Impact of Activity Tracker Usage in Combination with a Physical Activity Intervention on Physical and Cognitive Parameters in Healthy Adults Aged 60+: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Randomization
2.2. Measures
2.2.1. Grip Strength (Dynamometer)
2.2.2. Endurance (Two-Minute Step Test, TMST)
2.2.3. Gait Speed (Four-Meter Walk Test)
2.2.4. Physical Self-Concept (Physical Self-Description Questionnaire, PSDQ)
2.2.5. Cognition (Simon Task)
2.3. Procedure
2.4. Statistical Analysis
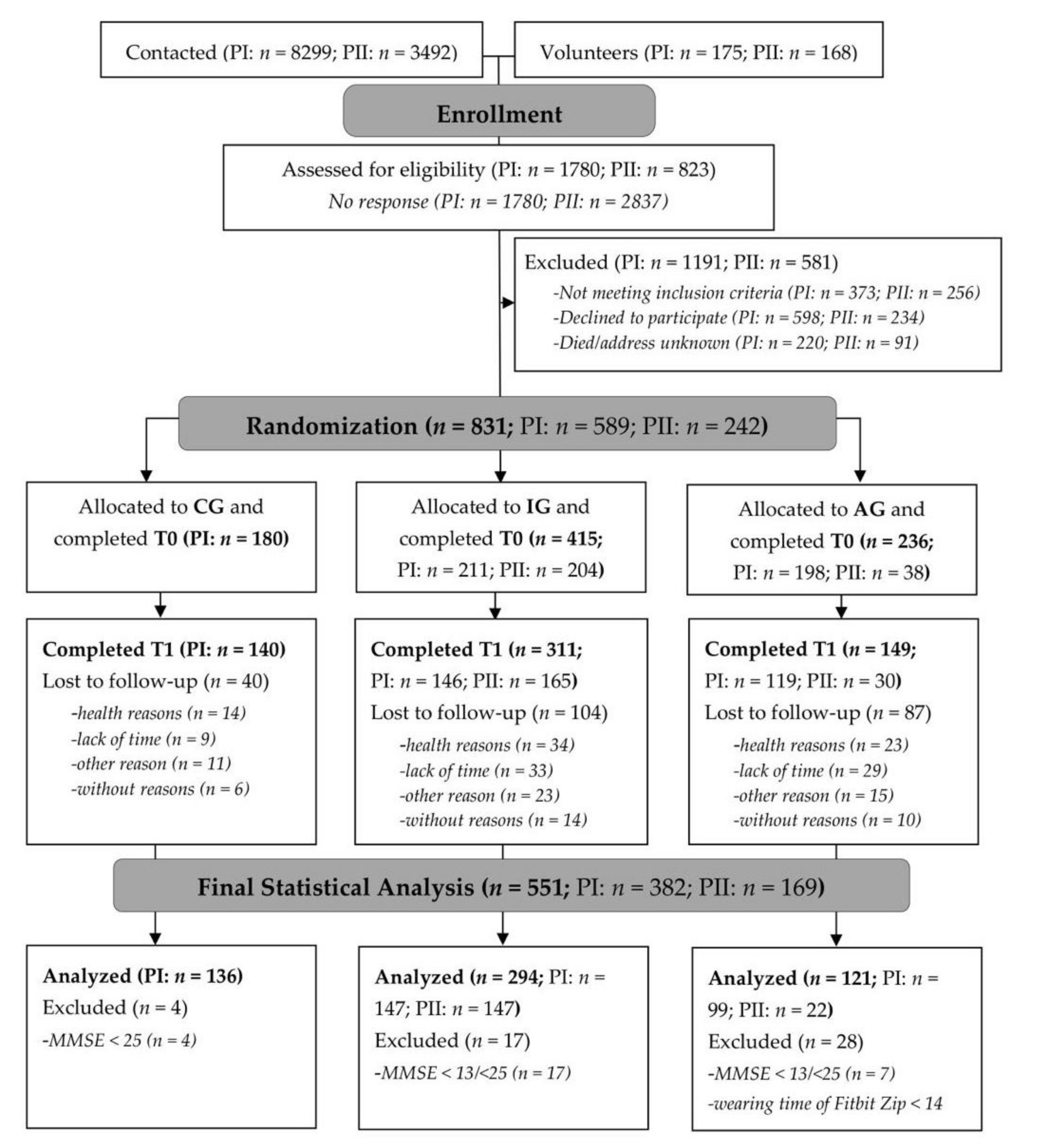
3. Results
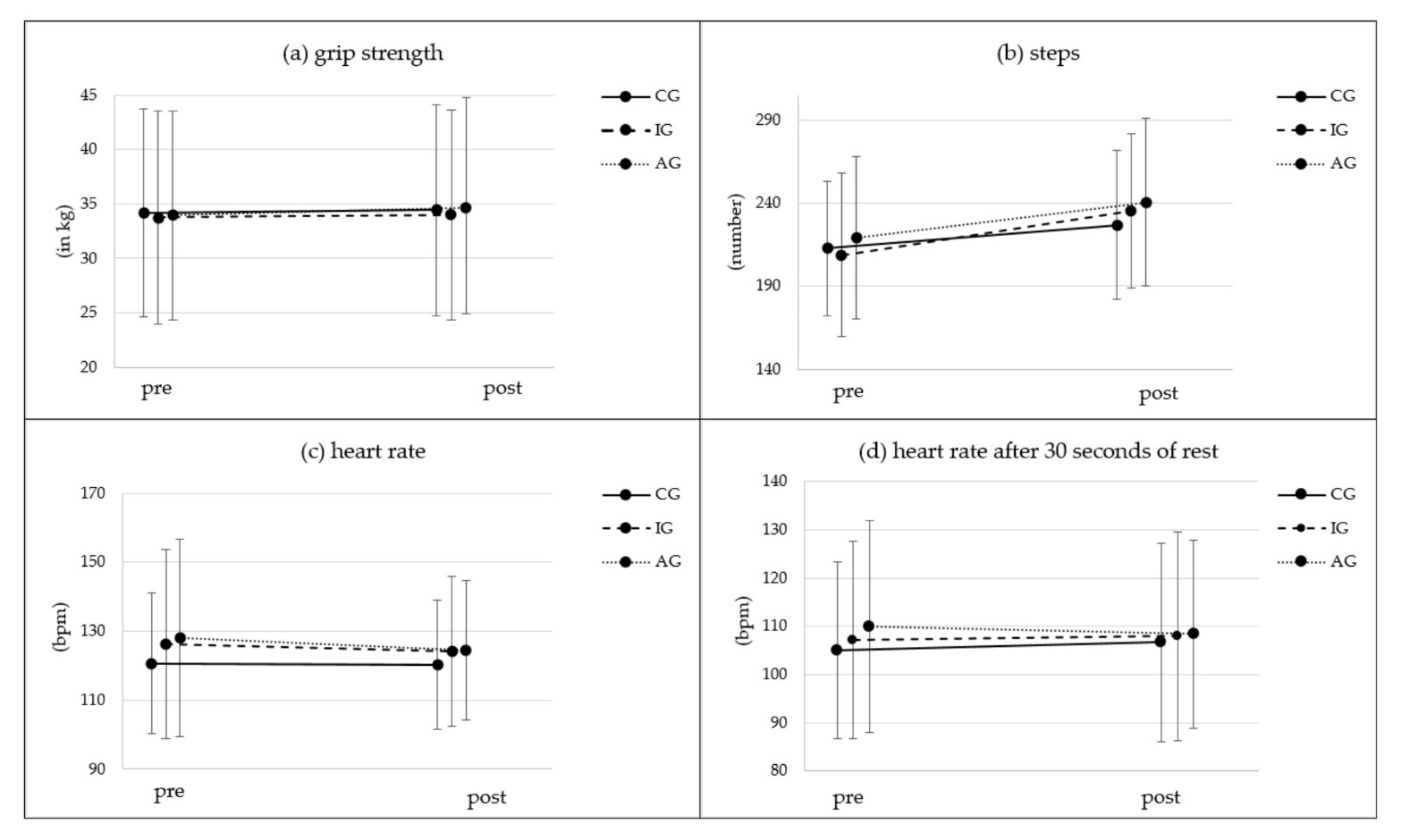
3.1. Grip Strength
3.2. Endurance/Cardiovascular Fitness
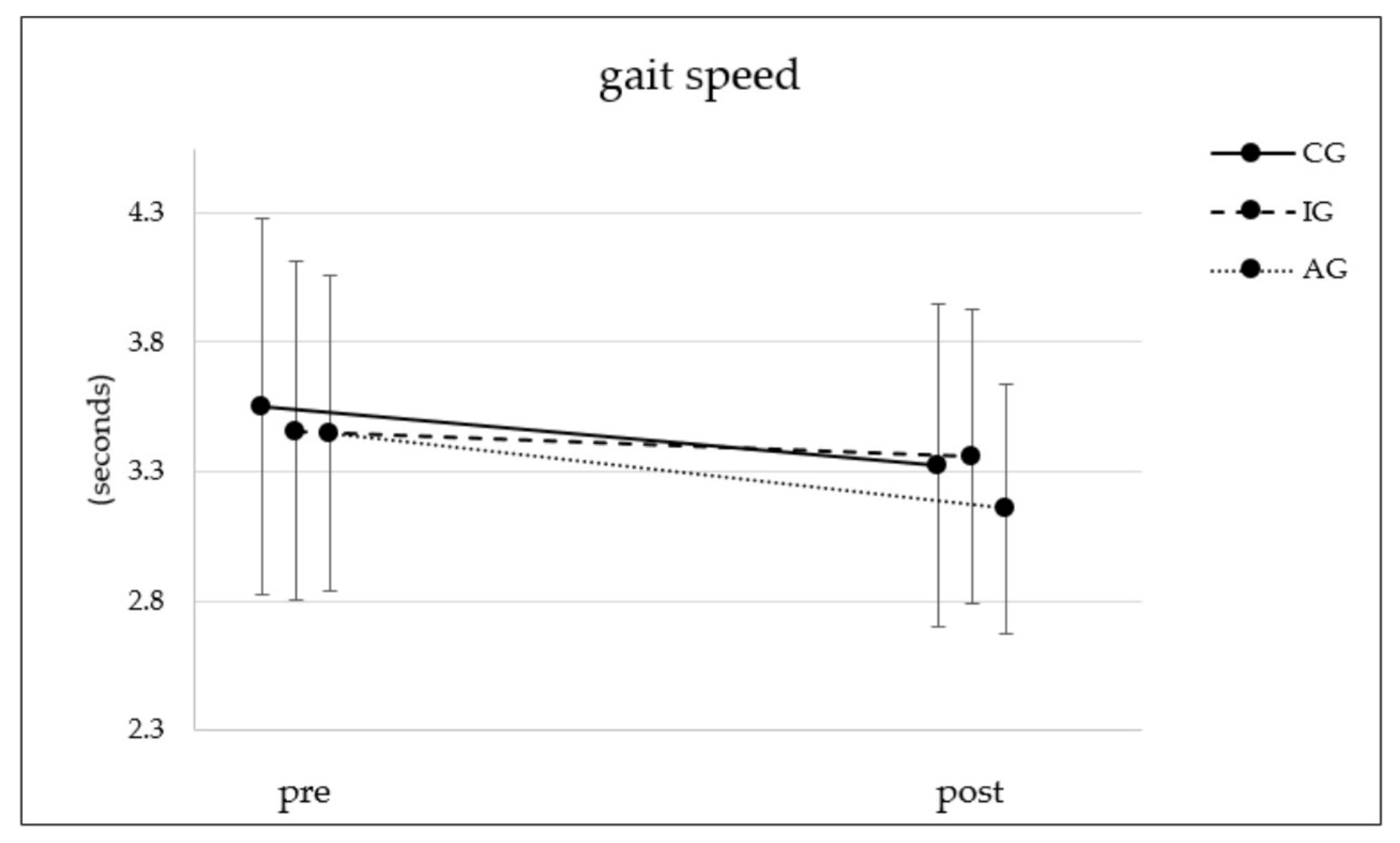
3.3. Gait
3.4. Physical Self-Concept
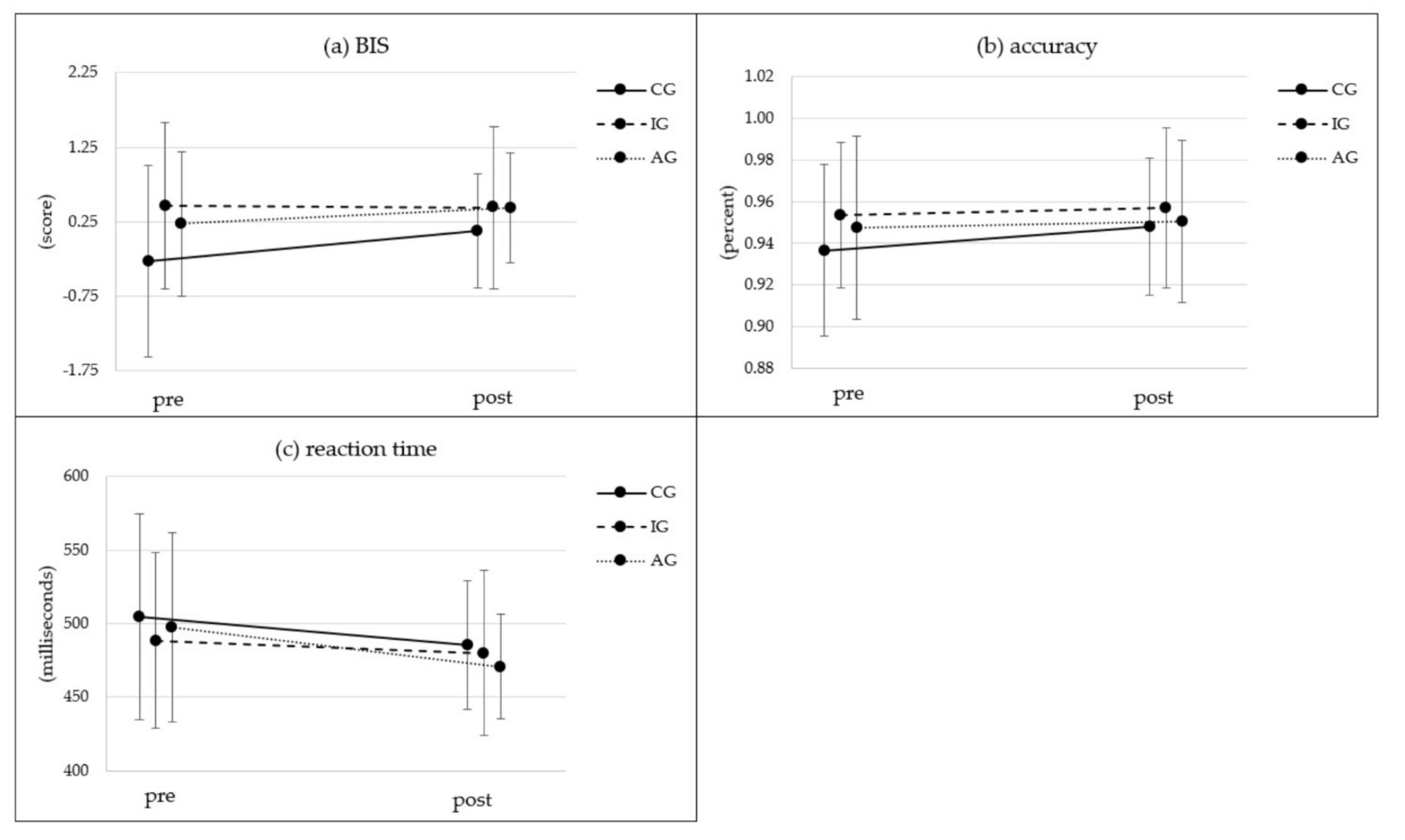
3.5. Cognition
4. Discussion
4.1. Impact of PA Intervention Primarily Conducted at Home (With or without Additional Use of an Activity Tracker) on Physical Parameters
4.2. Impact of PA Intervention Primarily Conducted at Home (With or without Additional Use of an Activity Tracker) on Cognitive Parameters
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avila-Funes, J.A.; Amieva, H.; Barberger-Gateau, P.; Le Goff, M.; Raoux, N.; Ritchie, K.; Carrière, I.; Tavernier, B.; Tzourio, C.; Gutiérrez-Robledo, L.M.; et al. Cognitive Impairment Improves the Predictive Validity of the Phenotype of Frailty for Adverse Health Outcomes: The Three-City Study. J. Am. Geriatr. Soc. 2009, 57, 453–461. [Google Scholar] [CrossRef]
- Preyde, M.; Brassard, K. Evidence-Based Risk Factors for Adverse Health Outcomes in Older Patients after Discharge Home and Assessment Tools: A Systematic Review. J. Evid. Based Soc. Work. 2011, 8, 445–468. [Google Scholar] [CrossRef]
- Schnitzer, S.; Blüher, S.; Teti, A.; Schaeffner, E.; Ebert, N.; Martus, P.; Suhr, R.; Kuhlmey, A. Risk Profiles for Care Dependency: Cross-Sectional Findings of a Population-Based Cohort Study in Germany. J. Aging Health 2020, 32, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Feil, D.; Marmon, T.; Unützer, J. Cognitive Impairment, Chronic Medical Illness, and Risk of Mortality in an Elderly Cohort. Am. J. Geriatr. Psychiatry 2003, 11, 551–560. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Fontana, L.; Trevisan, C.; Bolzetta, F.; Rui, M.; Sartori, L.; Musacchio, E.; Zambon, S.; Maggi, S.; et al. A Comparison of Objective Physical Performance Tests and Future Mortality in the Elderly People. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A Review of the Effects of Physical Activity and Exercise on Cognitive and Brain Functions in Older Adults. J. Aging Res. 2013, 2013, 657508. [Google Scholar] [CrossRef] [Green Version]
- Buman, M.P.; Hekler, E.B.; Haskell, W.L.; Pruitt, L.; Conway, T.L.; Cain, K.L.; Sallis, J.F.; Saelens, B.E.; Frank, L.D.; King, A.C. Objective Light-Intensity Physical Activity Associations with Rated Health in Older Adults. Am. J. Epidemiol. 2010, 172, 1155–1165. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Kim, J.; Han, E.S.; Chae, S.; Ryu, M.; Ahn, K.H.; Park, E.J. Changes in Physical Activity and Cognitive Decline in Older Adults Living in the Community. Age 2015, 37, 20. [Google Scholar] [CrossRef] [Green Version]
- Hamer, M.; Lavoie, K.L.; Bacon, S.L. Taking up Physical Activity in Later Life and Healthy Ageing: The English Longitudinal Study of Ageing. Br. J. Sports Med. 2014, 48, 239–243. [Google Scholar] [CrossRef]
- Voelcker-Rehage, C.; Godde, B.; Staudinger, U.M. Physical and Motor Fitness Are Both Related to Cognition in Old Age. Eur. J. Neurosci. 2010, 31, 167–176. [Google Scholar] [CrossRef] [PubMed]
- McAuley, E.; Morris, K.S. State of the Art Review: Advances in Physical Activity and Mental Health: Quality of Life. Am. J. Lifestyle Med. 2007, 1, 389–396. [Google Scholar] [CrossRef]
- Marcus, B.H.; Williams, D.M.; Dubbert, P.M.; Sallis, J.F.; King, A.C.; Yancey, A.K.; Franklin, B.A.; Buchner, D.; Daniels, S.R.; Claytor, R.P. Physical Activity Intervention Studies: What We Know and What We Need to Know: A Scientific Statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity); Council on Cardiovascular Disease in the Young; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. Circulation 2006, 114, 2739–2752. [Google Scholar] [CrossRef] [Green Version]
- Bowen, M.E. A Prospective Examination of the Relationship between Physical Activity and Dementia Risk in Later Life. Am. J. Health Promot. AJHP 2012, 26, 333–340. [Google Scholar] [CrossRef]
- Laurin, D.; Verreault, R.; Lindsay, J.; MacPherson, K.; Rockwood, K. Physical Activity and Risk of Cognitive Impairment and Dementia in Elderly Persons. Arch. Neurol. 2001, 58, 498–504. [Google Scholar] [CrossRef] [Green Version]
- Larson, E.B.; Wang, L.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Crane, P.; Kukull, W. Exercise Is Associated with Reduced Risk for Incident Dementia among Persons 65 Years of Age and Older. Ann. Intern. Med. 2006, 144, 73–81. [Google Scholar] [CrossRef]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Singh, M.A.F.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine Position Stand. Exercise and Physical Activity for Older Adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical Activity and Public Health in Older Adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1435–1445. [Google Scholar] [CrossRef] [Green Version]
- Rand, D.; Miller, W.C.; Yiu, J.; Eng, J.J. Interventions for Addressing Low Balance Confidence in Older Adults: A Systematic Review and Meta-Analysis. Age Ageing 2011, 40, 297–306. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-4-159997-9. [Google Scholar]
- RKI. Gesundheitsfördernde körperliche Aktivität in der Freizeit bei Erwachsenen in Deutschland; Robert Koch Institut: Berlin, Germany, 2017. [Google Scholar] [CrossRef]
- Giné-Garriga, M.; Roqué-Fíguls, M.; Coll-Planas, L.; Sitjà-Rabert, M.; Salvà, A. Physical Exercise Interventions for Improving Performance-Based Measures of Physical Function in Community-Dwelling, Frail Older Adults: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2014, 95, 753–769.e3. [Google Scholar] [CrossRef]
- Tseng, C.-N.; Gau, B.-S.; Lou, M.-F. The Effectiveness of Exercise on Improving Cognitive Function in Older People: A Systematic Review. J. Nurs. Res. JNR 2011, 19, 119–131. [Google Scholar] [CrossRef]
- Chase, J.-A.D.; Phillips, L.J.; Brown, M. Physical Activity Intervention Effects on Physical Function Among Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. J. Aging Phys. Act. 2017, 25, 149–170. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, S.E.; Reddy, S.; Lommel, T.S.; Fischer, J.G.; Speer, E.M.; Stephens, H.; Park, S.; Johnson, M.A. Physical Activity and Physical Function Improved Following a Community-Based Intervention in Older Adults in Georgia Senior Centers. J. Nutr. Elder. 2008, 27, 135–154. [Google Scholar] [CrossRef]
- Olanrewaju, O.; Kelly, S.; Cowan, A.; Brayne, C.; Lafortune, L. Physical Activity in Community Dwelling Older People: A Systematic Review of Reviews of Interventions and Context. PLoS ONE 2016, 11, e0168614. [Google Scholar] [CrossRef] [Green Version]
- Gates, N.; Singh, M.A.F.; Sachdev, P.S.; Valenzuela, M. The Effect of Exercise Training on Cognitive Function in Older Adults with Mild Cognitive Impairment: A Meta-Analysis of Randomized Controlled Trials. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2013, 21, 1086–1097. [Google Scholar] [CrossRef]
- Burke, L.; Lee, A.H.; Jancey, J.; Xiang, L.; Kerr, D.A.; Howat, P.A.; Hills, A.P.; Anderson, A.S. Physical Activity and Nutrition Behavioural Outcomes of a Home-Based Intervention Program for Seniors: A Randomized Controlled Trial. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Opdenacker, J.; Boen, F.; Coorevits, N.; Delecluse, C. Effectiveness of a Lifestyle Intervention and a Structured Exercise Intervention in Older Adults. Prev. Med. 2008, 46, 518–524. [Google Scholar] [CrossRef]
- Mouton, A.; Cloes, M. Efficacy of a Web-Based, Center-Based or Combined Physical Activity Intervention among Older Adults. Health Educ. Res. 2015, 30, 422–435. [Google Scholar] [CrossRef] [Green Version]
- Müller, A.M.; Khoo, S. Non-Face-to-Face Physical Activity Interventions in Older Adults: A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2014, 11, 35. [Google Scholar] [CrossRef] [Green Version]
- Muellmann, S.; Buck, C.; Voelcker-Rehage, C.; Bragina, I.; Lippke, S.; Meyer, J.; Peters, M.; Pischke, C.R. Effects of Two Web-Based Interventions Promoting Physical Activity among Older Adults Compared to a Delayed Intervention Control Group in Northwestern Germany: Results of the PROMOTE Community-Based Intervention Trial. Prev. Med. Rep. 2019, 15, 100958. [Google Scholar] [CrossRef]
- Jonkman, N.H.; van Schooten, K.S.; Maier, A.B.; Pijnappels, M. eHealth Interventions to Promote Objectively Measured Physical Activity in Community-Dwelling Older People. Maturitas 2018, 113, 32–39. [Google Scholar] [CrossRef]
- Frees, V.B.; Koch, W. Internetnutzung: Frequenz und Vielfalt nehmen in allen Altersgruppen zu. Media Perspekt. 2015, 9, 366–377. [Google Scholar]
- Gitlow, L. Technology Use by Older Adults and Barriers to Using Technology. Phys. Occup. Ther. Geriatr. 2014, 32, 271–280. [Google Scholar] [CrossRef]
- Ashe, M.C.; Edwards, N.Y.; Taylor, A.; Burnett, L.; Giangregorio, L.; Milne, K.; Clemson, L.; Fleig, L. Return to Everyday Activity in the Community and Home: A Feasibility Study for a Lifestyle Intervention to Sit Less, Move More, and Be Strong. Pilot Feasibility Stud. 2019, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.M.; Gianoudis, J.; Hall, T.; Mundell, N.L.; Maddison, R. Feasibility, Usability, and Enjoyment of a Home-Based Exercise Program Delivered via an Exercise App for Musculoskeletal Health in Community-Dwelling Older Adults: Short-Term Prospective Pilot Study. JMIR mHealth uHealth 2021, 9, e21094. [Google Scholar] [CrossRef]
- Lauzé, M.; Martel, D.D.; Agnoux, A.; Sirois, M.-J.; Émond, M.; Daoust, R.; Aubertin-Leheudre, M. Feasibility, Acceptability and Effects of a Home-Based Exercise Program Using a Gerontechnology on Physical Capacities after a Minor Injury in Community-Living Older Adults: A Pilot Study. J. Nutr. Health Aging 2018, 22, 16–25. [Google Scholar] [CrossRef]
- Geraedts, H.A.E.; Zijlstra, W.; Zhang, W.; Spoorenberg, S.L.W.; Báez, M.; Far, I.K.; Baldus, H.; Stevens, M. A Home-Based Exercise Program Driven by Tablet Application and Mobility Monitoring for Frail Older Adults: Feasibility and Practical Implications. Prev. Chronic Dis. 2017, 14, E12. [Google Scholar] [CrossRef] [Green Version]
- McAuley, E.; Wójcicki, T.R.; Gothe, N.P.; Mailey, E.L.; Szabo, A.N.; Fanning, J.; Olson, E.A.; Phillips, S.M.; Motl, R.W.; Mullen, S.P. Effects of a DVD-Delivered Exercise Intervention on Physical Function in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1076–1082. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.E.; Layne, J.E.; Bernstein, M.J.; Nuernberger, A.; Castaneda, C.; Kaliton, D.; Hausdorff, J.; Judge, J.O.; Buchner, D.M.; Roubenoff, R.; et al. The Effects of Multidimensional Home-Based Exercise on Functional Performance in Elderly People. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, 154–160. [Google Scholar] [CrossRef]
- Hsieh, T.-J.; Su, S.-C.; Chen, C.-W.; Kang, Y.-W.; Hu, M.-H.; Hsu, L.-L.; Wu, S.-Y.; Chen, L.; Chang, H.-Y.; Chuang, S.-Y.; et al. Individualized Home-Based Exercise and Nutrition Interventions Improve Frailty in Older Adults: A Randomized Controlled Trial. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 119. [Google Scholar] [CrossRef] [Green Version]
- Bernocchi, P.; Vitacca, M.; La Rovere, M.T.; Volterrani, M.; Galli, T.; Baratti, D.; Paneroni, M.; Campolongo, G.; Sposato, B.; Scalvini, S. Home-Based Telerehabilitation in Older Patients with Chronic Obstructive Pulmonary Disease and Heart Failure: A Randomised Controlled Trial. Age Ageing 2018, 47, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Liu-Ambrose, T.; Donaldson, M.G.; Ahamed, Y.; Graf, P.; Cook, W.L.; Close, J.; Lord, S.R.; Khan, K.M. Otago Home-Based Strength and Balance Retraining Improves Executive Functioning in Older Fallers: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2008, 56, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Hayden, K.M.; Baker, L.D.; Bray, G.; Carvajal, R.; Demos-McDermott, K.; Hergenroeder, A.L.; Hill, J.O.; Horton, E.; Jakicic, J.M.; Johnson, K.C.; et al. Long-Term Impact of Intensive Lifestyle Intervention on Cognitive Function Assessed with the National Institutes of Health Toolbox: The Look AHEAD Study. Alzheimers Dement. 2018, 10, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bravata, D.M.; Smith-Spangler, C.; Sundaram, V.; Gienger, A.L.; Lin, N.; Lewis, R.; Stave, C.D.; Olkin, I.; Sirard, J.R. Using Pedometers to Increase Physical Activity and Improve Health: A Systematic Review. JAMA 2007, 298, 2296–2304. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Stewart, J. Use of Consumer Wearable Devices to Promote Physical Activity: A Review of Health Intervention Studies. J. Environ. Health Sci. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Leskinen, T.; Suorsa, K.; Tuominen, M.; Pulakka, A.; Pentti, J.; Löyttyniemi, E.; Heinonen, I.; Vahtera, J.; Stenholm, S. The Effect of Consumer-Based Activity Tracker Intervention on Physical Activity among Recent Retirees—An RCT Study. Med. Sci. Sports Exerc. 2021, 53, 1756. [Google Scholar] [CrossRef] [PubMed]
- Auerswald, T.; Meyer, J.; von Holdt, K.; Voelcker-Rehage, C. Application of Activity Trackers among Nursing Home Residents—A Pilot and Feasibility Study on Physical Activity Behavior, Usage Behavior, Acceptance, Usability and Motivational Impact. Int. J. Environ. Res. Public Health 2020, 17, 7840. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, L.D.; Lansing, J.E.; DeShaw, K.J.; Peyer, K.L.; Bai, Y.; Perez, M.; Phillips, L.A.; Welk, G.J. Evaluating Motivational Interviewing and Habit Formation to Enhance the Effect of Activity Trackers on Healthy Adults’ Activity Levels: Randomized Intervention. JMIR mHealth uHealth 2019, 7, e10988. [Google Scholar] [CrossRef]
- Cadmus-Bertram, L.A.; Marcus, B.H.; Patterson, R.E.; Parker, B.A.; Morey, B.L. Randomized Trial of a Fitbit-Based Physical Activity Intervention for Women. Am. J. Prev. Med. 2015, 49, 414–418. [Google Scholar] [CrossRef] [Green Version]
- Compernolle, S.; Vandelanotte, C.; Cardon, G.; De Bourdeaudhuij, I.; De Cocker, K. Effectiveness of a Web-Based, Computer-Tailored, Pedometer-Based Physical Activity Intervention for Adults: A Cluster Randomized Controlled Trial. J. Med. Internet Res. 2015, 17, e38. [Google Scholar] [CrossRef] [Green Version]
- Franssen, W.M.A.; Franssen, G.H.L.M.; Spaas, J.; Solmi, F.; Eijnde, B.O. Can Consumer Wearable Activity Tracker-Based Interventions Improve Physical Activity and Cardiometabolic Health in Patients with Chronic Diseases? A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 57. [Google Scholar] [CrossRef]
- Snyder, A.; Colvin, B.; Gammack, J.K. Pedometer Use Increases Daily Steps and Functional Status in Older Adults. J. Am. Med. Dir. Assoc. 2011, 12, 590–594. [Google Scholar] [CrossRef]
- Talbot, L.A.; Gaines, J.M.; Huynh, T.N.; Metter, E.J. A Home-Based Pedometer-Driven Walking Program to Increase Physical Activity in Older Adults with Osteoarthritis of the Knee: A Preliminary Study. J. Am. Geriatr. Soc. 2003, 51, 387–392. [Google Scholar] [CrossRef]
- Bickmore, T.W.; Silliman, R.A.; Nelson, K.; Cheng, D.M.; Winter, M.; Henault, L.; Paasche-Orlow, M.K. A Randomized Controlled Trial of an Automated Exercise Coach for Older Adults. J. Am. Geriatr. Soc. 2013, 61, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Muellmann, S.; Bragina, I.; Voelcker-Rehage, C.; Rost, E.; Lippke, S.; Meyer, J.; Schnauber, J.; Wasmann, M.; Toborg, M.; Koppelin, F.; et al. Development and Evaluation of Two Web-Based Interventions for the Promotion of Physical Activity in Older Adults: Study Protocol for a Community-Based Controlled Intervention Trial. BMC Public Health 2017, 17, 512. [Google Scholar] [CrossRef] [Green Version]
- Pischke, C.R.; Voelcker-Rehage, C.; Peters, M.; Ratz, T.; Pohlabeln, H.; Meyer, J.; Holdt, K.; Lippke, S. Implementation and Effects of Information Technology-Based and Print-Based Interventions to Promote Physical Activity Among Community-Dwelling Older Adults: Protocol for a Randomized Crossover Trial. JMIR Res. Protoc. 2020, 9, e15168. [Google Scholar] [CrossRef] [PubMed]
- Pischke, C.; Voelcker-Rehage, C.; Peters, M.; Ratz, T.; Muellmann, S.; Meyer, J.; von Holdt, K.; Lippke, S. Die “Fit im Nordwesten” - Toolbox (PROMOTE). 2021. Available online: https://link.springer.com/article/10.1007/s11553-021-00926-w (accessed on 27 January 2022).
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-Mental State. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Mini-Mental State Examination Second Edition|MMSE-2. Available online: https://www.parinc.com/Products/Pkey/238 (accessed on 21 January 2022).
- Bohannon, R.W. Grip Strength: An Indispensable Biomarker For Older Adults. Clin. Interv. Aging 2019, 14, 1681. [Google Scholar] [CrossRef] [Green Version]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A Review of the Measurement of Grip Strength in Clinical and Epidemiological Studies: Towards a Standardised Approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Rikli, R.E.; Jones, C.J. Development and Validation of a Functional Fitness Test for Community-Residing Older Adults. J. Aging Phys. Act. 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Marsh, H.W. Physical Self Description Questionnaire: Stability and Discriminant Validity. Res. Q. Exerc. Sport 1996, 67, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Stiller, J.; Alfermann, D. Die Deutsche Übersetzung Des Physical Self-Description Questionnaire (PSDQ). Z. Sportpsychol. 2007, 14, 149–161. [Google Scholar] [CrossRef]
- Bialystok, E.; Craik, F.; Luk, G. Cognitive Control and Lexical Access in Younger and Older Bilinguals. J. Exp. Psychol. Learn. Mem. Cogn. 2008, 34, 859–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liesefeld, H.R.; Janczyk, M. Combining Speed and Accuracy to Control for Speed-Accuracy Trade-Offs(?). Behav. Res. Methods 2019, 51, 40–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farina, N.; Lowry, R.G. The Validity of Consumer-Level Activity Monitors in Healthy Older Adults in Free-Living Conditions. J. Aging Phys. Act. 2018, 26, 128–135. [Google Scholar] [CrossRef]
- Ferguson, T.; Rowlands, A.V.; Olds, T.; Maher, C. The Validity of Consumer-Level, Activity Monitors in Healthy Adults Worn in Free-Living Conditions: A Cross-Sectional Study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.S.; Tiedemann, A.; Hassett, L.M.; Ramsay, E.; Kirkham, C.; Chagpar, S.; Sherrington, C. Validity of the Fitbit Activity Tracker for Measuring Steps in Community-Dwelling Older Adults. BMJ Open Sport Exerc. Med. 2015, 1, e000013. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.; Holdt, K.; Beck, E.; Brandes, M.; Pischke, C.; Voelcker-Rehage, C. Toy or Tool? Activity Trackers for the Assessment of Physical Activity in the Wild. In Proceedings of the 2019 IEEE International Conference on Healthcare Informatics (ICHI), Xi’an, China, 10–13 June 2019; pp. 1–9. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Elsevier Science: Burlington, VT, USA, 2013; ISBN 978-1-4832-7648-9. [Google Scholar]
- Haider, S.; Dorner, T.E.; Luger, E.; Kapan, A.; Titze, S.; Lackinger, C.; Schindler, K.E. Impact of a Home-Based Physical and Nutritional Intervention Program Conducted by Lay-Volunteers on Handgrip Strength in Prefrail and Frail Older Adults: A Randomized Control Trial. PLoS ONE 2017, 12, e0169613. [Google Scholar] [CrossRef]
- Takahashi, P.Y.; Quigg, S.M.; Croghan, I.T.; Schroeder, D.R.; Ebbert, J.O. Effect of Pedometer Use and Goal Setting on Walking and Functional Status in Overweight Adults with Multimorbidity: A Crossover Clinical Trial. Clin. Interv. Aging 2016, 11, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Musalek, C.; Kirchengast, S. Grip Strength as an Indicator of Health-Related Quality of Life in Old Age-A Pilot Study. Int. J. Environ. Res. Public Health 2017, 14, 1447. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.L.; Nguyen, U.S.D.T.; Au, E.; Tan, K.C.B.; Kung, A.W.C. Association of Handgrip Strength with Chronic Diseases and Multimorbidity: A Cross-Sectional Study. Age 2013, 35, 929–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Kim, J.; Kim, S.W.; Kong, H.-J. Effects of Home-Based Tele-Exercise on Sarcopenia among Community-Dwelling Elderly Adults: Body Composition and Functional Fitness. Exp. Gerontol. 2017, 87, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Beishuizen, C.R.L.; Stephan, B.C.M.; van Gool, W.A.; Brayne, C.; Peters, R.J.G.; Andrieu, S.; Kivipelto, M.; Soininen, H.; Busschers, W.B.; van Moll Charante, E.P.; et al. Web-Based Interventions Targeting Cardiovascular Risk Factors in Middle-Aged and Older People: A Systematic Review and Meta-Analysis. J. Med. Internet Res. 2016, 18, e55. [Google Scholar] [CrossRef] [PubMed]
- Suboc, T.B.; Strath, S.J.; Dharmashankar, K.; Coulliard, A.; Miller, N.; Wang, J.; Tanner, M.J.; Widlansky, M.E. Relative Importance of Step Count, Intensity, and Duration on Physical Activity’s Impact on Vascular Structure and Function in Previously Sedentary Older Adults. J. Am. Heart Assoc. 2014, 3, e000702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, P.N.; Shumway-Cook, A.; Ciol, M.A. The Effects of a Home-Based Exercise Program on Physical Function in Frail Older Adults. J. Geriatr. Phys. Ther. 2010, 33, 78–84. [Google Scholar]
- Asiri, F.Y.; Marchetti, G.F.; Ellis, J.L.; Otis, L.; Sparto, P.J.; Watzlaf, V.; Whitney, S.L. Effect of Home-Based Rehabilitation on Activities of Daily Living and Gait in Older Adults with Heart Failure at Risk for Falling: A Retrospective Cohort Study. Physiother. Theory Pract. 2017, 33, 943–953. [Google Scholar] [CrossRef]
- Sen, E.I.; Eyigor, S.; Yagli, M.D.; Ozcete, Z.A.; Aydin, T.; Kesiktas, F.N.; Aydin, F.Y.; Vural, M.; Sahin, N.; Karan, A. Effect of Home-Based Exercise Program on Physical Function and Balance in Older Adults With Sarcopenia: A Multicenter Randomized Controlled Study. J. Aging Phys. Act. 2021, 29, 1010–1017. [Google Scholar] [CrossRef]
- Houston, D.K.; Neiberg, R.H.; Miller, M.E.; Hill, J.O.; Jakicic, J.M.; Johnson, K.C.; Gregg, E.W.; van Hubbard, S.; Pi-Sunyer, X.; Rejeski, W.J.; et al. Physical Function Following a Long-Term Lifestyle Intervention Among Middle Aged and Older Adults With Type 2 Diabetes: The Look AHEAD Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1552–1559. [Google Scholar] [CrossRef]
- Kim, I.; Ahn, J. The Effect of Changes in Physical Self-Concept through Participation in Exercise on Changes in Self-Esteem and Mental Well-Being. Int. J. Environ. Res. Public Health 2021, 18, 5224. [Google Scholar] [CrossRef]
- Griffith, K.; Wenzel, J.; Shang, J.; Thompson, C.; Stewart, K.; Mock, V. Impact of a Walking Intervention on Cardiorespiratory Fitness, Self-Reported Physical Function, and Pain in Patients Undergoing Treatment for Solid Tumors. Cancer 2009, 115, 4874–4884. [Google Scholar] [CrossRef]
- Matson, T.E.; Anderson, M.L.; Renz, A.D.; Greenwood-Hickman, M.A.; McClure, J.B.; Rosenberg, D.E. Changes in Self-Reported Health and Psychosocial Outcomes in Older Adults Enrolled in Sedentary Behavior Intervention Study. Am. J. Health Promot. AJHP 2019, 33, 1053–1057. [Google Scholar] [CrossRef]
- Conde-Pipó, J.; Melguizo-Ibáñez, E.; Mariscal-Arcas, M.; Zurita-Ortega, F.; Ubago-Jiménez, J.L.; Ramírez-Granizo, I.; González-Valero, G. Physical Self-Concept Changes in Adults and Older Adults: Influence of Emotional Intelligence, Intrinsic Motivation and Sports Habits. Int. J. Environ. Res. Public Health 2021, 18, 1711. [Google Scholar] [CrossRef]
- Wong, M.Y.C.; Chung, P.K.; Leung, K.M. Examining the Exercise and Self-Esteem Model Revised with Self-Compassion among Hong Kong Secondary School Students Using Structural Equation Modeling. Int. J. Environ. Res. Public Health 2021, 18, 3661. [Google Scholar] [CrossRef]
- Roh, S.Y. The Influence of Physical Self-Perception of Female College Students Participating in Pilates Classes on Perceived Health State and Psychological Wellbeing. J. Exerc. Rehabil. 2018, 14, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherder, E.J.A.; Van Paasschen, J.; Deijen, J.B.; Van Der Knokke, S.; Orlebeke, J.F.K.; Burgers, I.; Devriese, P.P.; Swaab, D.F.; Sergeant, J.A. Physical Activity and Executive Functions in the Elderly with Mild Cognitive Impairment. Aging Ment. Health 2010, 9, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Lautenschlager, N.T.; Cox, K.L.; Flicker, L.; Foster, J.K.; Van Bockxmeer, F.M.; Xiao, J.; Greenop, K.R.; Almeida, O.P. Effect of Physical Activity on Cognitive Function in Older Adults at Risk for Alzheimer Disease: A Randomized Trial. JAMA 2008, 300, 1027–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gelder, B.M.; Tijhuis, M.A.R.; Kalmijn, S.; Giampaoli, S.; Nissinen, A.; Kromhout, D. Physical Activity in Relation to Cognitive Decline in Elderly Men. Neurology 2004, 63, 2316–2321. [Google Scholar] [CrossRef]
- Hendker, A.; Jetzke, M.; Eils, E.; Voelcker-Rehage, C. The Implication of Wearables and the Factors Affecting Their Usage among Recreationally Active People. Int. J. Environ. Res. Public Health 2020, 17, 8532. [Google Scholar] [CrossRef] [PubMed]
- Marcus, B.H.; Lewis, B.A.; Williams, D.M.; Dunsiger, S.; Jakicic, J.M.; Whiteley, J.A.; Albrecht, A.E.; Napolitano, M.A.; Bock, B.C.; Tate, D.F.; et al. A Comparison of Internet and Print-Based Physical Activity Interventions. Arch. Intern. Med. 2007, 167, 944–949. [Google Scholar] [CrossRef] [Green Version]
- Peels, D.A.; Van Stralen, M.M.; Bolman, C.; Golsteijn, R.H.J.; De Vries, H.; Mudde, A.N.; Lechner, L. The Differentiated Effectiveness of a Printed versus a Web-Based Tailored Physical Activity Intervention among Adults Aged over 50. Health Educ. Res. 2014, 29, 870–882. [Google Scholar] [CrossRef] [Green Version]
- Ratz, T.; Lippke, S.; Muellmann, S.; Peters, M.; Pischke, C.R.; Meyer, J.; Bragina, I.; Voelcker-Rehage, C. Effects of Two Web-Based Interventions and Mediating Mechanisms on Stage of Change Regarding Physical Activity in Older Adults. Appl. Psychol. Health Well-Being 2020, 12, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Ratz, T.; Voelcker-Rehage, C.; Pischke, C.R.; Muellmann, S.; Peters, M.; Lippke, S. Health-Related Lifestyle and Dropout From a Web-Based Physical Activity Intervention Trial in Older Adults: A Latent Profile Analysis. Health Psychol. 2021, 40, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Brickwood, K.-J.; Williams, A.D.; Watson, G.; O’Brien, J. Older Adults’ Experiences of Using a Wearable Activity Tracker with Health Professional Feedback over a 12-Month Randomised Controlled Trial. Digit. Health 2020, 6, 2055207620921678. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Petersen, C.L.; Clark, M.M.; Cook, S.B.; Lopez-Jimenez, F.; Al-Nimr, R.I.; Pidgeon, D.; Kotz, D.; Mackenzie, T.A.; Bartels, S.J. A Weight Loss Intervention Augmented by a Wearable Device in Rural Older Adults With Obesity: A Feasibility Study. J. Gerontol. Ser. A 2021, 76, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.; Jarrahi, M.H.; Fei, Y.; Karami, A.; Gafinowitz, N.; Byun, A.; Lu, X. Wearable Activity Trackers, Accuracy, Adoption, Acceptance and Health Impact: A Systematic Literature Review. J. Biomed. Inform. 2019, 93, 103153. [Google Scholar] [CrossRef]

| CG | IG | AG | |
|---|---|---|---|
| N | 136 | 294 | 121 |
| Gender | |||
| female (n, %) | 71, 52.2% | 183, 62.2% | 66, 54.5% |
| male (n, %) | 61, 44.9% | 109, 37.1% | 55, 45.5% |
| Missing (n, %) | 4, 2.9% | 2, 0.7% | 0, 0.0% |
| age (years) (M ± SD) | 70.22 ± 3.02 | 68.71 ± 4.47 | 70.08 ± 4.02 |
| height (cm) (M ± SD) | 169.31 ± 8.72 | 169.88 ± 8.59 | 170.40 ± 8.48 |
| weight (kg) (M ± SD) | 80.23 ± 14.50 | 78.99 ± 14.04 | 78.60 ± 15.28 |
| BMI (kg/(height in m)2) (M ± SD) | 27.96 ± 4.57 | 27.36 ± 4.37 | 26.99 ± 4.40 |
| Inner Subject Contrasts | Between Subject Contrasts | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimension | Parameter | CG | IG | AG | Time | Time * Group | Group | |||||||||||||||
| n | M | SD | n | M | SD | n | M | SD | F-value | p-value | ηp2 | F-value | p-value | ηp2 | F-value | p-value | ηp2 | |||||
| Grip strength [dynamometer] | T0 [kg] | 134 | 34.18 | 9.61 | 198 | 33.76 | 9.82 | 103 | 34.03 | 9.49 | 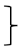 * * | (1, 432) = 8.88 | 0.003 * | 0.020 | (2, 432) = 1.06 | 0.349 | 0.005 | (2, 432) = 0.12 | 0.888 | 0.001 | ||
| T1 [kg] | 134 | 34.42 | 9.68 | 198 | 33.99 | 9.68 | 103 | 34.69 | 10.10 | |||||||||||||
| Endurance [two minute step test] | steps T0 [#] | 135 | 212.61 | 40.52 | 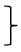 ** ** | 197 | 208.80 | 49.41 | 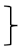 ** ** | 99 | 219.11 | 49.17 | 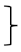 ** ** | (1, 428) = 117.90 | <0.001 ** | 0.216 | (2, 428) = 4.32 | 0.015 * | 0.02 | (2, 428) = 1.68 | 0.188 | 0.008 |
| steps T1 [#] | 135 | 226.75 | 44.97 | 197 | 235.21 | 46.62 | 99 | 240.35 | 50.64 | |||||||||||||
| heart rate T0 [bpm] | 121 | 120.57 | 20.46 | 186 | 126.24 | 27.58 | 96 | 128.01 | 28.77 | (1, 400) = 2.74 | 0.099 | 0.007 | (2, 400) = 0.58 | 0.563 | 0.003 | (2, 400) = 2.74 | 0.066 | 0.013 | ||||
| heart rate T1 [bpm] | 121 | 120.31 | 18.88 | 186 | 124.19 | 21.81 | 96 | 124.36 | 20.32 | |||||||||||||
| heart rate post T0 [bpm] | 119 | 105.03 | 18.30 | 186 | 107.16 | 20.59 | 96 | 109.95 | 22.14 | (1, 398) = 0.09 | 0.759 | 0.000 | (2, 398) = 0.93 | 0.396 | 0.005 | (2, 398) = 0.85 | 0.427 | 0.004 | ||||
| heart rate post T1 [bpm] | 119 | 106.65 | 20.56 | 186 | 107.96 | 21.74 | 96 | 108.38 | 19.54 | |||||||||||||
| Gait [four meter walk test] | T0 [seconds] | 136 | 3.50 | 0.73 | 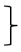 ** ** | 201 | 3.41 | 0.66 | 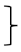 * * | 103 | 3.40 | 0.62 | 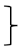 ** ** | (1, 437) = 37.73 | <0.001 ** | 0.079 | (2, 437) = 3.13 | 0.045 * | 0.014 | (2, 437) = 2.10 | 0.124 | 0.010 |
| T1 [seconds] | 136 | 3.27 | 0.63 | 201 | 3.31 | 0.57 | 103 | 3.11 | 0.48 | |||||||||||||
| Physical self-concept [PSDQ] | Score T0 [mean] | 126 | 3.88 | 0.87 | 282 | 3.73 | 0.83 | 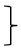 ** ** | 115 | 3.97 | 0.88 | 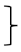 ** ** | (1, 520) = 92.90 | <0.001 ** | 0.152 | (2, 520) = 1.,03 | <0.001 ** | 0.048 | (2, 520) = 4.35 | 0.013 * | 0.016 | |
| Score T1 [mean] | 126 | 3.93 | 0.84 | 282 | 4.01 | 0.78 | 115 | 4.27 | 0.73 | |||||||||||||
| Endurance T0 [score] | 130 | 3.18 | 1.11 | 285 | 3.03 | 1.10 | 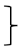 ** ** | 116 | 3.34 | 1.24 |  ** ** | (1, 528) = 39.87 | <0.001 ** | 0.070 | (2, 528) = 6.01 | 0.003 * | 0.022 | (2, 528) = 3.55 | 0.029 * | 0.013 | ||
| Endurance T1 [score] | 130 | 3.23 | 1.09 | 285 | 3.30 | 1.10 | 116 | 3.60 | 1.14 | |||||||||||||
| Coordination T0 [score] | 129 | 4.35 | 0.82 | 285 | 4.31 | 0.82 |  ** ** | 116 | 4.47 | 0.72 | 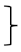 ** ** | (1, 527) = 46.47 | <0.001 ** | 0.081 | ( 2, 527) = 4.27 | 0.014 * | 0.016 | (2, 527) = 1.81 | 0.061 | 0.011 | ||
| Coordination T1 [score] | 129 | 4.41 | 0.75 | 285 | 4.53 | 0.76 | 116 | 4.69 | 0.61 | |||||||||||||
| Strength T0 [score] | 126 | 4.12 | 1.00 | 282 | 3.86 | 0.97 |  ** ** | 115 | 4.11 | 1.03 | 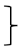 ** ** | (1, 520) = 93.23 | <0.001 ** | 0.152 | (2, 520) = 16.98 | <0.001 ** | 0.061 | (2, 520) = 3.85 | 0.022 * | 0.015 | ||
| Strength T1 [score] | 126 | 4.15 | 1.02 | 282 | 4.22 | 0.89 | 115 | 4.52 | 0.84 | |||||||||||||
| Cognition [Simon Task] | BIS1 T0 | 30 | −0.21 | 1.27 |  * * | 88 | 0.46 | 1.12 | 24 | 0.22 | 1.00 | (1, 139) = 5.79 | 0.017 * | 0.040 | (2, 139) = 3.02 | 0.052 | 0.042 | (2, 139) = 2.70 | 0.07 | 0.037 | ||
| BIS1 T1 | 30 | 0.13 | 0.78 | 88 | 0.44 | 1.10 | 24 | 0.43 | 0.75 | |||||||||||||
| Reaction time T0 [ms] | 30 | 504.55 | 71.20 | 88 | 488.38 | 59.97 |  * * | 24 | 497.61 | 65.72 |  * * | (1, 139) = 19.31 | <0.001 ** | 0.122 | (2, 139) = 2.12 | 0.124 | 0.030 | (2, 139) = 0.48 | 0.619 | 0.007 | ||
| Reaction time T1 [ms] | 30 | 485.51 | 44.37 | 88 | 479.99 | 56.69 | 24 | 470.81 | 36.35 | |||||||||||||
| Accuracy T0 [%] | 30 | 93.67 | 0.04 | 88 | 95.36 | 0.04 | 24 | 94.75 | 0.05 | (1, 139) = 5.22 | 0.024 * | 0.036 | (2, 139) = 1.14 | 0.322 | 0.016 | (2, 139) = 1.53 | 0.221 | 0.022 | ||||
| Accuracy T1 [%] | 30 | 94.80 | 0.03 | 88 | 95.69 | 0.04 | 24 | 95.04 | 0.04 | |||||||||||||
| Group | Parameter (pre * post) | t | Df | p-Value | Cohen’s d |
|---|---|---|---|---|---|
| CG | PSDQ Endurance | 0.688 | 129 | 0.493 | −0.060 |
| PSDQ Coordination | −1.346 | 128 | 0.181 | −0.199 | |
| PSDQ Strength | −0.621 | 125 | 0.536 | −0.055 | |
| PSDQ Overall Score | −1.154 | 125 | 0.251 | −0.103 | |
| IG | PSDQ Endurance | −7.184 | 284 | <0.001 * | −0.426 |
| PSDQ Coordination | −6.862 | 284 | <0.001 * | −0.406 | |
| PSDQ Strength | −10.288 | 281 | <0.001 * | −0.613 | |
| PSDQ Overall Score | −10.245 | 281 | <0.001 * | −0.610 | |
| AG | PSDQ Endurance | −4.639 | 115 | <0.001 * | −0.431 |
| PSDQ Coordination | −4.961 | 115 | <0.001 * | −0.461 | |
| PSDQ Strength | −7.355 | 114 | <0.001 * | −0.686 | |
| PSDQ Overall Score | −7.422 | 114 | <0.001 * | −0.692 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auerswald, T.; Hendker, A.; Ratz, T.; Lippke, S.; Pischke, C.R.; Peters, M.; Meyer, J.; Holdt, K.v.; Voelcker-Rehage, C. Impact of Activity Tracker Usage in Combination with a Physical Activity Intervention on Physical and Cognitive Parameters in Healthy Adults Aged 60+: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 3785. https://doi.org/10.3390/ijerph19073785
Auerswald T, Hendker A, Ratz T, Lippke S, Pischke CR, Peters M, Meyer J, Holdt Kv, Voelcker-Rehage C. Impact of Activity Tracker Usage in Combination with a Physical Activity Intervention on Physical and Cognitive Parameters in Healthy Adults Aged 60+: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2022; 19(7):3785. https://doi.org/10.3390/ijerph19073785
Chicago/Turabian StyleAuerswald, Tina, Anna Hendker, Tiara Ratz, Sonia Lippke, Claudia R. Pischke, Manuela Peters, Jochen Meyer, Kai von Holdt, and Claudia Voelcker-Rehage. 2022. "Impact of Activity Tracker Usage in Combination with a Physical Activity Intervention on Physical and Cognitive Parameters in Healthy Adults Aged 60+: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 19, no. 7: 3785. https://doi.org/10.3390/ijerph19073785
APA StyleAuerswald, T., Hendker, A., Ratz, T., Lippke, S., Pischke, C. R., Peters, M., Meyer, J., Holdt, K. v., & Voelcker-Rehage, C. (2022). Impact of Activity Tracker Usage in Combination with a Physical Activity Intervention on Physical and Cognitive Parameters in Healthy Adults Aged 60+: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 19(7), 3785. https://doi.org/10.3390/ijerph19073785









