The Relationship between Alexithymia and Mental Health Is Fully Mediated by Anxiety and Depression in Patients with Psoriasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Measures
2.3. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Differences between Alexithymic and Non-Alexithymic Patients
3.3. Correlations between Variables
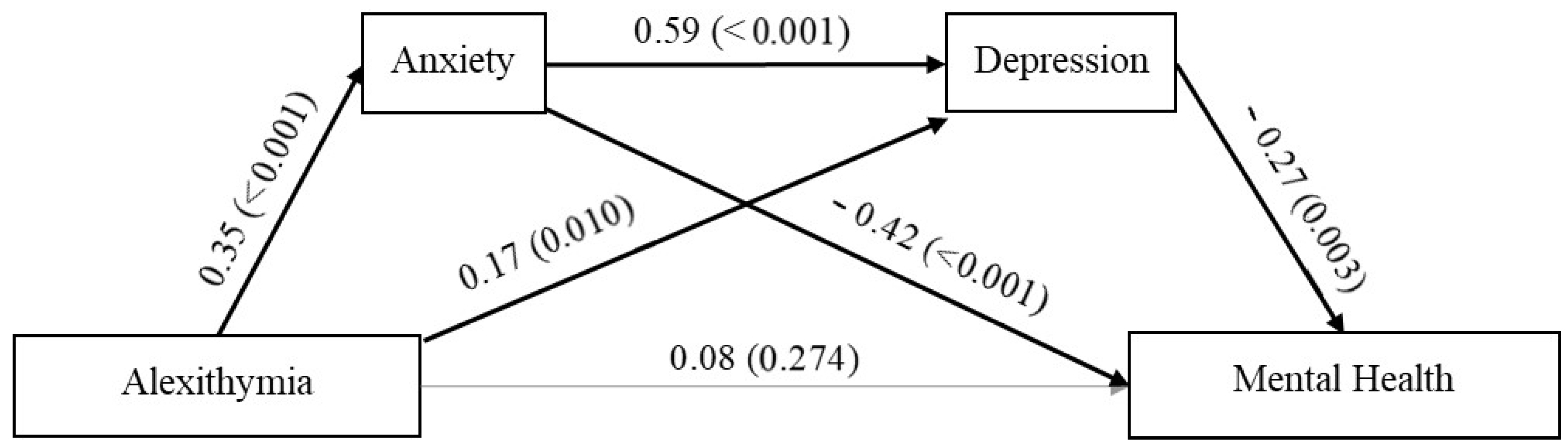
3.4. Serial Mediation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Augustin, M.; Radtke, M.A. Quality of life in psoriasis patients. Expert. Rev. Pharmacoecon. Outcomes Res. 2014, 14, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Innamorati, M.; Quinto, R.M.; Lester, D.; Iani, L.; Graceffa, D.; Bonifati, C. Cognitive impairment in patients with psoriasis: A matched case-control study. J. Psychosom. Res. 2018, 105, 99–105. [Google Scholar] [CrossRef] [PubMed]
- De Korte, J.; Mombers, F.M.C.; Bos, J.D.; Sprangers, M.A.G. Quality of Life in Patients with Psoriasis: A Systematic Literature Review. J. Investig. Dermatol. 2004, 9, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pompili, M.; Innamorati, M.; Forte, A.; Erbuto, D.; Lamis, D.A.; Narcisi, A.; Rea, C.; Orsini, D.; D’Arino, D.; Arcese, A.; et al. Psychiatric comorbidity and suicidal ideation in psoriasis, melanoma and allergic disorders. Int. J. Psychiatry Clin. Pract. 2017, 21, 209–214. [Google Scholar] [CrossRef]
- Fleming, P.; Bai, J.W.; Pratt, M.; Sibbald, C.; Lynde, C.; Gulliver, W.P. The prevalence of anxiety in patients with psoriasis: A systematic review of observational studies and clinical trials. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 798–807. [Google Scholar] [CrossRef]
- Dowlatshahi, E.A.; Wakkee, M.; Arends, L.R.; Nijsten, T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: A systematic review and meta-analysis. J. Investig. Dermatol. 2014, 134, 1542–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iani, L.; Quinto, R.M.; Porcelli, P.; Angeramo, A.R.; Schiralli, A.; Abeni, D. Positive psychological factors are associated with better spiritual well-being and lower distress in individuals with skin diseases. Front. Psychol. 2020, 11, 552764. [Google Scholar] [CrossRef] [PubMed]
- Innamorati, M.; Quinto, R.M.; Imperatori, C.; Lora, V.; Graceffa, G.; Fabbricatore, M.; Lester, D.; Contardi, A.; Bonifati, C. Health-related quality of life and its association with alexithymia and difficulties in emotion regulation in patients with psoriasis. Compr. Psychiatry 2016, 70, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Taylor, C.; Kornmehl, H.; Armstrong, A.W. Psoriasis and suicidality: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 425–440. [Google Scholar] [CrossRef]
- Liang, S.E.; Cohen, J.M.; Ho, R.S. Psoriasis and suicidality: A review of the literature. Dermatol. Ther. 2019, 32, e12771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, C.C.; Chen, T.H.; Wang, S.H.; Tung, T.H. Risk of suicidality in people with psoriasis: A systematic review and meta-analysis of cohort studies. Am. J. Clin. Dermatol. 2017, 18, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Nowowiejska, J.; Baran, A.; Grabowska, P.; Lewoc, M.; Kaminski, T.W.; Flisiak, I. Assessment of life quality, stress and physical activity among patients with psoriasis. Dermatol. Ther. 2022, 12, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.; Yen, H.; Chi, C.C. Is psoriasis associated with dementia or cognitive impairment? A Critically Appraised Topic. Br. J. Dermatol. 2021, 184, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Hedemann, T.L.; Liu, X.; Kang, C.N.; Husain, M.I. Associations between psoriasis and mental illness: An update for clinicians. Gen. Hosp. Psychiatry 2022, 75, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Thorslund, K.; Amatya, B.; Dufva, A.E.; Nordlind, K. The expression of serotonin transporter protein correlates with the severity of psoriasis and chronic stress. Arch. Dermatol. Res. 2013, 305, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Bagby, R.M.; Taylor, G.J. Affect dysregulation and alexithymia. In Disorders of Affect Regulation. Alexithymia in Medical and Psychiatric Illness; Taylor, G.J., Bagby, R.M., Parker, J.D.A., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 26–45. [Google Scholar]
- Cherrez-Ojeda, I.; Vanegas, E.; Felix, M.; Cherrez, S.; Suárez-Almendariz, D.; Ponton, J.; Preciado, V.; Ollague-Cordova, E.; Loayza, E. Alexithymia in Patients with Psoriasis: A Cross-Sectional Study from Ecuador. Psychol. Res. Behav. Manag. 2019, 12, 1121–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korkoliakou, P.; Christodoulou, C.; Kouris, A.; Porichi, E.; Efstathiou, V.; Kaloudi, E.; Kokkevi, A.; Stavrianeas, N.; Papageorgiou, C.; Douzenis, A. Alexithymia, anxiety and depression in patients with psoriasis: A case–control study. Ann. Gen. Psychiatry 2014, 13, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinto, R.M.; Sampogna, F.; Fania, L.; Ciccone, D.; Fusari, R.; Mastroeni, S.; Iani, L.; Abeni, D. Alexithymia, psychological distress, and social impairment in patients with hidradenitis suppurativa. Dermatology 2021, 237, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Sampogna, F.; Puig, L.; Spuls, P.; Girolomoni, G.; Radtke, M.A.; Kirby, B.; Brunori, M.; Bergmans, P.; Smirnov, P.; Rundle, J.; et al. EPIDEPSO Investigators. Prevalence of alexithymia in patients with psoriasis and its association with disease burden: A multicentre observational study. Br. J. Dermatol. 2017, 176, 1195–1203. [Google Scholar] [CrossRef]
- Founta, O.; Adamzik, K.; Tobin, A.M.; Kirby, B.; Hevey, D. Psychological distress, alexithymia and alcohol misuse in patients with psoriasis: A cross-sectional study. J. Clin. Psychol. Med. Settings 2019, 26, 200–219. [Google Scholar] [CrossRef]
- Korkoliakou, P.; Efstathiou, V.; Giannopoulou, I.; Christodoulou, C.; Kouris, A.; Rigopoulos, D.; Douzenis, A. Psychopathology and alexithymia in patients with psoriasis. Ann. Bras. Dermatol. 2017, 92, 510–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honkalampi, K.; De Berardis, D.; Vellante, F.; Viinamäki, H. Relations between alexithymia and depressive and anxiety disorders and personality. In Alexithymia: Advances in Research, Theory, and Clinical; Luminet, O., Bagby, R.M., Taylor, G.J., Eds.; Cambridge University Press: Cambridge, UK, 2018; pp. 142–157. [Google Scholar]
- Nonterah, C.W.; Marek, R.J.; Borckardt, J.J.; Balliet, W.E. Impact of Alexithymia on Organ Transplant Candidates’ Quality of Life: The Mediating Role of Depressive Symptoms. Psychol. Rep. 2020, 123, 1614–1634. [Google Scholar] [CrossRef] [PubMed]
- Lumley, M.A. Alexithymia and negative emotional conditions. J. Psychosom. Res. 2000, 49, 51–54. [Google Scholar] [CrossRef]
- Freyberger, H. Supportive psychotherapy techniques in primary and secondary alexithymia. Psychother. Psychosom. 1977, 28, 337–342. [Google Scholar] [CrossRef] [PubMed]
- De Vente, W.; Kamphuis, J.H.; Emmelkamp, P.M. Alexithymia, risk factor or consequence of work-related stress? Psychother. Psychosom. 2006, 75, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Wise, T.N.; Mann, L.S.; Mitchell, J.D.; Hryvniak, M.; Hill, B. Secondary alexithymia: An empirical validation. Compr. Psychiatry 1990, 31, 284–288. [Google Scholar] [CrossRef]
- Hemming, L.; Haddock, G.; Shaw, J.; Pratt, D. Alexithymia and its associations with depression, suicidality, and aggression: An overview of the literature. Front. Psychiatry 2019, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, C.; Brusamonti, E.; Maggini, C. Are alexithymia, depression, and anxiety distinct constructs in affective disorders? J. Psychosom. Res. 2000, 49, 43–49. [Google Scholar] [CrossRef]
- Tesio, V.; Di Tella, M.; Ghiggia, A.; Romeo, A.; Colonna, F.; Fusaro, E.; Geminiani, G.C.; Castelli, L. Alexithymia and depression affect quality of life in patients with chronic pain: A study on 205 patients with fibromyalgia. Front. Psychol. 2018, 9, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbasio, C.; Vagelli, R.; Marengo, D.; Querci, F.; Settanni, M.; Tani, C.; Mosca, M.; Granieri, M. Illness perception in systemic lupus erythematosus patients: The roles of alexithymia and depression. Compr. Psychiatry 2015, 63, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Lanzara, R.; Conti, C.; Camelio, M.; Cannizzaro, P.; Lalli, V.; Bellomo, R.G.; Saggini, R.; Porcelli, P. Alexithymia and Somatization in Chronic Pain Patients: A Sequential Mediation Model. Front. Psychol. 2020, 11, 2929. [Google Scholar] [CrossRef] [PubMed]
- Bressi, C.; Taylor, G.; Parker, J.; Bressi, S.; Brambilla, V.; Aguglia, E.; Allegranti, I.; Bongiorno, A.; Giberti, F.; Bucca, M.; et al. Cross validation of the factor structure of the 20-item Toronto Alexithymia Scale: An Italian multicenter study. J. Psychosom. Res. 1996, 41, 551–559. [Google Scholar] [CrossRef]
- Apolone, G.; Mosconi, P.; Quattrociocchi, L.; Gianicolo, E.A.L.; Groth, N.; Ware, J.E. Questionario sullo Stato di Salute Versione Italiana [Questionnaire on Health Status Italian Version]; Istituto di Ricerche Farmacologiche Mario Negri: Milano, Italy, 2005. [Google Scholar]
- Iani, L.; Lauriola, M.; Costantini, M. A confirmatory bifactor analysis of the hospital anxiety and depression scale in an Italian community sample. Health Qual. Life Outcomes 2014, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, T.; Pettersson, U. Severe psoriasis: Oral therapy with a new retinoid. Dermatologica 1978, 157, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 2nd ed.; Guilford Publications: New York, NY, USA, 2017. [Google Scholar]
- Fava, M.; Rankin, M.A.; Wright, E.C.; Alpert, J.E.; Nierenberg, A.A.; Pava, J.; Rosenbaum, J.F. Anxiety disorders in major depression. Compr. Psychiatry 2000, 41, 97–102. [Google Scholar] [CrossRef]
- Lamers, F.; van Oppen, P.; Comijs, H.C.; Smit, J.H.; Spinhoven, P.; van Balkom, A.J.; Nolen, W.A.; Zitman, F.G.; Beekman, A.T.F.; Penninx, B.W. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: The Netherlands Study of Depression and Anxiety (NESDA). J. Clin. Psychiatry 2011, 72, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Panasiti, M.S.; Ponsi, G.; Violani, C. Emotions, Alexithymia, and Emotion Regulation in Patients with Psoriasis. Front. Psychol. 2020, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- Picardi, A.; Mazzoti, E.; Gaetano, P.; Cattaruzza, M.S.; Baliva, G.; Melchi, C.F.; Pasquini, P. Stress, social support, emotional regulation and exacerbation of diffuse plaque psoriasis. Psychosomatics 2005, 46, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczak, M.; Luminet, O. Is alexithymia affected by situational stress or is it a stable trait related to emotion regulation? Pers. Individ. Dif. 2006, 40, 1399–1408. [Google Scholar] [CrossRef]
- Larsen, M.H.; Krogstad, A.L.; Wahl, A.K. Alexithymia, illness perception and self-management competency in psoriasis. Acta Derm. Venereol. 2017, 97, 934–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, H.L.; Fortune, D.G.; Griffiths, C.E.M.; Main, C. Alexithymia in patients with psoriasis: Clinical correlates and psychometric properties of the Toronto Alexithymia Scale-20. J. Psychosom. Res. 2005, 58, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Nandrino, J.L.; Baracca, M.; Antoine, P.; Paget, V.; Bydlowski, S.; Carton, S. Level of emotional awareness in the general French population: Effects of gender, age, and education level. Int. J. Psychol. 2013, 48, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Preece, D.A.; Mehta, A.; Becerra, R.; Chen, W.; Allan, A.; Robinson, K.; Boyes, M.; Hasking, P.; Gross, J.J. Why is Alexithymia a Risk Factor for Affective Disorder Symptoms? The Role of Emotion Regulation. J. Affect. Disord. 2021, 296, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Norman, H.; Marzano, L.; Coulson, M.; Oskis, A. Effects of mindfulness-based interventions on alexithymia: A systematic review. Evid. Based Ment. Health 2019, 22, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Yap, K.; Batalha, L. Mindfulness-based interventions and their effects on emotional clarity: A systematic review and meta-analysis. J. Affect. Disord. 2018, 235, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.; Ogrodniczuk, J.; Hadjipavlou, G. Changes in alexithymia following psychological intervention: A review. Harv. Rev. Psychiatry 2014, 22, 162–178. [Google Scholar] [CrossRef] [PubMed]
| Variable, M (SD) | No | Borderline/High | Total | t or χ2 | p | |
|---|---|---|---|---|---|---|
| Alexithymia | Alexithymia | Sample | ||||
| (N = 84) | (N = 66) | (N = 150) | ||||
| Age | 45.94 (13.83) | 49.80 (15.79) | 47.64 (14.80) | t(129.9) = −1.57 | 0.119 | |
| Sex, N (%) | 0.566 | |||||
| Male | 47 (54.0) | 40 (46.0) | 87 (58.0) | |||
| Female | 37 (58.7) | 26 (41.3) | 63 (42.0) | |||
| Marital status, N (%) | χ2(1, N = 150) = 0.15 | 0.928 | ||||
| Not married | 26 (55.3) | 21 (44.7) | 47 (31.3) | |||
| Married | 46 (55.4) | 37 (44.6) | 83 (55.4) | |||
| Divorced/widowed | 12 (60.0) | 8 (40.0) | 20 (13.3) | |||
| Years of education | 13.44 (3.12) | 11.23 (3.53) | 12.47 (3.47) | t(130.7) = 4.01 | <0.001 | |
| Job status, N (%) | χ2 (1, N = 150) = 1.74 | 0.627 | ||||
| Not employed | 13 (59.1) | 9 (40.9) | 22 (14.7) | |||
| Employed | 59 (57.8) | 43 (42.2) | 102 (68.0) | |||
| Retired | 7 (41.2) | 10 (58.8) | 17 (11.3) | |||
| Student | 5 (55.6) | 4 (44.4) | 9 (6.0) | |||
| Disease duration | 17.10 (14.08) | 19.36 (12.84) | 18.10 (13.55) | t(140.0) = −1.00 | 0.315 | |
| PASI | 4.31 (2.89) | 4.11 (2.67) | 4.22 (2.79) | t(144.2) = 0.45 | 0.653 | |
| Family history of psoriasis, N (%) | 38 (45.2) | 38 (58.5) | χ2 (1, N = 150) = 2.56 | 0.109 | ||
| BMI | χ2 (1, N = 150) = 2.63 | 0.268 | ||||
| BMI ≤ 25 | 35 (59.3) | 24 (40.7) | 59 (39.3) | |||
| BMI 25.1–29.9 | 32 (60.4) | 21 (39.6) | 53 (35.4) | |||
| BMI ≥ 30 | 17 (44.7) | 21 (55.3) | 38 (25.3) | |||
| Anxiety | 5.46 (3.05) | 7.65 (4.10) | 6.43 (3.70) | t(116.5) = −3.62 | <0.001 | |
| Depression | 4.69 (3.29) | 7.20 (3.90) | 5.79 (3.77) | t(126.9) = −4.18 | <0.001 | |
| PCS | 50.22 (6.84) | 46.61 (8.63) | 48.62 (7.87) | t(122.1) = 2.77 | 0.006 | |
| MCS | 43.26 (9.69) | 40.06 (8.97) | 41.84 (9.48) | t(143.6) = 2.08 | 0.039 | |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1 Disease duration | 1 | ||||||
| 2 PASI | 0.13 | ||||||
| 3 BMI | 0.28 ** | 0.17 * | |||||
| 4 Anxiety | 0.05 | 0.04 | 0.04 | ||||
| 5 Depression | 0.05 | 0.05 | 0.09 | 0.65 *** | |||
| 6 PCS | −0.17 * | −0.18 * | −0.28 ** | −0.24 ** | −0.32 *** | ||
| 7 MCS | 0.06 | −0.12 | -0.01 | −0.57 *** | −0.51 *** | −0.10 | |
| 8 TAS-Tot | 0.16 | 0.03 | 0.11 | 0.36 *** | 0.38 *** | −0.23 ** | −0.17 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinto, R.M.; De Vincenzo, F.; Graceffa, D.; Bonifati, C.; Innamorati, M.; Iani, L. The Relationship between Alexithymia and Mental Health Is Fully Mediated by Anxiety and Depression in Patients with Psoriasis. Int. J. Environ. Res. Public Health 2022, 19, 3649. https://doi.org/10.3390/ijerph19063649
Quinto RM, De Vincenzo F, Graceffa D, Bonifati C, Innamorati M, Iani L. The Relationship between Alexithymia and Mental Health Is Fully Mediated by Anxiety and Depression in Patients with Psoriasis. International Journal of Environmental Research and Public Health. 2022; 19(6):3649. https://doi.org/10.3390/ijerph19063649
Chicago/Turabian StyleQuinto, Rossella Mattea, Francesco De Vincenzo, Dario Graceffa, Claudio Bonifati, Marco Innamorati, and Luca Iani. 2022. "The Relationship between Alexithymia and Mental Health Is Fully Mediated by Anxiety and Depression in Patients with Psoriasis" International Journal of Environmental Research and Public Health 19, no. 6: 3649. https://doi.org/10.3390/ijerph19063649
APA StyleQuinto, R. M., De Vincenzo, F., Graceffa, D., Bonifati, C., Innamorati, M., & Iani, L. (2022). The Relationship between Alexithymia and Mental Health Is Fully Mediated by Anxiety and Depression in Patients with Psoriasis. International Journal of Environmental Research and Public Health, 19(6), 3649. https://doi.org/10.3390/ijerph19063649







