Influence of Carbon Sources on Biomass and Biomolecule Accumulation in Picochlorum sp. Cultured under the Mixotrophic Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Collection, Culturing and Microscopic Observation
2.2. Experimental Methodology
2.2.1. Media Selection and Growth Phase
2.2.2. Establishment of Mixotrophic Growth Mode
2.2.3. Effects of Different Carbon Sources in Picochlorum sp.
2.2.4. Effects of Different Concentrations of Sodium Acetate in Picochlorum sp.
2.3. Analytical Methods
Picochlorum sp. BDUG 100241 Growth, Biomass Yield and Productivity
2.4. Harvesting, Extractions and Determination of Lipids and Pigments
2.4.1. Harvesting of Picochlorum sp. Biomass
2.4.2. Lipid Extraction and Quantification
2.4.3. UV-Spectrometric Analysis of Pigments
2.4.4. Extraction of Chlorophyll a and b
2.4.5. Extraction of Total Carotenoids
2.4.6. Extraction and Quantification of Astaxanthin
2.4.7. Extraction and Quantification of β-Carotene
2.5. Statistical Analysis
3. Results and Discussions
3.1. Microscopic Observation and Morphology
3.2. Determination of Pigment Present in Picochlorum sp. Biomass
3.3. Impact of Cultivations Media and Growth Conditions in Picochlorum sp. BDUG100241
3.4. Impact of Organic Carbon Sources on Growth and Biomass Yield
3.5. Impact of Organic Carbon Sources on Lipids and Pigments’ Accumulation
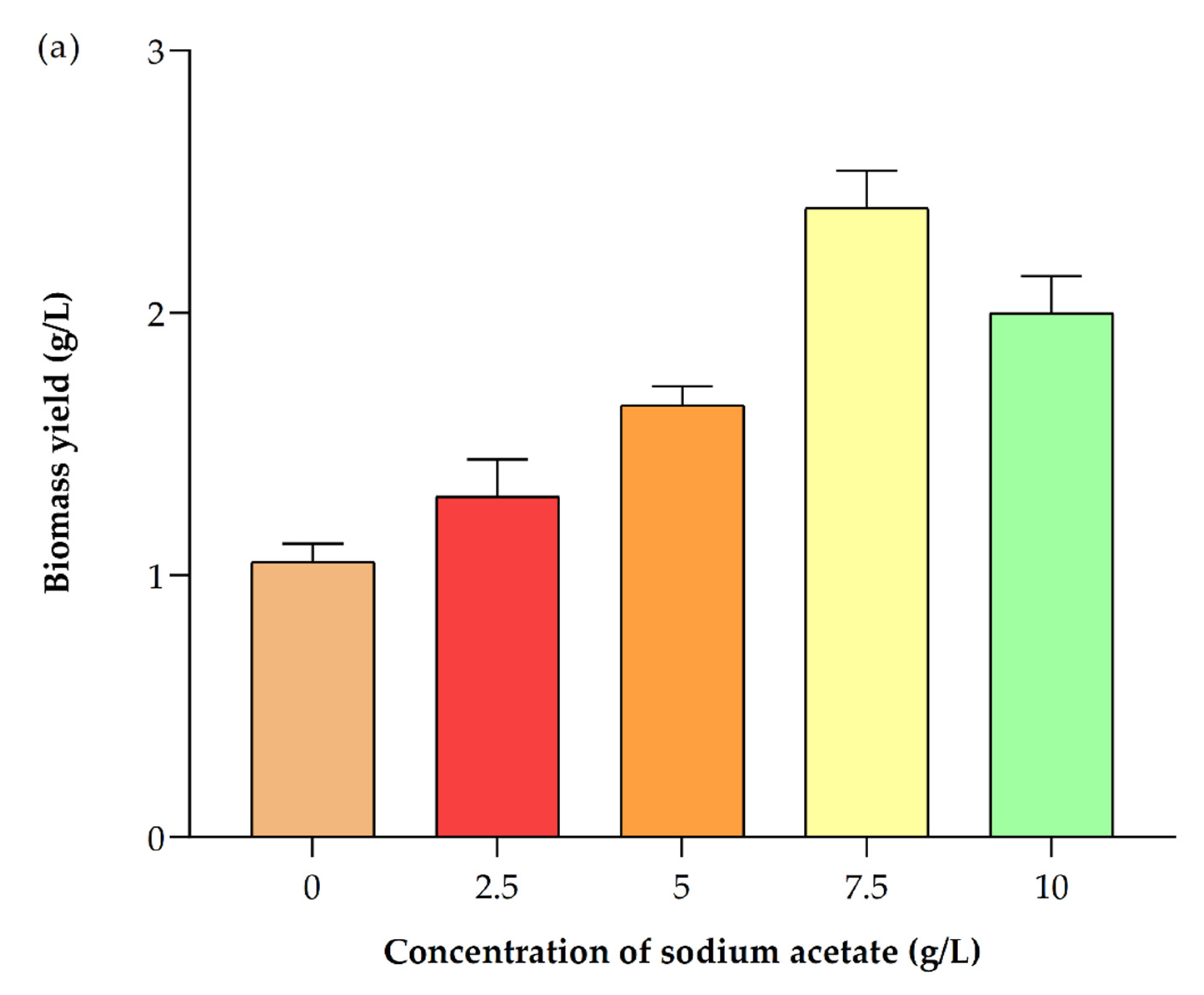
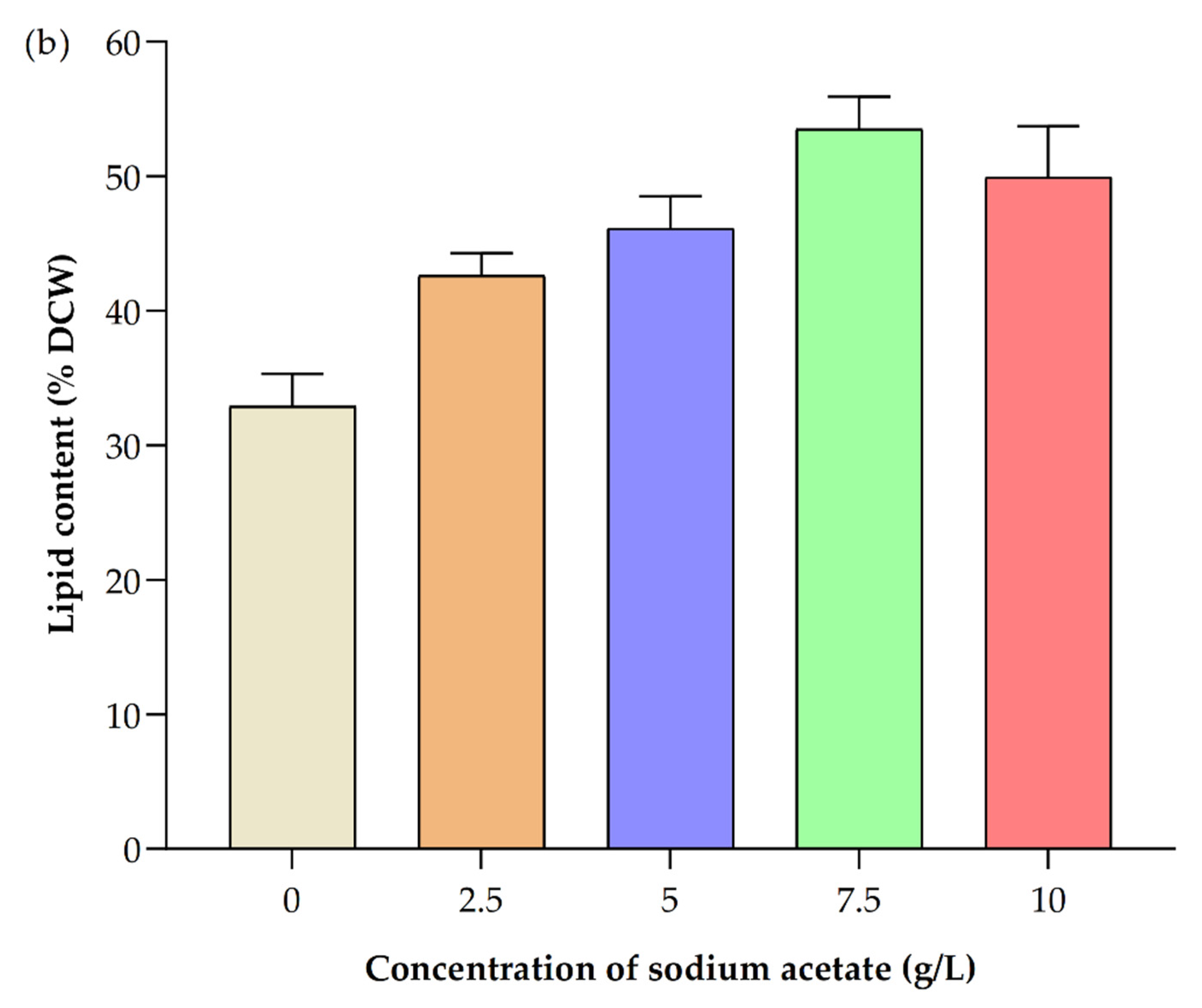
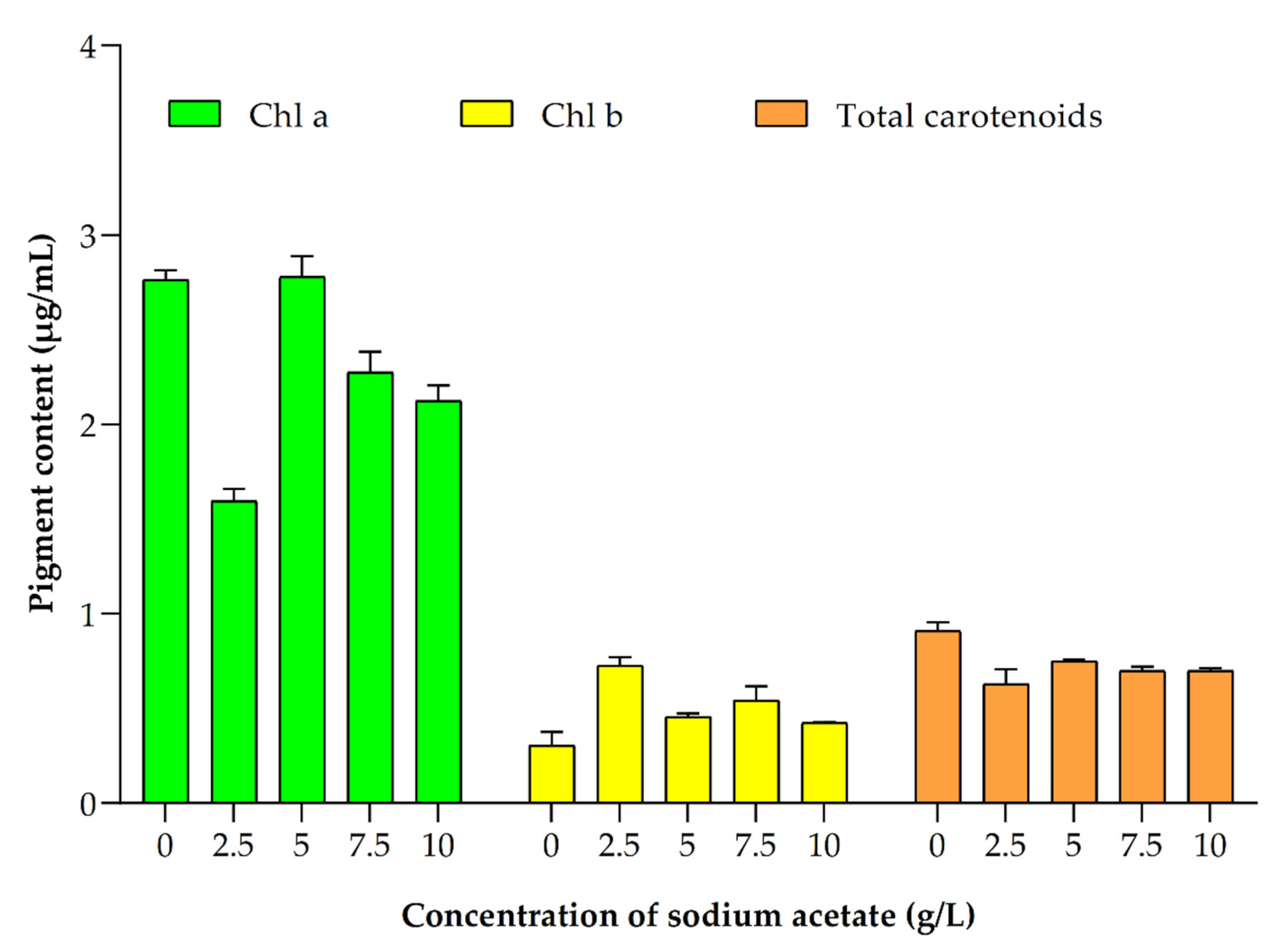
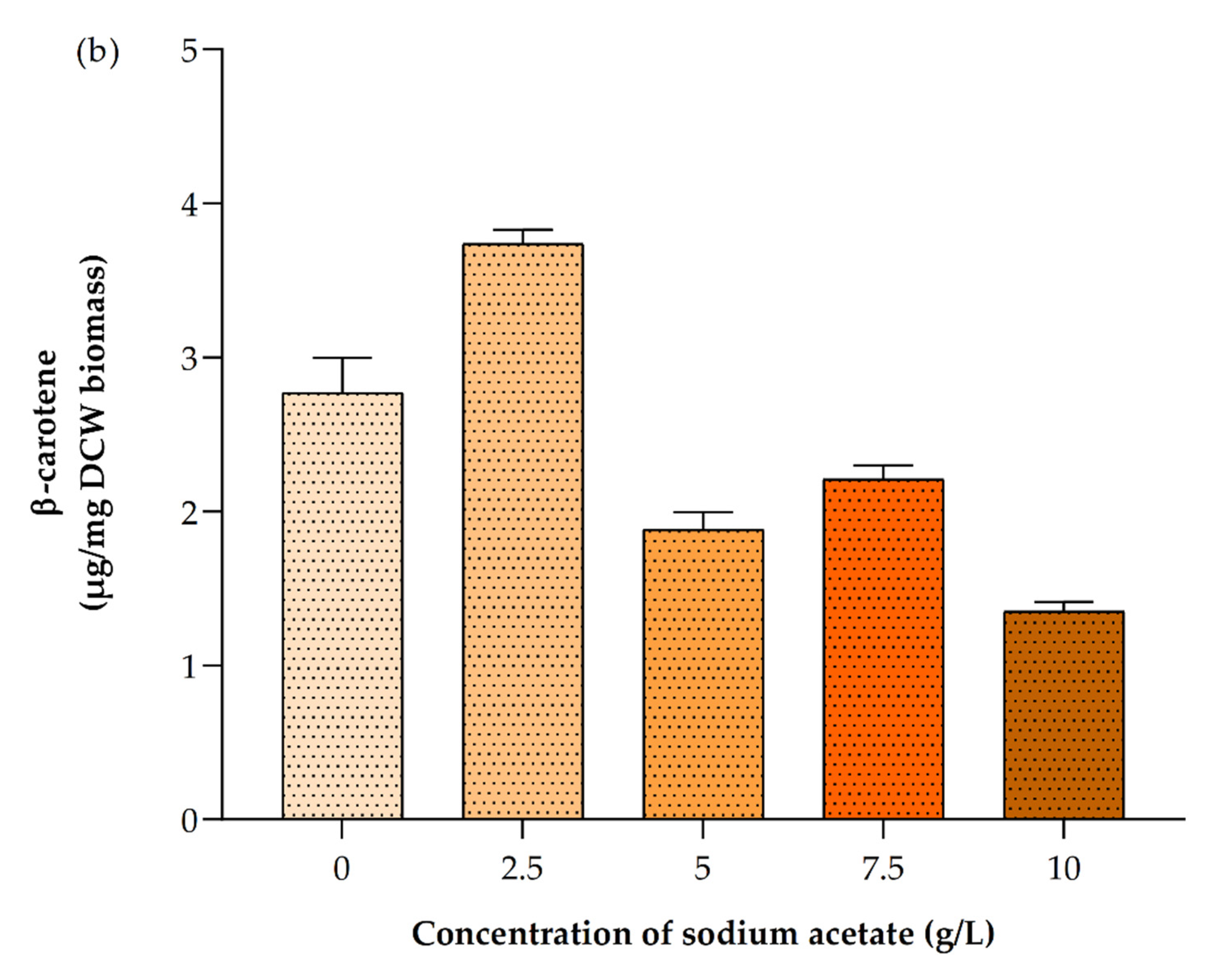
3.6. Impact of Different Concentrations of Sodium Acetate
3.6.1. Impact on Biomass Production
3.6.2. Impact on Lipid Production
3.6.3. Impact on Pigments Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, J.; Fan, W.; Zheng, L. Development of a mixotrophic cultivation strategy for simultaneous improvement of biomass and photosynthetic efficiency in freshwater microalga Scenedesmus obliquus by adding appropriate concentration of sodium acetate. Biochem. Eng. J. 2021, 176, 108177. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Karthikeyan, O.P.; Gupta, V.K.; Verma, P. Multifaceted application of microalgal biomass integrated with carbon dioxide reduction and wastewater remediation: A flexible concept for sustainable environment. J. Clean. Prod. 2022, 339, 130654. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Verma, P. Multifaceted role of microalgae for municipal wastewater treatment: A futuristic outlook towards wastewater management. Clean Soil Air Water 2021, 2021, 2100286. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Verma, P. Microalgae Dunaliella as biofuel feedstock and β-carotene production: An influential step towards environmental sustainability. Energy Convers. Manag. 2021, 13, 100154. [Google Scholar] [CrossRef]
- Huang, A.; Sun, L.; Wu, S.; Liu, C.; Zhao, P.; Xie, X.; Wang, G. Utilization of glucose and acetate by Chlorella and the effect of multiple factors on cell composition. J. Appl. Phycol. 2017, 29, 23–33. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Mehariya, S.; Verma, P. Current perspective on wastewater treatment using photobioreactor for Tetraselmis sp.: An emerging and foreseeable sustainable approach. Environ. Sci. Pollut. Res. 2021, 2021, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Agrawal, K.; Mehariya, S.; Molino, A.; Musmarra, D.; Verma, P. Microalgae-based biorefinery for utilization of carbon dioxide for production of valuable bioproducts. In Chemo-Biological Systems for CO2 Utilization; Kumar, A., Sharma, S., Eds.; CRC Press: Hoboken, NJ, USA, 2020; pp. 203–228. [Google Scholar]
- Vega, D.L.M.; Díaz, E.; Vila, M.; León, R. Isolation of a new strain of Picochlorum Sp. and characterization of its potential biotechnological applications. Biotechnol. Prog. 2011, 27, 1535–1543. [Google Scholar] [CrossRef]
- Marudhupandi, T.; Sathishkumar, R.; Kumar, T.T.A. Heterotrophic cultivation of Nannochloropsis salina for enhancing biomass and lipid production. Biotechnol. Rep. 2016, 10, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Pawar, S.B. Mixotrophic cultivation of microalgae to enhance the quality of lipid for biodiesel application: Effects of scale of cultivation and light spectrum on reduction of α-linolenic acid. Bioprocess Biosyst. Eng. 2018, 41, 531–542. [Google Scholar] [CrossRef]
- Foflonker, F.; Ananyev, G.; Qiu, H.; Morrison, A.; Palenik, B.; Dismukes, G.C.; Bhattacharya, D. The unexpected extremophile: Tolerance to fluctuating salinity in the green alga Picochlorum. Algal Res. 2016, 16, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Foflonker, F.; Mollegard, D.; Ong, M.; Yoon, H.S.; Bhattacharya, D. Genomic analysis of Picochlorum species reveals how microalgae may adapt to variable environments. Mol. Biol. Evol. 2018, 35, 2702–2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, R.K.; Agrawal, K.; Verma, P. Phycoremediation of nitrogen and phosphate from wastewater using Picochlorum Sp.: A Tenable Approach. J. Basic Microbiol. 2021, 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, L.R.; Gerritsen, A.T.; Henard, C.A.; van Wychen, S.; Linger, J.G.; Kunde, Y.; Hovde, B.T.; Starkenburg, S.R.; Posewitz, M.C.; Guarnieri, M.T. Development of a high-productivity, halophilic, thermotolerant microalga Picochlorum Renovo. Commun. Biol. 2019, 2, 388. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Esquer, C.R.; Wright, K.T.; Sudasinghe, N.; Carr, C.K.; Sanders, C.K.; Turmo, A.; Kerfeld, C.A.; Twary, S.; Dale, T. Demonstration of the potential of Picochlorum Soloecismus as a microalgal platform for the production of renewable fuels. Algal Res. 2019, 43, 101658. [Google Scholar] [CrossRef]
- Moussa, I.D.-B.; Chtourou, H.; Hassairi, I.; Sayadi, S.; Dhouib, A. The Effect of Switching Environmental Conditions on Content and Structure of Lipid Produced by a Wild Strain Picochlorum sp. Renew. Energy 2019, 134, 406–415. [Google Scholar] [CrossRef]
- Rippka, E.; Deruelles, J.; Waterbury, N.B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Sathasivam, R.; Juntawong, N. Modified medium for enhanced growth of Dunaliella Strains. Int. J. Curr. Sci. 2013, 5, 67–73. [Google Scholar]
- Bhatnagar, A.; Chinnasamy, S.; Singh, M.; Das, K.C. Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl. Energy 2011, 88, 3425–3431. [Google Scholar] [CrossRef]
- Mitra, M.; Shah, F.; Bharadwaj, S.V.V.; Patidar, S.K.; Mishra, S. Cultivation of Nannochloropsis Oceanica biomass rich in eicosapentaenoic acid utilizing wastewater as nutrient resource. Bioresour. Technol. 2016, 218, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Patel, A.; Pruthi, P.A.; Pruthi, V. Synergistic dynamics of nitrogen and phosphorous influences lipid productivity in Chlorella Minutissima for biodiesel production. Bioresour. Technol. 2016, 213, 79–87. [Google Scholar] [CrossRef]
- Arora, N.; Jaiswal, K.K.; Kumar, V.; Vlaskin, M.S.; Nanda, M.; Pruthi, V.; Chauhan, P.K. Small-scale phyco-mitigation of raw urban wastewater integrated with biodiesel production and its utilization for aquaculture. Bioresour. Technol. 2020, 297, 122489. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Pub. Health 2018, 15, 2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriques, M.; Silva, A.; Rocha, J. Extraction and quantification of pigments from a marine microalga: A simple and reproducible method. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. Formatex 2007, 2, 586–593. [Google Scholar]
- Morowvat, M.H.; Ghasemi, Y. Culture medium optimization for enhanced β-carotene and biomass production by Dunaliella Salina in mixotrophic culture. Biocatal. Agric. Biotechnol. 2016, 7, 217–223. [Google Scholar] [CrossRef]
- Hashemi, A.; Pajoum Shariati, F.; Heydarinasab, A. The effect of instantaneous and slow-release salt stress methods on beta-carotene production within Dunaliella Salina Cells. Iran. J. Chem. Chem. Eng. 2021, 40, 1642–1652. [Google Scholar]
- Jain, A.; Behera, B.; Paramasivan, B. Evaluation of physicochemical procedures for pigment extraction from mixed microalgal consortium. Bioresour. Technol. Rep. 2021, 15, 100775. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Cano, M.; Karns, D.A.J.; Weissman, J.C.; Heinnickel, M.L.; Posewitz, M.C. Pigment modulation in response to irradiance intensity in the fast-growing alga Picochlorum celeri. Algal Res. 2021, 58, 102370. [Google Scholar] [CrossRef]
- Barten, R.; Kleisman, M.; D’Ermo, G.; Nijveen, H.; Wijffels, R.H.; Barbosa, M.J. K Short-Term Physiologic Physiologic Response of the Green Microalga Picochlorum Sp. (BPE23) to Supra-Optimal Temperature. Sci. Rep. 2022, 12, 3290. [Google Scholar] [CrossRef]
- Pereira, H.; Custódio, L.; Rodrigues, M.J.; de Sousa, C.B.; Oliveira, M.; Barreira, L.; Neng, N.D.R.; Nogueira, J.M.F.; Alrokayan, S.A.; Mouffouk, F.; et al. Biological activities and chemical composition of methanolic extracts of selected autochthonous microalgae strains from the red sea. Mar. Drugs 2015, 13, 3531–3549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, M.; Liu, K.; Liu, H. Evidence for mixotrophy in pico-chlorophytes from a new Picochlorum (Trebouxiophyceae) Strain. J. Phycol. 2021, 58, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Olia, M.S.J.; Azin, M.; Sepahi, A.A.; Moazami, N. Miniaturized culture method for the statistical study of growth rate and carbohydrate content of Picochlorum Sp. D8 isolated from the Persian Gulf. Renew. Energy 2020, 149, 479–488. [Google Scholar] [CrossRef]
- Abreu, A.P.; Fernandes, B.; Vicente, A.A.; Teixeira, J.; Dragone, G. Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 2012, 118, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.S.; Wu, J.Y. Effect of carbon sources on growth and lipid accumulation of newly isolated microalgae cultured under mixotrophic condition. Bioresour. Technol. 2015, 184, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Yu, S.W. Influence of crude glycerol on the biomass and lipid content of microalgae. Biotechnol. Biotechnol. Equip. 2015, 29, 506–513. [Google Scholar] [CrossRef]
- Skorupskaite, V.; Makareviciene, V.; Levisauskas, D. Optimization of mixotrophic cultivation of microalgae Chlorella sp. for biofuel production using response surface methodology. Algal Res. 2015, 7, 45–50. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Verma, P. An overview of microalgal carotenoids: Advances in the production and its impact on sustainable development. In Bioenergy Research: Evaluating Strategies for Commercialization and Sustainability; Wiley: Hoboken, NJ, USA, 2021; pp. 105–128. [Google Scholar]
- Kong, W.; Song, H.; Cao, Y.; Yang, H.; Hua, S.; Xia, C. The characteristics of biomass production, lipid accumulation and chlorophyll biosynthesis of Chlorella Vulgaris under mixotrophic cultivation. Afr. J. Biotechnol. 2011, 10, 11620–11630. [Google Scholar]
- Wen, X.; Wang, Z.; Ding, Y.; Geng, Y.; Li, Y. Enhancing the production of astaxanthin by mixotrophic cultivation of Haematococcus pluvialis in open raceway ponds. Aquac. Int. 2020, 28, 625–638. [Google Scholar] [CrossRef]
- Rai, M.P.; Nigam, S.; Sharma, R. Response of growth and fatty acid compositions o6f Chlorella Pyrenoidosa under mixotrophic cultivation with acetate and glycerol for bioenergy application. Biomass Bioener. 2013, 58, 251–257. [Google Scholar] [CrossRef]
- García, M.C.C.; Mirón, A.S.; Sevilla, J.M.F.; Grima, E.M.; Camacho, F.G. Mixotrophic growth of the microalga Phaeodactylum tricornutum: Influence of different nitrogen and organic carbon sources on productivity and biomass composition. Process Biochem. 2005, 40, 297–305. [Google Scholar] [CrossRef]
- White, D.A.; Pagarette, A.; Rooks, P.; Ali, S.T. The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures. J. Appl. Phycol. 2013, 25, 153–165. [Google Scholar] [CrossRef]
- Ghosh, A.; Sangtani, R.; Samadhiya, K.; Kiran, B. Maximizing intrinsic value of microalgae using multi-parameter study: Conjoint effect of organic carbon, nitrate, and phosphate supplementation. Clean Technol. Environ. Policy 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S.; Lin, Z.; Lin, M. Enhanced microalgae growth for biodiesel production and nutrients removal in raw swine wastewater by carbon sources supplementation. Waste Biomass Valor. 2021, 12, 1991–1999. [Google Scholar] [CrossRef]
- Azma, M.; Mohamed, R.; Rahim, R.A.; Ariff, A.B. Improvement of medium composition for heterotrophic cultivation of green microalgae, Tetraselmis suecica, using response surface methodology. Biochem. Eng. J. 2011, 53, 187–195. [Google Scholar] [CrossRef]
- Silva, H.R.; Prete, C.E.C.; Zambrano, F.; de Mello, V.H.; Tischer, C.A.; Andrade, D.S. Combining glucose and sodium acetate improves the growth of Neochloris oleoabundans under mixotrophic conditions. AMB Express 2016, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Ra, C.H.; Kang, C.H.; Jung, J.H.; Jeong, G.T.; Kim, S.K. Enhanced biomass production and lipid accumulation of Picochlorum atomus using light-emitting diodes (LEDs). Bioresour. Technol. 2016, 218, 1279–1283. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Goswami, S. Microalgae—A green multi-product biorefinery for future industrial prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Verma, P. Microalgae-based biofuel-integrated biorefinery approach as sustainable feedstock for resolving energy crisis. In Bioenergy Research: Commercial Opportunities & Challenges; Srivastava, M., Srivastava, N., Singh, R., Eds.; Springer: Singapore, 2021; pp. 267–293. [Google Scholar]
- Heo, J.; Shin, D.S.; Cho, K.; Cho, D.H.; Lee, Y.J.; Kim, H.S. Indigenous microalga Parachlorella sp. JD-076 as a potential source for lutein production: Optimization of lutein productivity via regulation of light intensity and carbon source. Algal Res. 2018, 33, 1–7. [Google Scholar] [CrossRef]

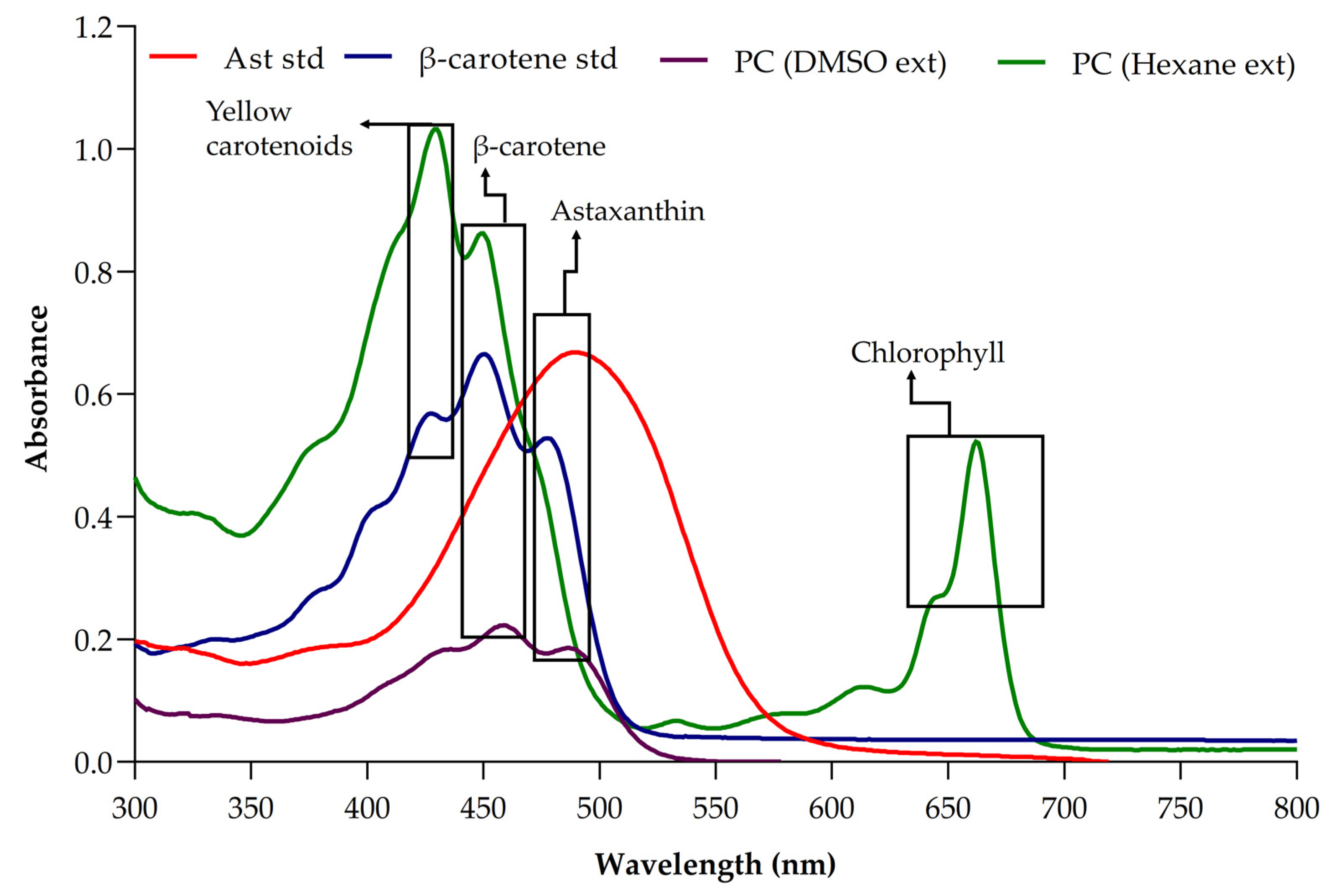
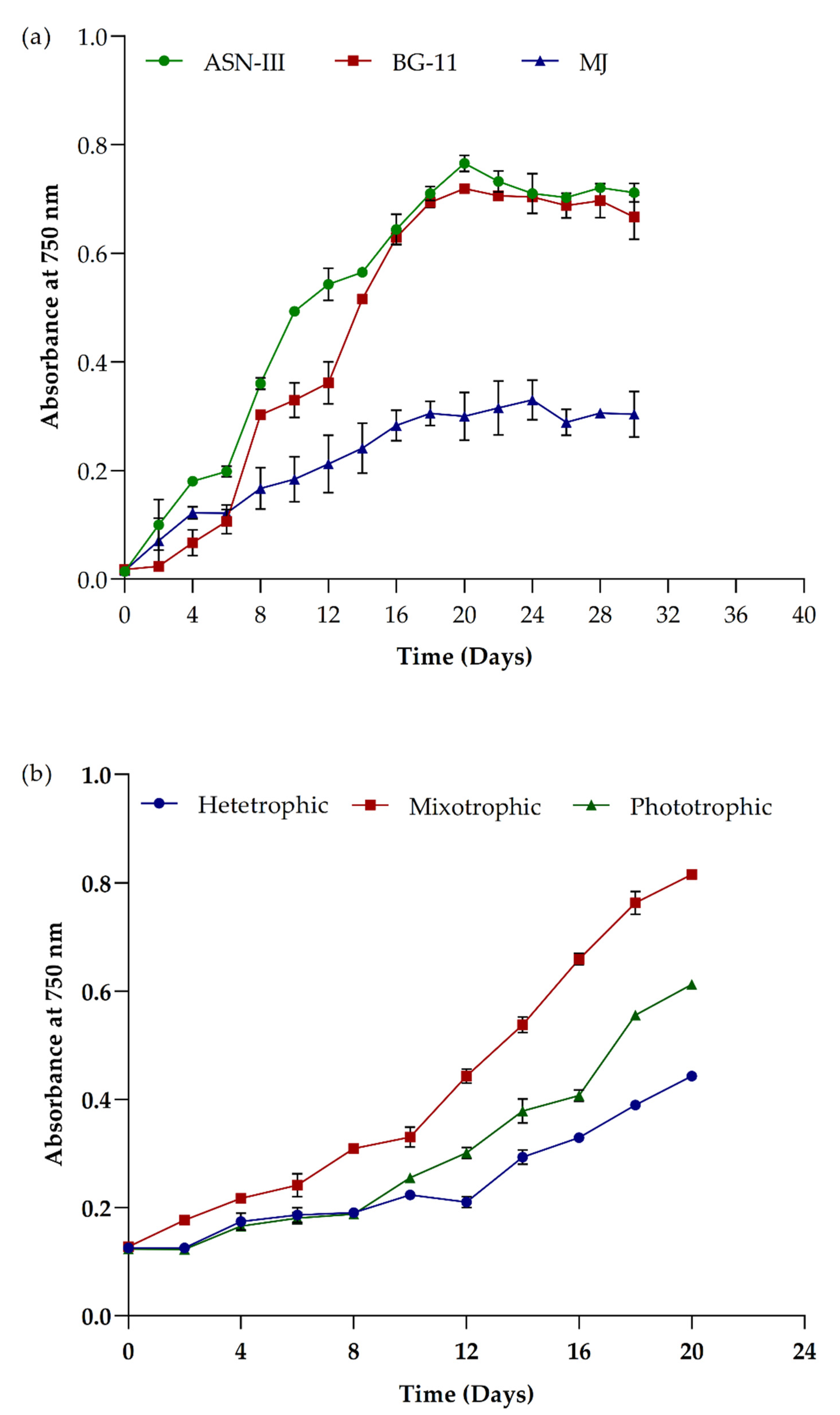
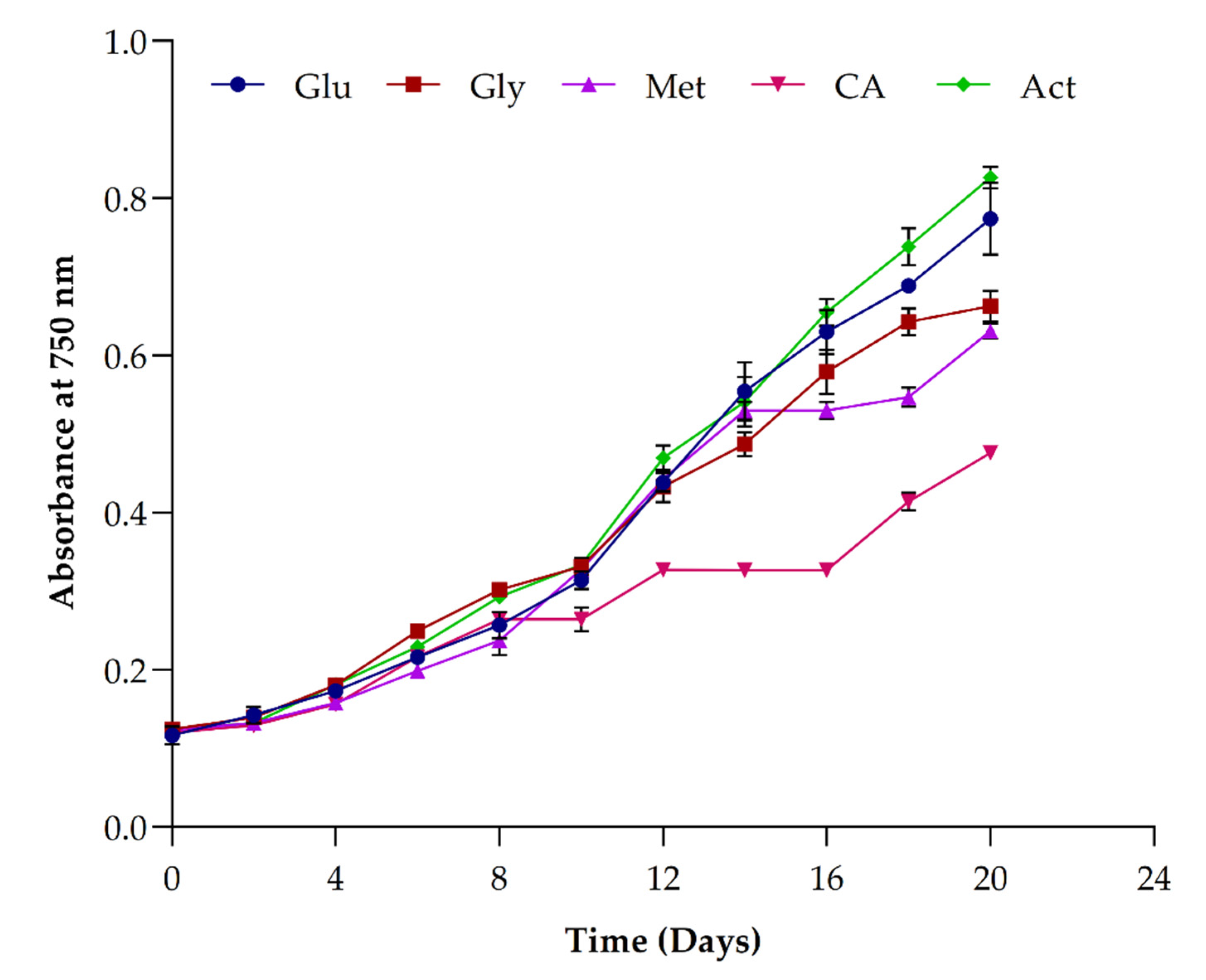
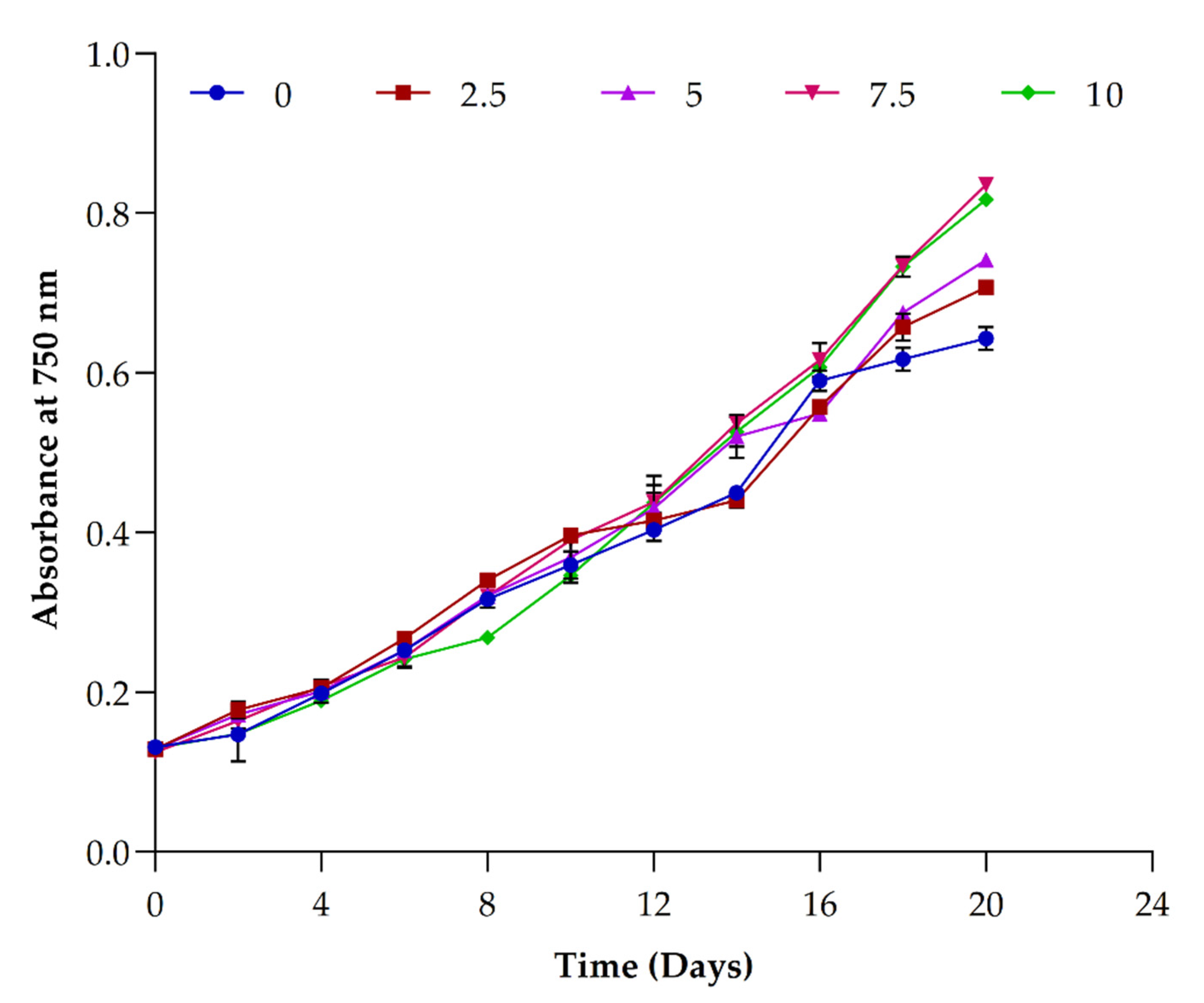

| Carbon Sources | Biomass Yield (g/L) | Biomass Productivity (mg/L/d) | Lipid Content (%) in Dry Cell Weight (DCW) | Chla (µg/mL) | Chlb (µg/mL) | Total Carotenoids (µg/mL) | Astaxanthin (µg/mg DCW Biomass) | β-Carotene (µg/mg DCW Biomass) |
|---|---|---|---|---|---|---|---|---|
| Glu | 1.30 ± 0.12 | 50.00 ± 6.01 | 52 ± 2.8 | 2.49 ± 0.21 | 0.35 ± 0.09 | 0.71 ± 0.10 | 0.53 ± 0.01 | 1.30 ± 0.37 |
| Gly | 0.76 ± 0.20 | 22.78 ± 9.77 | 51 ± 1.4 | 1.48 ± 0.03 | 0.32 ± 0.015 | 0.56 ± 0.07 | 0.35 ± 0.02 | 1.89 ± 0.21 |
| Met | 0.74 ± 0.17 | 22.22 ± 8.55 | 50 ± 2.8 | 1.44 ± 0.35 | 0.30 ± 0.007 | 0.60 ± 0.06 | 0.64 ± 0.04 | 2.46 ± 0.12 |
| CA | 0.68 ± 0.25 | 18.89 ± 12.62 | 50 ± 2.8 | 0.92 ± 0.05 | 0.24 ± 0.013 | 0.44 ± 0.04 | 0.37 ± 0.04 | 2.72 ± 0.04 |
| Act | 1.67 ± 0.18 | 68.33 ± 8.82 | 51 ± 2.8 | 2.68 ± 0.29 | 0.40 ± 0.046 | 0.71 ± 0.06 | 0.59 ± 0.02 | 1.89 ± 0.07 |
| Sodium Acetate Concentration g/L | Biomass Yield (g/L) | Biomass Productivity (mg/L/d) | Lipid Content (%) in Dry Cell Weight (DCW) | Chla (µg/mL) | Chlb (µg/mL) | Total Carotenoids (µg/mL) | Astaxanthin (µg/mg DCW Biomass) | β-Carotene (µg/mg DCW Biomass) |
|---|---|---|---|---|---|---|---|---|
| 0 | 1.10 ± 0.07 | 27.50 ± 3.54 | 32.90 ± 2.40 | 2.76 ± 0.07 | 0.30 ± 0.10 | 0.91 ± 0.06 | 0.35 ± 0.01 | 2.77 ± 0.32 |
| 2.5 | 1.30 ± 0.14 | 40.00 ± 7.07 | 42.60 ± 1.70 | 1.60 ± 0.09 | 0.73 ± 0.06 | 0.63 ± 0.11 | 0.37 ± 0.02 | 3.74 ± 0.12 |
| 5 | 1.65 ± 0.07 | 57.50 ± 3.54 | 46.10 ± 2.40 | 2.78 ± 0.15 | 0.46 ± 0.02 | 0.75 ± 0.01 | 0.38 ± 0.03 | 1.88 ± 0.16 |
| 7.5 | 2.40 ± 0.14 | 95.00 ± 7.07 | 53.50 ± 2.4 | 2.82 ± 0.15 | 0.54 ± 0.10 | 0.70 ± 0.03 | 0.044 ± 0.01 | 2.21 ± 0.12 |
| 10 | 2.00 ± 0.14 | 75.00 ± 7.07 | 49.90 ± 3.82 | 2.13 ± 0.11 | 0.42 ± 0.00 | 0.70 ± 0.02 | 0.55 ± 0.03 | 1.35 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goswami, R.K.; Mehariya, S.; Karthikeyan, O.P.; Verma, P. Influence of Carbon Sources on Biomass and Biomolecule Accumulation in Picochlorum sp. Cultured under the Mixotrophic Condition. Int. J. Environ. Res. Public Health 2022, 19, 3674. https://doi.org/10.3390/ijerph19063674
Goswami RK, Mehariya S, Karthikeyan OP, Verma P. Influence of Carbon Sources on Biomass and Biomolecule Accumulation in Picochlorum sp. Cultured under the Mixotrophic Condition. International Journal of Environmental Research and Public Health. 2022; 19(6):3674. https://doi.org/10.3390/ijerph19063674
Chicago/Turabian StyleGoswami, Rahul Kumar, Sanjeet Mehariya, Obulisamy Parthiba Karthikeyan, and Pradeep Verma. 2022. "Influence of Carbon Sources on Biomass and Biomolecule Accumulation in Picochlorum sp. Cultured under the Mixotrophic Condition" International Journal of Environmental Research and Public Health 19, no. 6: 3674. https://doi.org/10.3390/ijerph19063674
APA StyleGoswami, R. K., Mehariya, S., Karthikeyan, O. P., & Verma, P. (2022). Influence of Carbon Sources on Biomass and Biomolecule Accumulation in Picochlorum sp. Cultured under the Mixotrophic Condition. International Journal of Environmental Research and Public Health, 19(6), 3674. https://doi.org/10.3390/ijerph19063674









