khatri, M.; Bansal, M.; Saxena, S. Assessment of the width of attached gingiva using different methods in various age groups: A clinical study. J. Indian Soc. Periodontol. 2015, 19, 199–202. [Google Scholar] [CrossRef]
- Srivastava, B.; Chandra, S.; Jaiswal, J.N.; Saimbi, C.S.; Srivastava, D. Cross-sectional study to evaluate variations in attached gingiva and gingival sulcus in the three periods of dentition. J. Clin. Pediatr. Dent. 1990, 15, 17–24. [Google Scholar]
- Wyrębek, B.; Orzechowska, A.; Cudziło, D.; Plakwicz, P. Evaluation of changes in the width of gingiva in children and youth. Review of literature. Dev. Period. Med. 2015, 19, 212–216. [Google Scholar]
- Tenenbaum, H.; Tenenbaum, M. A clinical study of the width of the attached gingiva in the deciduous, transitional and permanent dentitions. J. Clin. Periodontol. 1986, 13, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, M.; Sruthi, R.; Ramakrishnan, T.; Emmadi, P.; Ambalavanan, N. An overview of frenal attachments. J. Indian. Soc. Periodontol. 2013, 17, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Alwan, A.H. Prevalence of types of labial frenum attachement and frenectomy with conventional scalpel technique in a sample of Iraqi population. Intern. Med. J. 2020, 25, 2079–2088. [Google Scholar]
- Divater, V.; Bali, P.; Nawab, A.; Hiremath, N.; Jain, J.; Kalaivanan, D. Frenal attachment and its association with oral hygiene status among adolescents in Dakshina Kannada population: A cross-sectional study. J. Fam. Med. Prim. Care 2019, 15, 3664–3667. [Google Scholar] [CrossRef]

| Variables | Examination I | Examination II | p Value | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Me | Mean ± SD | Me | |||
| Pl. I | 0.70 ± 0.62 | 0.6 | 0.76 ± 0.66 | 0.8 | p = 0.464 | |
| GI | 0.18 ± 0.26 | 0 | 0.04 ± 0.11 | 0 | p < 0.001 * | |
| GT (mm) | ||||||
| all incisors | 0.76 ± 0.36 | 0.7 | 0.73 ± 0.19 | 0.74 | p = 0.02 * | |
| central incisors | 0.67 ± 0.34 | 0.61 | 0.71 ± 0.18 | 0.74 | p < 0.001 * | |
| lateral incisors | 0.88 ± 0.35 | 0.86 | 0.76 ± 0.19 | 0.75 | p = 0.064 | |
| AGW (mm) | ||||||
| all incisors | 3.33 ± 1.01 | 3 | 2.7 ± 0.97 | 3 | p < 0.001 * | |
| central incisors | 3.28 ± 1.07 | 3 | 2.57 ± 0.96 | 3.5 | p < 0.001 * | |
| lateral incisors | 3.4 ± 0.93 | 2.5 | 2.83 ± 0.97 | 3 | p < 0.001 * | |
| PD (mm) | ||||||
| all incisors | 1.47 ± 0.76 | 1 | 1.25 ± 0.51 | 1 | p < 0.001 * | |
| central incisors | 1.47 ± 0.69 | 1 | 1.14 ± 0.39 | 1 | p < 0.001 * | |
| lateral incisors | 1.47 ± 0.84 | 1 | 1.37 ± 0.58 | 1 | p = 0.746 | |
| VD (mm) | 7.76 ± 1.86 | 8 | 7.22 ± 1.47 | 8 | p > 0.05 | |
| Exchange/Eruption Degree | Examination I | Examination II | p Value | ||
|---|---|---|---|---|---|
| Mean ± SD | Me | Mean ± SD | Me | ||
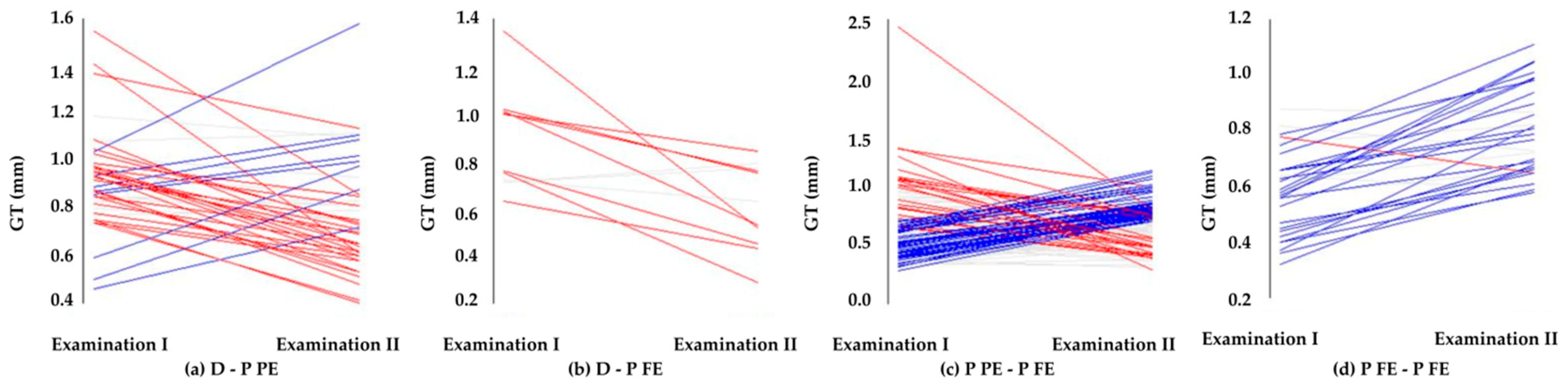
| D-P PE | 0.92 ± 0.20 | 0.89 | 0.78 ± 0.23 | 0.75 | p = 0.001 * |
| D-P FE | 0.88 ± 0.22 | 0.76 | 0.61 ± 0.18 | 0.63 | p = 0.005 * |
| P PE-P FE | 0.65 ± 0.25 | 0.62 | 0.72 ± 0.17 | 0.74 | p < 0.001 * |
| P FE-P FE | 0.59 ± 0.15 | 0.58 | 0.80 ± 0.16 | 0.78 | p < 0.001 * |
| Exchange/Eruption Degree | Examination I | Examination II | p Value | ||
|---|---|---|---|---|---|
| Mean ± SD | Me | Mean ± SD | Me | ||
| D-P PE | 3.80 ± 0.85 | 4 | 2.96 ± 1.17 | 3 | p < 0.001 * |
| D-P FE | 4.36 ± 1.03 | 4 | 2.82 ± 0.98 | 3 | p = 0.007 * |
| P PE-P FE | 3.17 ± 0.98 | 3 | 2.53 ± 0.93 | 2.5 | p < 0.001 * |
| P FE-P FE | 2.75 ± 1.09 | 2.5 | 2.60 ± 0.88 | 2 | p = 0.372 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kus-Bartoszek, A.; Lipski, M.; Jarząbek, A.; Manowiec, J.; Droździk, A. Gingival Phenotype Changes and the Prevalence of Mucogingival Deformities during the Early Transitional Dentition Phase—A Two-Year Longitudinal Study. Int. J. Environ. Res. Public Health 2022, 19, 3899. https://doi.org/10.3390/ijerph19073899
Kus-Bartoszek A, Lipski M, Jarząbek A, Manowiec J, Droździk A. Gingival Phenotype Changes and the Prevalence of Mucogingival Deformities during the Early Transitional Dentition Phase—A Two-Year Longitudinal Study. International Journal of Environmental Research and Public Health. 2022; 19(7):3899. https://doi.org/10.3390/ijerph19073899
Chicago/Turabian StyleKus-Bartoszek, Agnieszka, Mariusz Lipski, Anna Jarząbek, Joanna Manowiec, and Agnieszka Droździk. 2022. "Gingival Phenotype Changes and the Prevalence of Mucogingival Deformities during the Early Transitional Dentition Phase—A Two-Year Longitudinal Study" International Journal of Environmental Research and Public Health 19, no. 7: 3899. https://doi.org/10.3390/ijerph19073899
APA StyleKus-Bartoszek, A., Lipski, M., Jarząbek, A., Manowiec, J., & Droździk, A. (2022). Gingival Phenotype Changes and the Prevalence of Mucogingival Deformities during the Early Transitional Dentition Phase—A Two-Year Longitudinal Study. International Journal of Environmental Research and Public Health, 19(7), 3899. https://doi.org/10.3390/ijerph19073899






