Time to Sleep?—A Review of the Impact of the COVID-19 Pandemic on Sleep and Mental Health
Abstract
1. Introduction
2. Materials and Methods
3. Epidemiology of Sleep Deprivation before the Pandemic
3.1. Epidemiology of Sleep Deprivation before the Pandemic
3.2. Is Sleep Deprivation an Epidemic?
4. Epidemiology of Sleep Deprivation during the Pandemic
4.1. Direct Comparisons Drawn before and during the Pandemic
4.2. Data from Systematic Reviews and Meta-Analyses during the Pandemic
4.3. Objective Sleep Data during the Pandemic
5. Effects of Sleep Deficiencies on Risk, Severity of COVID-19 and Vaccine Efficiency
6. Quantitative and Qualitative Alterations of Sleep in Acute and Long COVID Patients
6.1. Sleep Deficiencies during Acute COVID-19
6.2. Sleep Deficiencies during Long COVID
7. Mental Health during the Pandemic
7.1. Mental Health Impact in the General Population and HCW
7.2. Mental Health Impact in Acute and Surviving COVID Patients
7.3. Mental Health Impact on Pre-Existing Mental Health Conditions and Vulnerable Groups
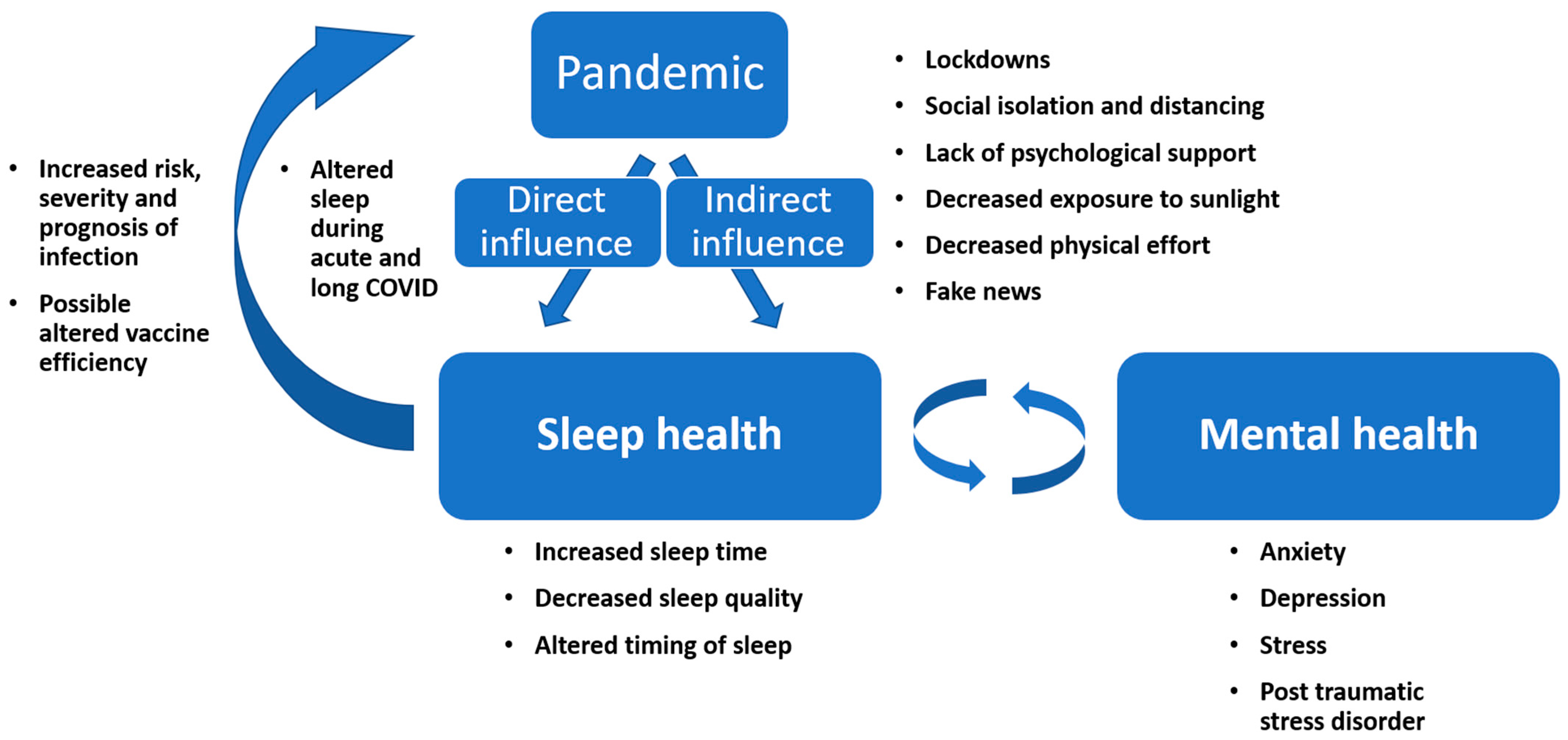
8. Mental Health and Other Factors That Can Influence Sleep during the Pandemic
9. Limitations in the Interpretation and Comparison of Literature Data
9.1. Heterogeneity Regarding Sleep Health Terminology
9.2. Subjective and Objective Sleep Data
9.3. Heterogeneity Regarding Used Sleep Questionnaires
9.4. Diagnosed vs. Total COVID-19 Cases
10. Conclusions
11. Further Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s Sleep Time Duration Recommendations: Methodology and Results Summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Harvey, A.G.; Murray, G.; Chandler, R.A.; Soehner, A. Sleep Disturbance as Transdiagnostic: Consideration of Neurobiological Mechanisms. Clin. Psychol. Rev. 2011, 31, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.E.; Kamerow, D.B. Epidemiologic Study of Sleep Disturbances and Psychiatric Disorders. An Opportunity for Prevention? JAMA 1989, 262, 1479–1484. [Google Scholar] [CrossRef]
- Blackwelder, A.; Hoskins, M.; Huber, L. Effect of Inadequate Sleep on Frequent Mental Distress. Prev. Chronic Dis. 2021, 18, E61. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Nanovska, S.; Regen, W.; Spiegelhalder, K.; Feige, B.; Nissen, C.; Reynolds, C.F.; Riemann, D. Sleep and Mental Disorders: A Meta-Analysis of Polysomnographic Research. Psychol. Bull. 2016, 142, 969–990. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.J.; Webb, T.L.; Martyn-St James, M.; Rowse, G.; Weich, S. Improving Sleep Quality Leads to Better Mental Health: A Meta-Analysis of Randomised Controlled Trials. Sleep Med. Rev. 2021, 60, 101556. [Google Scholar] [CrossRef]
- World Health Organisation. ICD-11 for Mortality and Morbidity Statistics (ICD-11 MMS). Available online: https://icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1980306606 (accessed on 12 September 2021).
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014; ISBN 9780991543410. [Google Scholar]
- Singh, B.; McArdle, N.; Hillman, D. Chapter 21—Psychopharmacology of Sleep Disorders. Handb. Clin. Neurol. 2019, 165, 345–364. [Google Scholar]
- National Heart, Lung, and Blood Institute. Sleep Deprivation and Deficiency. Available online: https://www.nhlbi.nih.gov/health-topics/sleep-deprivation-and-deficiency (accessed on 20 September 2020).
- Morse, A.M.; Bender, E. Sleep in Hospitalized Patients. Clocks Sleep 2019, 1, 151–165. [Google Scholar] [CrossRef]
- Dzierzewski, J.M.; Sabet, S.M.; Ghose, S.M.; Perez, E.; Soto, P.; Ravyts, S.G.; Dautovich, N.D. Lifestyle Factors and Sleep Health across the Lifespan. Int. J. Environ. Res. Public Health 2021, 18, 6626. [Google Scholar] [CrossRef]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef]
- Kohyama, J. Which Is More Important for Health: Sleep Quantity or Sleep Quality? Children 2021, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- Bin, Y.S. Is Sleep Quality More Important than Sleep Duration for Public Health? Sleep 2016, 39, 1629–1630. [Google Scholar] [CrossRef] [PubMed]
- Medic, G.; Wille, M.; Hemels, M.E. Short- and Long-Term Health Consequences of Sleep Disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A. Sleep, Health, and Society. Sleep Med. Clin. 2017, 12, 1–22. [Google Scholar] [CrossRef]
- Chattu, V.K.; Sakhamuri, S.M.; Kumar, R.; Spence, D.W.; BaHammam, A.S.; Pandi-Perumal, S.R. Insufficient Sleep Syndrome: Is It Time to Classify It as a Major Noncommunicable Disease? Sleep Sci. 2018, 11, 56–64. [Google Scholar] [CrossRef]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-NCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2018, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Stranges, S.; Dorn, J.M.; Shipley, M.J.; Kandala, N.B.; Trevisan, M.; Miller, M.A.; Donahue, R.P.; Hovey, K.M.; Ferrie, J.E.; Marmot, M.G.; et al. Correlates of Short and Long Sleep Duration: A Cross-Cultural Comparison between the United Kingdom and the United States: The Whitehall II Study and the Western New York Health Study. Am. J. Epidemiol. 2008, 168, 1353–1364. [Google Scholar] [CrossRef]
- Kronholm, E.; Härmä, M.; Hublin, C.; Aro, A.R.; Partonen, T. Self-Reported Sleep Duration in Finnish General Population. J. Sleep Res. 2006, 15, 276–290. [Google Scholar] [CrossRef]
- Groeger, J.A.; Zijlstra, F.R.; Dijk, D.J. Sleep Quantity, Sleep Difficulties and Their Perceived Consequences in a Representative Sample of Some 2000 British Adults. J. Sleep Res. 2004, 13, 359–371. [Google Scholar] [CrossRef]
- Kronholm, E.; Partonen, T.; Laatikainen, T.; Peltonen, M.; Härmä, M.; Hublin, C.; Kaprio, J.; Aro, A.R.; Partinen, M.; Fogelholm, M.; et al. Trends in Self-Reported Sleep Duration and Insomnia-Related Symptoms in Finland from 1972 to 2005: A Comparative Review and Re-Analysis of Finnish Population Samples. J. Sleep Res. 2008, 17, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Krueger, P.M.; Friedman, E.M. Sleep Duration in the United States: A Cross-Sectional Population-Based Study. Am. J. Epidemiol. 2009, 169, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Cunningham, T.J.; Croft, J.B. Trends in Self-Reported Sleep Duration among US Adults from 1985 to 2012. Sleep 2015, 38, 829–832. [Google Scholar] [CrossRef] [PubMed]
- National Sleep Foundation. 2013 International Bedroom Poll. Available online: https://www.sleepfoundation.org/wp-content/uploads/2018/10/RPT495a.pdf (accessed on 1 October 2021).
- Kocevska, D.; Lysen, T.S.; Dotinga, A.; Koopman-Verhoeff, M.E.; Luijk, M.P.C.M.; Antypa, N.; Biermasz, N.R.; Blokstra, A.; Brug, J.; Burk, W.J.; et al. Sleep Characteristics across the Lifespan in 1.1 Million People from the Netherlands, United Kingdom and United States: A Systematic Review and Meta-Analysis. Nat. Hum. Behav. 2021, 5, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, C.M.; Frochen, S.E.; Walsemann, K.M.; Ailshire, J.A. Are US Adults Reporting Less Sleep?: Findings from Sleep Duration Trends in the National Health Interview Survey, 2004–2017. Sleep 2019, 42, zsy221. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, G.A. Epidemiology of Sleep and Sleep Disorders in The Netherlands. Sleep Med. 2017, 30, 229–239. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Sleep and Sleep Disorders: Data and Statistics. Available online: https://www.cdc.gov/sleep/data_statistics.html (accessed on 29 September 2021).
- Seoane, H.A.; Moschetto, L.; Orliacq, F.; Orliacq, J.; Serrano, E.; Cazenave, M.I.; Vigo, D.E.; Perez-Lloret, S. Sleep Disruption in Medicine Students and Its Relationship with Impaired Academic Performance: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2020, 53, 101333. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, A.G.; Jones, S.E.; Cooper, A.C.; Croft, J.B. Short Sleep Duration Among Middle School and High School Students—United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Patte, K.A.; Qian, W.; Leatherdale, S.T. Sleep Duration Trends and Trajectories among Youth in the COMPASS Study. Sleep Health 2017, 3, 309–316. [Google Scholar] [CrossRef]
- Hysing, M.; Harvey, A.G.; Linton, S.J.; Askeland, K.G.; Sivertsen, B. Sleep and Academic Performance in Later Adolescence: Results from a Large Population-Based Study. J. Sleep Res. 2016, 25, 318–324. [Google Scholar] [CrossRef]
- Saxvig, I.W.; Bjorvatn, B.; Hysing, M.; Sivertsen, B.; Gradisar, M.; Pallesen, S. Sleep in Older Adolescents. Results from a Large Cross-Sectional, Population-Based Study. J. Sleep Res. 2021, 30, e13263. [Google Scholar] [CrossRef] [PubMed]
- De Souza Neto, J.M.; da Costa, F.F.; Barbosa, A.O.; Prazeres Filho, A.; Santos, E.V.O.D.; de Farias Júnior, J.C. Physical activity, screen time, nutritional status and sleep in adolescents in northeast Brazil. Rev. Paul Pediatr. 2021, 39, e2019138. [Google Scholar] [CrossRef] [PubMed]
- Nasim, M.; Saade, M.; AlBuhairan, F. Sleep Deprivation: Prevalence and Associated Factors among Adolescents in Saudi Arabia. Sleep Med. 2019, 53, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-L.; Zheng, X.-Y.; Yang, J.; Ye, C.-P.; Chen, Y.-Y.; Zhang, Z.-G.; Xiao, Z.-J. A Systematic Review of Studies on the Prevalence of Insomnia in University Students. Public Health 2015, 129, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.-Y.; Wang, S.-B.; Zhang, L.; Li, L.; Xu, D.-D.; Ng, C.H.; Ungvari, G.S.; Cui, X.; Liu, Z.-M.; et al. Prevalence of Sleep Disturbances in Chinese University Students: A Comprehensive Meta-Analysis. J. Sleep Res. 2018, 27, e12648. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Miao, M.; Yuan, W.; Sun, J. Sleep Duration and All-Cause Mortality in the Elderly in China: A Population-Based Cohort Study. BMC Geriatr. 2020, 20, 541. [Google Scholar] [CrossRef] [PubMed]
- López-García, E.; Faubel, R.; León-Muñoz, L.; Zuluaga, M.C.; Banegas, J.R.; Rodríguez-Artalejo, F. Sleep Duration, General and Abdominal Obesity, and Weight Change among the Older Adult Population of Spain. Am. J. Clin. Nutr. 2008, 87, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Su, T.P.; Chou, P. A Nine-Year Follow-up Study of Sleep Patterns and Mortality in Community-Dwelling Older Adults in Taiwan. Sleep 2013, 36, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Zawisza, K.; Tobiasz-Adamczyk, B.; Galas, A.; Brzyska, M. Sleep Duration and Mortality among Older Adults in a 22-Year Follow-up Study: An Analysis of Possible Effect Modifiers. Eur. J. Ageing 2015, 12, 119–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, L.; Wang, S.-B.; Rao, W.-W.; Ungvari, G.S.; Ng, C.H.; Chiu, H.F.K.; Zhang, J.; Kou, C.; Jia, F.-J.; Xiang, Y.-T. Sleep Duration and Patterns in Chinese Older Adults: A Comprehensive Meta-Analysis. Int. J. Biol. Sci. 2017, 13, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.-Y.; Lan, T.-H.; Wen, C.-P.; Lin, Y.-H.; Chuang, Y.-L. Nighttime Sleep, Chinese Afternoon Nap, and Mortality in the Elderly. Sleep 2007, 30, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Nakakubo, S.; Makizako, H.; Doi, T.; Tsutsumimoto, K.; Hotta, R.; Lee, S.; Lee, S.; Bae, S.; Makino, K.; Suzuki, T.; et al. Long and Short Sleep Duration and Physical Frailty in Community-Dwelling Older Adults. J. Nutr. Health Aging 2018, 22, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Castro-Costa, E.; Dewey, M.E.; Ferri, C.P.; Uchôa, E.; Firmo, J.O.A.; Rocha, F.L.; Prince, M.; Lima-Costa, M.F.; Stewart, R. Association between Sleep Duration and All-Cause Mortality in Old Age: 9-Year Follow-up of the Bambuí Cohort Study, Brazil. J. Sleep Res. 2011, 20, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Miner, B.; Kryger, M.H. Sleep in the Aging Population. Sleep Med. Clin. 2017, 12, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Stranges, S.; Tigbe, W.; Gómez-Olivé, F.X.; Thorogood, M.; Kandala, N.B. Sleep Problems: An Emerging Global Epidemic? Findings from the INDEPTH WHO-SAGE Study among More than 40,000 Older Adults from 8 Countries across Africa and Asia. Sleep 2012, 35, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Yetish, G.; Kaplan, H.; Gurven, M.; Wood, B.; Pontzer, H.; Manger, P.R.; Wilson, C.; McGregor, R.; Siegel, J.M. Natural Sleep and Its Seasonal Variations in Three Pre-Industrial Societies. Curr. Biol. 2015, 25, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Youngstedt, S.D.; Goff, E.E.; Reynolds, A.M.; Kripke, D.F.; Irwin, M.R.; Bootzin, R.R.; Khan, N.; Jean-Louis, G. Has Adult Sleep Duration Declined over the Last 50+ Years? Sleep Med. Rev. 2016, 28, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Bonke, J. Trends in Short and Long Sleep in Denmark from 1964 to 2009, and the Associations with Employment, SES (Socioeconomic Status) and BMI. Sleep Med. 2015, 16, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Van Cauter, E.; Rathouz, P.J.; DeLeire, T.; Lauderdale, D.S. Trends in the Prevalence of Short Sleepers in the USA: 1975–2006. Sleep 2010, 33, 37–45. [Google Scholar] [CrossRef]
- Rowshan Ravan, A.; Bengtsson, C.; Lissner, L.; Lapidus, L.; Björkelund, C. Thirty-Six-Year Secular Trends in Sleep Duration and Sleep Satisfaction, and Associations with Mental Stress and Socioeconomic Factors-Results of the Population Study of Women in Gothenburg, Sweden. J. Sleep Res. 2010, 19, 496–503. [Google Scholar] [CrossRef]
- Bin, Y.S.; Marshall, N.S.; Glozier, N. Secular Trends in Adult Sleep Duration: A Systematic Review. Sleep Med. Rev. 2012, 16, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Bin, Y.S.; Marshall, N.S.; Glozier, N. Sleeping at the Limits: The Changing Prevalence of Short and Long Sleep Durations in 10 Countries. Am. J. Epidemiol. 2013, 177, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Matricciani, L.; Olds, T.; Petkov, J. In Search of Lost Sleep: Secular Trends in the Sleep Time of School-Aged Children and Adolescents. Sleep Med. Rev. 2012, 16, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Kellner, S. “Coronasomnia”—Resilienzförderung durch Insomniebehandlung [“Coronasomnia”-promoting resilience through insomnia treatment]. Somnologie 2021, 25, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Mamedova, A.; Vinnik, V.; Klimova, M.; Saranceva, E.; Ageev, V.; Yu, T.; Zhu, D.; Penzel, T.; Kurths, J. Brain Mechanisms of COVID-19-Sleep Disorders. Int. J. Mol. Sci. 2021, 22, 6917. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Torres, A.; Giménez-Llort, L. Confinement and the Hatred of Sound in Times of COVID-19: A Molotov Cocktail for People With Misophonia. Front. Psychiatry 2021, 12, 627044. [Google Scholar] [CrossRef] [PubMed]
- Hisler, G.C.; Twenge, J.M. Sleep Characteristics of U.S. Adults before and during the COVID-19 Pandemic. Soc. Sci. Med. 2021, 276, 113849. [Google Scholar] [CrossRef] [PubMed]
- Trakada, A.; Nikolaidis, P.T.; Andrade, M.D.S.; Puccinelli, P.J.; Economou, N.-T.; Steiropoulos, P.; Knechtle, B.; Trakada, G. Sleep During “Lockdown” in the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 9094. [Google Scholar] [CrossRef]
- Trabelsi, K.; Ammar, A.; Masmoudi, L.; Boukhris, O.; Chtourou, H.; Bouaziz, B.; Brach, M.; Bentlage, E.; How, D.; Ahmed, M.; et al. Globally Altered Sleep Patterns and Physical Activity Levels by Confinement in 5056 Individuals: ECLB COVID-19 International Online Survey. Biol. Sport 2021, 38, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Gauthier-Gagne, G.; Voutou, D.; Somerville, G.; Saha, S.; Boursier, J. Pre-Pandemic Sleep Behavior and Adolescents’ Stress during COVID-19: A Prospective Longitudinal Study. Child Adolesc. Psychiatry Ment. Health 2021, 15, 43. [Google Scholar] [CrossRef]
- Trabelsi, K.; Ammar, A.; Masmoudi, L.; Boukhris, O.; Chtourou, H.; Bouaziz, B.; Brach, M.; Bentlage, E.; How, D.; Ahmed, M.; et al. Sleep Quality and Physical Activity as Predictors of Mental Wellbeing Variance in Older Adults during COVID-19 Lockdown: ECLB COVID-19 International Online Survey. Int. J. Environ. Res. Public Health 2021, 18, 4329. [Google Scholar] [CrossRef] [PubMed]
- Albakri, U.; Drotos, E.; Meertens, R. Sleep Health Promotion Interventions and Their Effectiveness: An Umbrella Review. Int. J. Environ. Res. Public Health 2021, 18, 5533. [Google Scholar] [CrossRef] [PubMed]
- Jahrami, H.; BaHammam, A.S.; Bragazzi, N.L.; Saif, Z.; Faris, M.; Vitiello, M.V. Sleep Problems during the COVID-19 Pandemic by Population: A Systematic Review and Meta-Analysis. J. Clin. Sleep Med. 2021, 17, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.N.; Liu, Z.R.; Li, S.Q.; Li, C.X.; Zhang, L.; Li, N.; Sun, X.W.; Li, H.P.; Zhou, J.P.; Li, Q.Y. Burden of Sleep Disturbance During COVID-19 Pandemic: A Systematic Review. Nat. Sci. Sleep 2021, 13, 933–966. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Chen, C.; Liu, Z.; Luo, X.; Guo, C.; Liu, Z.; Zhang, K.; Liu, H. Prevalence of Sleep Disturbances and Sleep Quality in Chinese Healthcare Workers During the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Front. Psychiatry 2021, 12, 646342. [Google Scholar] [CrossRef] [PubMed]
- Marvaldi, M.; Mallet, J.; Dubertret, C.; Moro, M.R.; Guessoum, S.B. Anxiety, Depression, Trauma-Related, and Sleep Disorders among Healthcare Workers during the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 126, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Alimoradi, Z.; Broström, A.; Tsang, H.W.H.; Griffiths, M.D.; Haghayegh, S.; Ohayon, M.M.; Lin, C.-Y.; Pakpour, A.H. Sleep Problems during COVID-19 Pandemic and Its’ Association to Psychological Distress: A Systematic Review and Meta-Analysis. EClinicalMedicine 2021, 36, 100916. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Aggarwal, S.; Madaan, P.; Saini, L.; Bhutani, M. Impact of COVID-19 Pandemic on Sleep in Children and Adolescents: A Systematic Review and Meta-Analysis. Sleep Med. 2021, 84, 259–267. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, G.M.; Tavares, V.D.O.; de Meiroz Grilo, M.L.P.; Coelho, M.L.G.; de Lima-Araújo, G.L.; Schuch, F.B.; Galvão-Coelho, N.L. Mental Health in COVID-19 Pandemic: A Meta-Review of Prevalence Meta-Analyses. Front. Psychol. 2021, 12, 703838. [Google Scholar] [CrossRef]
- Yuksel, D.; McKee, G.B.; Perrin, P.B.; Alzueta, E.; Caffarra, S.; Ramos-Usuga, D.; Arango-Lasprilla, J.C.; Baker, F.C. Sleeping When the World Locks down: Correlates of Sleep Health during the COVID-19 Pandemic across 59 Countries. Sleep Health 2021, 7, 134–142. [Google Scholar] [CrossRef]
- Cellini, N.; Canale, N.; Mioni, G.; Costa, S. Changes in Sleep Pattern, Sense of Time and Digital Media Use during COVID-19 Lockdown in Italy. J. Sleep Res. 2020, 29, e13074. [Google Scholar] [CrossRef]
- Cellini, N.; Conte, F.; De Rosa, O.; Giganti, F.; Malloggi, S.; Reyt, M.; Guillemin, C.; Schmidt, C.; Muto, V.; Ficca, G. Changes in Sleep Timing and Subjective Sleep Quality during the COVID-19 Lockdown in Italy and Belgium: Age, Gender and Working Status as Modulating Factors. Sleep Med. 2021, 77, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Schmidt, M.H.; Cajochen, C. Effects of the COVID-19 Lockdown on Human Sleep and Rest-Activity Rhythms. Curr. Biol. 2020, 30, R795–R797. [Google Scholar] [CrossRef]
- Yu, B.Y.; Yeung, W.F.; Lam, J.C.; Yuen, S.C.-S.; Lam, S.C.; Chung, V.C.-H.; Chung, K.-F.; Lee, P.H.; Ho, F.Y.-Y.; Ho, J.Y.-S. Prevalence of Sleep Disturbances during COVID-19 Outbreak in an Urban Chinese Population: A Cross-Sectional Study. Sleep Med. 2020, 74, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Sigodo, K.O.; Al-Ghadban, F.; Al-Lahou, B.; Alnashmi, M.; Hermassi, S.; Chun, S. Effects of COVID-19 Lockdown on Physical Activity and Dietary Behaviors in Kuwait: A Cross-Sectional Study. Nutrients 2021, 13, 2252. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, N. Generalized Anxiety Disorder, Depressive Symptoms and Sleep Quality during COVID-19 Outbreak in China: A Web-Based Cross-Sectional Survey. Psychiatry Res. 2020, 288, 112954. [Google Scholar] [CrossRef]
- Cui, X.; He, Y.; Gong, J.; Luo, X.; Liu, J. Epidemiology of Sleep Disturbances and Their Effect on Psychological Distress During the COVID-19 Outbreak: A Large National Study in China. Front. Psychol. 2021, 12, 615867. [Google Scholar] [CrossRef] [PubMed]
- Voitsidis, P.; Gliatas, I.; Bairachtari, V.; Papadopoulou, K.; Papageorgiou, G.; Parlapani, E.; Syngelakis, M.; Holeva, V.; Diakogiannis, I. Insomnia during the COVID-19 Pandemic in a Greek Population. Psychiatry Res. 2020, 289, 113076. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, L.; Gao, Y.; Gao, X.; Lei, X. Effects of Physical Activity and Sleep Quality on Well-Being: A Wrist Actigraphy Study during the Pandemic. Appl. Psychol. Health Well Being 2021, 13, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Folarin, A.A.; Ranjan, Y.; Rashid, Z.; Conde, P.; Stewart, C.; Cummins, N.; Matcham, F.; Dalla Costa, G.; Simblett, S.; et al. Using Smartphones and Wearable Devices to Monitor Behavioral Changes During COVID-19. J. Med. Internet Res. 2020, 22, e19992. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, N.; Grandner, M.A. Changes in Sleep Duration, Timing, and Variability during the COVID-19 Pandemic: Large-Scale Fitbit Data from 6 Major US Cities. Sleep Health 2021, 7, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef] [PubMed]
- Prather, A.A.; Leung, C.W. Association of Insufficient Sleep With Respiratory Infection Among Adults in the United States. JAMA Intern. Med. 2016, 176, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Malhotra, A.; Gao, X.; Hu, F.B.; Neuman, M.I.; Fawzi, W.W. A Prospective Study of Sleep Duration and Pneumonia Risk in Women. Sleep 2012, 35, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Irwin, M.R.; Krueger, J.M.; Gaddameedhi, S.; Van Dongen, H.P.A. Night Shift Schedule Alters Endogenous Regulation of Circulating Cytokines. Neurobiol. Sleep Circadian Rhythms 2021, 10, 100063. [Google Scholar] [CrossRef]
- Rizza, S.; Coppeta, L.; Grelli, S.; Ferrazza, G.; Chiocchi, M.; Vanni, G.; Bonomo, O.C.; Bellia, A.; Andreoni, M.; Magrini, A.; et al. High Body Mass Index and Night Shift Work Are Associated with COVID-19 in Health Care Workers. J. Endocrinol. Investig. 2021, 44, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hegde, S.; LaFiura, C.; Raghavan, M.; Luong, E.; Cheng, S.; Rebholz, C.M.; Seidelmann, S.B. COVID-19 Illness in Relation to Sleep and Burnout. BMJ Nutr. Prev. Health 2021, 4, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ni, S.-Y.; Yan, W.; Lu, Q.-D.; Zhao, Y.-M.; Xu, Y.-Y.; Mei, H.; Shi, L.; Yuan, K.; Han, Y.; et al. Mental and Neurological Disorders and Risk of COVID-19 Susceptibility, Illness Severity and Mortality: A Systematic Review, Meta-Analysis and Call for Action. EClinicalMedicine 2021, 40, 101111. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Niu, Y.; Zhao, W.; Bao, P.; Li, D. Reduced Sleep in the Week Prior to Diagnosis of COVID-19 Is Associated with the Severity of COVID-19. Nat. Sci. Sleep 2020, 12, 999–1007. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, D.; Xie, B.; Zhang, Y.; Huang, H.; Liu, H.; Chen, H.; Sun, Y.; Shang, Y.; Hashimoto, K.; et al. Poor-Sleep Is Associated with Slow Recovery from Lymphopenia and an Increased Need for ICU Care in Hospitalized Patients with COVID-19: A Retrospective Cohort Study. Brain Behav. Immun. 2020, 88, 50–58. [Google Scholar] [CrossRef]
- Benedict, C.; Cedernaes, J. Could a Good Night’s Sleep Improve COVID-19 Vaccine Efficacy? Lancet Respir. Med. 2021, 9, 447–448. [Google Scholar] [CrossRef]
- Lammers-van der Holst, H.M.; Lammers, G.J.; van der Horst, G.T.J.; Chaves, I.; de Vries, R.D.; GeurtsvanKessel, C.H.; Koch, B.; van der Kuy, H.M. Understanding the Association between Sleep, Shift Work and COVID-19 Vaccine Immune Response Efficacy: Protocol of the S-CORE Study. J. Sleep Res. 2021, 31, e13496. [Google Scholar] [CrossRef] [PubMed]
- Mullington, J.; Korth, C.; Hermann, D.M.; Orth, A.; Galanos, C.; Holsboer, F.; Pollmächer, T. Dose-Dependent Effects of Endotoxin on Human Sleep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R947–R955. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, L.; Schreiber, W.; Holsboer, F.; Pollmächer, T. Endotoxin Enhances EEG Alpha and Beta Power in Human Sleep. Sleep 1994, 17, 132–139. [Google Scholar] [CrossRef]
- Drake, C.L.; Roehrs, T.A.; Royer, H.; Koshorek, G.; Turner, R.B.; Roth, T. Effects of an Experimentally Induced Rhinovirus Cold on Sleep, Performance, and Daytime Alertness. Physiol. Behav. 2000, 71, 75–81. [Google Scholar] [CrossRef]
- Lasselin, J.; Ingre, M.; Regenbogen, C.; Olsson, M.J.; Garke, M.; Brytting, M.; Edgar, R.; Lekander, M.; Axelsson, J. Sleep during Naturally Occurring Respiratory Infections: A Pilot Study. Brain Behav. Immun. 2019, 79, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, M.; Möller, A.A.; Schreiber, W.; Krieg, J.C.; Fuchs, D.; Wachter, H.; Holsboer, F. Nocturnal Sleep EEG in Patients with HIV Infection. Eur. Arch. Psychiatry Clin. Neurosci. 1991, 240, 153–158. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 14 September 2021).
- Vitalakumar, D.; Sharma, A.; Kumar, A.; Flora, S.J.S. Neurological Manifestations in COVID-19 Patients: A Meta-Analysis. ACS Chem. Neurosci. 2021, 12, 2776–2797. [Google Scholar] [CrossRef]
- Rogers, J.P.; Watson, C.J.; Badenoch, J.; Cross, B.; Butler, M.; Song, J.; Hafeez, D.; Morrin, H.; Rengasamy, E.R.; Thomas, L.; et al. Neurology and Neuropsychiatry of COVID-19: A Systematic Review and Meta-Analysis of the Early Literature Reveals Frequent CNS Manifestations and Key Emerging Narratives. J. Neurol. Neurosurg. Psychiatry 2021, 92, 932–941. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The Prevalence of Depression, Anxiety, and Sleep Disturbances in COVID-19 Patients: A Meta-Analysis. Ann. N. Y. Acad. Sci. 2021, 1486, 90–111. [Google Scholar] [CrossRef]
- Wesselius, H.M.; van den Ende, E.S.; Alsma, J.; Ter Maaten, J.C.; Schuit, S.C.E.; Stassen, P.M.; de Vries, O.J.; Kaasjager, K.H.A.H.; Haak, H.R.; van Doormaal, F.F.; et al. Quality and Quantity of Sleep and Factors Associated With Sleep Disturbance in Hospitalized Patients. JAMA Intern. Med. 2018, 178, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Manian, F.A.; Manian, C.J. Sleep Quality in Adult Hospitalized Patients with Infection: An Observational Study. Am. J. Med. Sci. 2015, 349, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Y.; Wu, D.; Lin, R.; Wang, Z.; Pan, L. Effects of Progressive Muscle Relaxation on Anxiety and Sleep Quality in Patients with COVID-19. Complement. Ther. Clin. Pract. 2020, 39, 101132. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.X.; Lin, Y.J.; Lin, R.Q.; Liu, A.N.; Zhong, G.Q.; Lan, C.F. Effects of Progressive Muscle Relaxation Training on Negative Emotions and Sleep Quality in COVID-19 Patients: A Clinical Observational Study. Medicine 2020, 99, e23185. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, X.; Kumar, P.; Cao, B.; Ma, X.; Li, T. Social Support and Clinical Improvement in COVID-19 Positive Patients in China. Nurs. Outlook 2020, 68, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Tam, W.; Hu, X.; Tan, W.; Jiang, L.; Jiang, X.; Zhang, L.; Zhao, X.; Zou, Y.; Hu, Y.; et al. A Quantitative and Qualitative Study on the Neuropsychiatric Sequelae of Acutely Ill COVID-19 Inpatients in Isolation Facilities. Transl. Psychiatry 2020, 10, 355. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, P.; Wang, L.; Hu, Y.; Pang, M.; Ma, S.; Tang, X. Psychological Distress and Sleep Quality of COVID-19 Patients in Wuhan, a Lockdown City as the Epicenter of COVID-19. J. Psychiatr. Res. 2021, 136, 595–602. [Google Scholar] [CrossRef]
- Vitale, J.A.; Perazzo, P.; Silingardi, M.; Biffi, M.; Banfi, G.; Negrini, F. Is Disruption of Sleep Quality a Consequence of Severe COVID-19 Infection? A Case-Series Examination. Chronobiol. Int. 2020, 37, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Tansey, C.M.; Louie, M.; Loeb, M.; Gold, W.L.; Muller, M.P.; de Jager, J.; Cameron, J.I.; Tomlinson, G.; Mazzulli, T.; Walmsley, S.L.; et al. One-Year Outcomes and Health Care Utilization in Survivors of Severe Acute Respiratory Syndrome. Arch. Intern. Med. 2007, 167, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Moldofsky, H.; Patcai, J. Chronic Widespread Musculoskeletal Pain, Fatigue, Depression and Disordered Sleep in Chronic Post-SARS Syndrome; a Case-Controlled Study. BMC Neurol. 2011, 11, 37. [Google Scholar] [CrossRef]
- National Institute for Health Research. Living with COVID19—Second Review. Available online: https://evidence.nihr.ac.uk/themedreview/living-with-covid19-second-review/ (accessed on 16 September 2021).
- Goërtz, Y.M.J.; Van Herck, M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.C.; Houben-Wilke, S.; Burtin, C.; Posthuma, R.; Franssen, F.M.E.; et al. Persistent Symptoms 3 Months after a SARS-CoV-2 Infection: The Post-COVID-19 Syndrome? ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Stavem, K.; Ghanima, W.; Olsen, M.K.; Gilboe, H.M.; Einvik, G. Persistent Symptoms 1.5-6 Months after COVID-19 in Non-Hospitalised Subjects: A Population-Based Cohort Study. Thorax 2021, 76, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Yang, D.; Lewis, A.; Patel, P.; Medicherla, C.; Arena, V.; Fang, T.; Andino, A.; Snyder, T.; Madhavan, M.; et al. A Prospective Study of Long-Term Outcomes among Hospitalized COVID-19 Patients with and without Neurological Complications. J. Neurol. Sci. 2021, 426, 117486. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.M.; Abd-Elrahman, N.M.; Bakheet, T.M. Persistence of Symptoms after Improvement of Acute COVID19 Infection, a Longitudinal Study. J. Med. Virol. 2021, 93, 5942–5946. [Google Scholar] [CrossRef]
- Lombardo, M.D.M.; Foppiani, A.; Peretti, G.M.; Mangiavini, L.; Battezzati, A.; Bertoli, S.; Martinelli Boneschi, F.; Zuccotti, G.V. Long-Term Coronavirus Disease 2019 Complications in Inpatients and Outpatients: A One-Year Follow-up Cohort Study. Open Forum Infect Dis. 2021, 8, ofab384. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-Year Outcomes in Hospital Survivors with COVID-19: A Longitudinal Cohort Study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Blomberg, B.; Mohn, K.G.-I.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.-A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a Prospective Cohort of Home-Isolated Patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef]
- August, D.; Stete, K.; Hilger, H.; Götz, V.; Biever, P.; Hosp, J.; Wagner, D.; Köhler, T.C.; Gerstacker, K.; Seufert, J.; et al. Persistierende Beschwerden 6 Monate nach COVID-19—Erfahrungen aus der COVID-19-Nachsorgeambulanz des Universitätsklinikums Freiburg (Complaints and clinical findings six months after COVID-19: Outpatient follow-up at the University Medical Center Freiburg). Dtsch. Med. Wochenschr. 2021, 146, e65–e73. [Google Scholar] [CrossRef]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and Depression in COVID-19 Survivors: Role of Inflammatory and Clinical Predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef]
- Kessler, R.; Knutson, K.L.; Mokhlesi, B.; Anderson, S.L.; Shah, M.; Meltzer, D.O.; Arora, V.M. Sleep and Activity Patterns in Older Patients Discharged from the Hospital. Sleep 2019, 42, zsz153. [Google Scholar] [CrossRef] [PubMed]
- Altman, M.T.; Knauert, M.P.; Pisani, M.A. Sleep Disturbance after Hospitalization and Critical Illness: A Systematic Review. Ann. Am. Thorac. Soc. 2017, 14, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, J.K.; Kumar, R.; Gupta, N.K.; Ish, P.; Yadav, S.R.; Chakrabarti, S.; Gupta, N. A Prospective Study Evaluating Sleep Quality and Disorders in Post-ARDS Patients. Sleep Vigil. 2021, 5, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Bozan, Ö.; Atiş, Ş.E.; Çekmen, B.; Şentürk, M.; Kalkan, A. Healthcare Workers’ Sleep Quality after COVID-19 Infection: A Cross-Sectional Study. Int. J. Clin. Pract. 2021, 75, e14772. [Google Scholar] [CrossRef] [PubMed]
- Gaber, T.A.K.; Ashish, A.; Unsworth, A. Persistent Post-Covid Symptoms in Healthcare Workers. Occup. Med. 2021, 71, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients With COVID-19: A Systematic Review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-Acute COVID-19 Syndrome (PCS) and Health-Related Quality of Life (HRQoL)-A Systematic Review and Meta-Analysis. J. Med. Virol. 2021, 94, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Kar, A.; Saxena, K. COVID Eyes: REM in COVID-19 Survivors. Sleep Vigil. 2021, 5, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Heidbreder, A.; Sonnweber, T.; Stefani, A.; Ibrahim, A.; Cesari, M.; Bergmann, M.; Brandauer, E.; Tancevski, I.; Löffler-Ragg, J.; Högl, B. Video-Polysomnographic Findings after Acute COVID-19: REM Sleep without Atonia as Sign of CNS Pathology? Sleep Med. 2021, 80, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Saxena, K.; Kar, A.; Khurana, A.; Bhagtana, P.K.; Sridevi, C.S.K.R.; Pakhare, A. Obstructive Sleep Apnea Is Highly Prevalent in COVID19 Related Moderate to Severe ARDS Survivors: Findings of Level I Polysomnography in a Tertiary Care Hospital. Sleep Med. 2021; in press. [Google Scholar] [CrossRef]
- Singh, R.K.; Bajpai, R.; Kaswan, P. COVID-19 Pandemic and Psychological Wellbeing among Health Care Workers and General Population: A Systematic-Review and Meta-Analysis of the Current Evidence from India. Clin. Epidemiol. Glob. Health 2021, 11, 100737. [Google Scholar] [CrossRef] [PubMed]
- Pashazadeh Kan, F.; Raoofi, S.; Rafiei, S.; Khani, S.; Hosseinifard, H.; Tajik, F.; Raoofi, N.; Ahmadi, S.; Aghalou, S.; Torabi, F.; et al. A Systematic Review of the Prevalence of Anxiety among the General Population during the COVID-19 Pandemic. J. Affect. Disord. 2021, 293, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Neelam, K.; Duddu, V.; Anyim, N.; Neelam, J.; Lewis, S. Pandemics and Pre-Existing Mental Illness: A Systematic Review and Meta-Analysis. Brain Behav. Immun. Health 2021, 10, 100177. [Google Scholar] [CrossRef]
- Gobbi, S.; Płomecka, M.B.; Ashraf, Z.; Radziński, P.; Neckels, R.; Lazzeri, S.; Dedić, A.; Bakalović, A.; Hrustić, L.; Skórko, B.; et al. Worsening of Preexisting Psychiatric Conditions During the COVID-19 Pandemic. Front. Psychiatry 2020, 11, 581426. [Google Scholar] [CrossRef]
- Murphy, L.; Markey, K.; O’ Donnell, C.; Moloney, M.; Doody, O. The Impact of the COVID-19 Pandemic and Its Related Restrictions on People with Pre-Existent Mental Health Conditions: A Scoping Review. Arch. Psychiatr. Nurs. 2021, 35, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-H.; Nam, J.-H.; Kwon, C.-Y. Comparison of the Mental Health Impact of COVID-19 on Vulnerable and Non-Vulnerable Groups: A Systematic Review and Meta-Analysis of Observational Studies. Int. J. Environ. Res. Public Health 2021, 18, 10830. [Google Scholar] [CrossRef]
- Hards, E.; Loades, M.E.; Higson-Sweeney, N.; Shafran, R.; Serafimova, T.; Brigden, A.; Reynolds, S.; Crawley, E.; Chatburn, E.; Linney, C.; et al. Loneliness and Mental Health in Children and Adolescents with Pre-Existing Mental Health Problems: A Rapid Systematic Review. Br. J. Clin. Psychol. 2021. [Google Scholar] [CrossRef]
- Haack, M.; Simpson, N.; Sethna, N.; Kaur, S.; Mullington, J. Sleep Deficiency and Chronic Pain: Potential Underlying Mechanisms and Clinical Implications. Neuropsychopharmacology 2020, 45, 205–216. [Google Scholar] [CrossRef]
- Lauderdale, D.S.; Knutson, K.L.; Yan, L.L.; Rathouz, P.J.; Hulley, S.B.; Sidney, S.; Liu, K. Objectively Measured Sleep Characteristics among Early-Middle-Aged Adults: The CARDIA Study. Am. J. Epidemiol. 2006, 164, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.E.; Goodwin, J.L.; Sherrill, D.L.; Arnold, J.L.; Bootzin, R.R.; Smith, T.; Walsleben, J.A.; Baldwin, C.M.; Quan, S.F. Relationship between Reported and Measured Sleep Times: The Sleep Heart Health Study (SHHS). J. Clin. Sleep Med. 2007, 3, 622–630. [Google Scholar] [CrossRef]
- Jackson, C.L.; Ward, J.B.; Johnson, D.A.; Sims, M.; Wilson, J.; Redline, S. Concordance between Self-Reported and Actigraphy-Assessed Sleep Duration among African-American Adults: Findings from the Jackson Heart Sleep Study. Sleep 2020, 43, zsz246. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Patel, S.R.; Pantesco, E.J.; Buysse, D.J.; Kamarck, T.W.; Lee, L.; Hall, M.H. Similarities and Differences in Estimates of Sleep Duration by Polysomnography, Actigraphy, Diary, and Self-Reported Habitual Sleep in a Community Sample. Sleep Health 2018, 4, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, E.M.; Hu, F.B.; Redline, S.; Rosner, B.; Alcantara, C.; Cai, J.; Hall, M.H.; Loredo, J.S.; Mossavar-Rahmani, Y.; Ramos, A.R.; et al. Comparison of Self-Reported Sleep Duration With Actigraphy: Results From the Hispanic Community Health Study/Study of Latinos Sueño Ancillary Study. Am. J. Epidemiol. 2016, 183, 561–573. [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J.; Calhoun, S.L.; Bixler, E.O.; Karataraki, M.; Liao, D.; Vela-Bueno, A.; Jose Ramos-Platon, M.; Sauder, K.A.; Basta, M.; Vgontzas, A.N. Sleep Misperception and Chronic Insomnia in the General Population: Role of Objective Sleep Duration and Psychological Profiles. Psychosom. Med. 2011, 73, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.T.; Williams, K.L.; McKinney, S.; Ellenbogen, J.M. The Subjective-Objective Mismatch in Sleep Perception among Those with Insomnia and Sleep Apnea. J. Sleep Res. 2013, 22, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jeon, S.; Lee, H.-J. Mild Obstructive Sleep Apnea Tends to Be Associated with Sleep State Misperception. Chronobiol. Med. 2020, 2, 115–122. [Google Scholar] [CrossRef]
- Rezaie, L.; Fobian, A.D.; McCall, W.V.; Khazaie, H. Paradoxical Insomnia and Subjective-Objective Sleep Discrepancy: A Review. Sleep Med. Rev. 2018, 40, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Herbert, V.; Pratt, D.; Emsley, R.; Kyle, S.D. Predictors of Nightly Subjective-Objective Sleep Discrepancy in Poor Sleepers over a Seven-Day Period. Brain Sci. 2017, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhu, S.; Li, X. Anxiety and Depression in Paradoxical Insomnia: A Case-Control Study. Neuropsychiatr. Dis. Treat. 2018, 14, 231–238. [Google Scholar] [CrossRef]
- Castelnovo, A.; Ferri, R.; Galbiati, A.; Rossi, A.; Zucconi, M.; Castronovo, V.; Strambi, L.-F.; Manconi, M. Extreme Sleep State Misperception: From Psychopathology to Objective-Subjective Sleep Measures. Int. J. Psychophysiol. 2021, 167, 77–85. [Google Scholar] [CrossRef]
- Saline, A.; Goparaju, B.; Bianchi, M.T. Sleep Fragmentation Does Not Explain Misperception of Latency or Total Sleep Time. J. Clin. Sleep Med. 2016, 12, 1245–1255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baillet, M.; Cosin, C.; Schweitzer, P.; Pérès, K.; Catheline, G.; Swendsen, J.; Mayo, W. Mood Influences the Concordance of Subjective and Objective Measures of Sleep Duration in Older Adults. Front. Aging Neurosci. 2016, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Javaheri, S.; Guo, N.; Champion, C.L.; Sims, J.F.; Brock, M.P.; Sims, M.; Patel, S.R.; Williams, D.R.; Wilson, J.G.; et al. Objective Measures of Sleep Apnea and Actigraphy-Based Sleep Characteristics as Correlates of Subjective Sleep Quality in an Epidemiologic Study: The Jackson Heart Sleep Study. Psychosom. Med. 2020, 82, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Khosrawipour, T.; Kocbach, P.; Ichii, H.; Bania, J.; Khosrawipour, V. Evaluating the Massive Underreporting and Undertesting of COVID-19 Cases in Multiple Global Epicenters. Pulmonology 2021, 27, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Krantz, S.G.; Rao, A.S.R.S. Level of Underreporting Including Underdiagnosis before the First Peak of COVID-19 in Various Countries: Preliminary Retrospective Results Based on Wavelets and Deterministic Modeling. Infect. Control Hosp. Epidemiol. 2020, 41, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.J.; MacKenna, B.; Inglesby, P.; Tomlinson, L.; Rentsch, C.T.; Curtis, H.J.; Morton, C.E.; Morley, J.; Mehrkar, A.; Bacon, S.; et al. Clinical Coding of Long COVID in English Primary Care: A Federated Analysis of 58 Million Patient Records in Situ Using OpenSAFELY. Br. J. Gen. Pract. 2021, 71, e806–e814. [Google Scholar] [CrossRef] [PubMed]
| Country | Datapoint | Prevalence (%) of Short Sleep | Reference |
|---|---|---|---|
| USA | 1985 | 22 | [26] |
| 1990s | 13.7 | [21] | |
| 2004–2007 | 28.3 | [25] | |
| 2012 | 29.2 | [26] | |
| 2013 | 21 | [27] | |
| 2014 | 35.2 * | [31] | |
| 2017 | 32.9 | [29] | |
| Canada | 2013 | 7 | [27] |
| Mexico | 2013 | 11 | [27] |
| The Netherlands, UK, USA | 2000–2017 | 6.5 47 ** | [28] |
| The Netherlands | 2012 | 30.4 | [30] |
| Britain | 1990s | 7.5 | [21] |
| Early 2000s | ~13 | [23] | |
| UK | 2013 | 18 | [27] |
| Finland | Early 2000s | 14.5 | [22] |
| 1972–2005 | ~8–12 ^ | [24] | |
| Germany | 2013 | 10 | [27] |
| Japan | 2013 | 19 | [27] |
| Country | Age Group | Datapoint | Prevalence of Short Sleep (%) | Definition of Insufficient Sleep or Sleep Duration | Reference |
|---|---|---|---|---|---|
| Multiple countries | Medical students | 2001–2018 | 29 | <6–8 h or NS | [32] |
| The Netherlands, UK, USA | 14–17 years | 2000–2017 | 51.5 | <8–10 h | [28] |
| USA | High school students | 2014 | 68.8 * | <8 h | [31] |
| Middle school students High school students | 2015 | 57.8 * 72.7 * | <8 h/<9 h ^ | [33] | |
| Canada | Secondary school students | 2013–2016 | 49.7–54.7 | <8–10 h | [34] |
| Norway | 16–19 years | 2012 | 53.8 | <7 h | [35] |
| 16–17 years | 2019 | 49.4 * 11.7 ** | <7 h | [36] | |
| Brazil | 10–14 years | 2014 | 12.6 | <8 h | [37] |
| Saudi Arabia | 10–19 years | 2011–2012 | 45.6 * 33.4 ** | <7 h | [38] |
| Country | Datapoint | Prevalence of Short Sleep (%) | Reference |
|---|---|---|---|
| USA | 2014 | 26.3 | [31] |
| Spain | 2001 | 21.2 | [42] |
| Poland | 1980–1987 | 26.5 | [44] |
| China | 2005–2014 | 11.9 | [41] |
| 1997–2016 | 26.7 | [45] | |
| Taiwan | 1999–2002 | 53.9 | [43] |
| 1993 | 14.6 | [46] | |
| Japan | 2011–2013 | 21.6 | [47] |
| Brazil | 1997 | 17.7 | [48] |
| Country | General Population (95% CI) | Health Care Workers (95% CI) | Reference | ||
|---|---|---|---|---|---|
| Total Pooled Prevalence | PSQI Pooled Prevalence | Total Pooled Prevalence | PSQI Pooled Prevalence | ||
| Multiple countries | 32.3% (25.3–40.2) | 37.9% (25.2–52.4) | 36% (21.1–54.2) | 39.7% (21.2–61.6) | [68] |
| Multiple countries | 18% (15–21) | - | 31% (27–36) | - | [72] |
| Multiple countries | - | - | 44% (24.6–64.5) | - | [71] |
| China | - | - | 45.1% (37.2–53.1) | 58% (43.4–71.9) | [70] |
| Timeframe | 3 Months | 1.5–6 Months | 6 Months | 8–10 Months | 12 Months |
|---|---|---|---|---|---|
| Prevalence of at least one symptom | 99.3% [118] | 41% [119] | 91% [120] | 61% [121] | 81% [122] |
| - | - | 76% [123] | - | - | |
| - | - | 68% [124] | - | 49% [124] | |
| - | - | 61% [125] | - | - |
| Timeframe | 1 Month | 6 Months | 8–10 Months | 12 Months |
|---|---|---|---|---|
| Prevalence of insomnia or sleep difficulties | 40% [127] | 43% [126] | 13.4% [121] | 47% [122] |
| - | 38% [120] | - | - | |
| - | 27% [124] | - | 17% [124] | |
| - | 26% [123] | - | - | |
| - | 5% * [125] | - | - |
| Mental Health Symptoms | General Population | Health Care Workers |
|---|---|---|
| Anxiety | 27.3–28.33% [74,138,139] | 27.5–35.3% [71,74,138] |
| Depression | 24.9, 26.7% [74,138] | 27.05–35.4% [71,74,138] |
| Stress | 51.7% [138] | 56.5, 65.1% [71,138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neculicioiu, V.S.; Colosi, I.A.; Costache, C.; Sevastre-Berghian, A.; Clichici, S. Time to Sleep?—A Review of the Impact of the COVID-19 Pandemic on Sleep and Mental Health. Int. J. Environ. Res. Public Health 2022, 19, 3497. https://doi.org/10.3390/ijerph19063497
Neculicioiu VS, Colosi IA, Costache C, Sevastre-Berghian A, Clichici S. Time to Sleep?—A Review of the Impact of the COVID-19 Pandemic on Sleep and Mental Health. International Journal of Environmental Research and Public Health. 2022; 19(6):3497. https://doi.org/10.3390/ijerph19063497
Chicago/Turabian StyleNeculicioiu, Vlad Sever, Ioana Alina Colosi, Carmen Costache, Alexandra Sevastre-Berghian, and Simona Clichici. 2022. "Time to Sleep?—A Review of the Impact of the COVID-19 Pandemic on Sleep and Mental Health" International Journal of Environmental Research and Public Health 19, no. 6: 3497. https://doi.org/10.3390/ijerph19063497
APA StyleNeculicioiu, V. S., Colosi, I. A., Costache, C., Sevastre-Berghian, A., & Clichici, S. (2022). Time to Sleep?—A Review of the Impact of the COVID-19 Pandemic on Sleep and Mental Health. International Journal of Environmental Research and Public Health, 19(6), 3497. https://doi.org/10.3390/ijerph19063497







