Cognitive Function among World Trade Center-Exposed Community Members with Mental Health Symptoms
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Assessments
2.3. Statistical Methods
3. Results
3.1. Patient Characteristics
3.2. Characteristics Associated with MoCA Scores < 26
3.3. Multivariable Logistic Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clouston, S.A.P.; Hall, C.B.; Kritikos, M.; Bennett, D.A.; DeKosky, S.; Edwards, J.; Finch, C.; Kreisl, W.C.; Mielke, M.; Peskind, E.R.; et al. Cognitive impairment and World Trade Centre-related exposures. Nat. Rev. Neurol. 2021, 18, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Kritikos, M.; Clouston, S.A.P.; Deri, Y.; Serrano-Sosa, M.; Bangiyev, L.; Santiago-Michels, S.; Gandy, S.; Sano, M.; Bromet, E.J.; et al. White Matter Connectivity in Incident Mild Cognitive Impairment: A Diffusion Spectrum Imaging Study of World Trade Center Responders at Midlife. J. Alzheimers Dis. 2021, 80, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Kritikos, M.; Clouston, S.A.P.; Huang, C.; Pellecchia, A.C.; Mejia-Santiago, S.; Carr, M.A.; Kotov, R.; Lucchini, R.G.; Gandy, S.E.; Bromet, E.J.; et al. Cortical complexity in world trade center responders with chronic posttraumatic stress disorder. Transl. Psychiatry 2021, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Clouston, S.; Pietrzak, R.H.; Kotov, R.; Richards, M.; Spiro, A., 3rd; Scott, S.; Deri, Y.; Mukherjee, S.; Stewart, C.; Bromet, E.; et al. Traumatic exposures, posttraumatic stress disorder, and cognitive functioning in World Trade Center responders. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 593–602. [Google Scholar] [CrossRef]
- Clouston, S.A.; Kotov, R.; Pietrzak, R.H.; Luft, B.J.; Gonzalez, A.; Richards, M.; Ruggero, C.J.; Spiro, A., 3rd; Bromet, E.J. Cognitive impairment among World Trade Center responders: Long-term implications of re-experiencing the 9/11 terrorist attacks. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2016, 4, 67–75. [Google Scholar] [CrossRef]
- Clouston, S.A.P.; Diminich, E.D.; Kotov, R.; Pietrzak, R.H.; Richards, M.; Spiro, A., 3rd; Deri, Y.; Carr, M.; Yang, X.; Gandy, S.; et al. Incidence of mild cognitive impairment in World Trade Center responders: Long-term consequences of re-experiencing the events on 9/11/2001. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2019, 11, 628–636. [Google Scholar] [CrossRef]
- Diminich, E.D.; Clouston, S.A.P.; Kranidis, A.; Kritikos, M.; Kotov, R.; Kuan, P.; Carr, M.; Bromet, E.J.; Luft, B.J. Chronic Posttraumatic Stress Disorder and Comorbid Cognitive and Physical Impairments in World Trade Center Responders. J. Trauma. Stress 2021, 34, 616–627. [Google Scholar] [CrossRef]
- Kritikos, M.; Clouston, S.A.P.; Diminich, E.D.; Deri, Y.; Yang, X.; Carr, M.; Gandy, S.; Sano, M.; Bromet, E.J.; Luft, B.J. Pathway Analysis for Plasma beta-Amyloid, Tau and Neurofilament Light (ATN) in World Trade Center Responders at Midlife. Neurol. Ther. 2020, 9, 159–171. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Alper, H.E.; Tuly, R.A.; Seil, K.; Brite, J. Post-9/11 Mental Health Comorbidity Predicts Self-Reported Confusion or Memory Loss in World Trade Center Health Registry Enrollees. Int. J. Environ. Res. Public Health 2020, 17, 7330. [Google Scholar] [CrossRef]
- Seil, K.; Yu, S.; Alper, H. A Cognitive Reserve and Social Support-Focused Latent Class Analysis to Predict Self-Reported Confusion or Memory Loss among Middle-Aged World Trade Center Health Registry Enrollees. Int. J. Environ. Res. Public Health 2019, 16, 1401. [Google Scholar] [CrossRef] [PubMed]
- Lioy, P.J.; Georgopoulos, P. The anatomy of the exposures that occurred around the World Trade Center site: 9/11 and beyond. Ann. N. Y. Acad. Sci. 2006, 1076, 54–79. [Google Scholar] [CrossRef] [PubMed]
- Maslow, C.B.; Friedman, S.M.; Pillai, P.S.; Reibman, J.; Berger, K.I.; Goldring, R.; Stellman, S.D.; Farfel, M. Chronic and acute exposures to the world trade center disaster and lower respiratory symptoms: Area residents and workers. Am. J. Public Health 2012, 102, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Farfel, M.; DiGrande, L.; Brackbill, R.; Prann, A.; Cone, J.; Friedman, S.; Walker, D.J.; Pezeshki, G.; Thomas, P.; Galea, S.; et al. An overview of 9/11 experiences and respiratory and mental health conditions among World Trade Center Health Registry enrollees. J. Urban Health 2008, 85, 880–909. [Google Scholar] [CrossRef] [PubMed]
- Jordan, H.T.; Osahan, S.; Li, J.; Stein, C.R.; Friedman, S.M.; Brackbill, R.M.; Cone, J.E.; Gwynn, C.; Mok, H.K.; Farfel, M.R. Persistent mental and physical health impact of exposure to the September 11, 2001 World Trade Center terrorist attacks. Environ. Health 2019, 18, 12. [Google Scholar] [CrossRef]
- Li, J.; Brackbill, R.M.; Stellman, S.D.; Farfel, M.R.; Miller-Archie, S.A.; Friedman, S.; Walker, D.J.; Thorpe, L.E.; Cone, J. Gastroesophageal reflux symptoms and comorbid asthma and posttraumatic stress disorder following the 9/11 terrorist attacks on World Trade Center in New York City. Am. J. Gastroenterol. 2011, 106, 1933–1941. [Google Scholar] [CrossRef]
- Reibman, J.; Lin, S.; Hwang, S.A.; Gulati, M.; Bowers, J.A.; Rogers, L.; Berger, K.I.; Hoerning, A.; Gomez, M.; Fitzgerald, E.F. The World Trade Center residents’ respiratory health study: New-onset respiratory symptoms and pulmonary function. Environ. Health Perspect. 2005, 113, 406–411. [Google Scholar] [CrossRef]
- Reibman, J.; Liu, M.; Cheng, Q.; Liautaud, S.; Rogers, L.; Lau, S.; Berger, K.I.; Goldring, R.M.; Marmor, M.; Fernandez-Beros, M.E.; et al. Characteristics of a residential and working community with diverse exposure to World Trade Center dust, gas, and fumes. J. Occup. Environ. Med. 2009, 51, 534–541. [Google Scholar] [CrossRef]
- Shao, Y.; Durmus, N.; Zhang, Y.; Pehlivan, S.; Fernandez-Beros, M.E.; Umana, L.; Corona, R.; Addessi, A.; Abbott, S.A.; Smyth-Giambanco, S.; et al. The Development of a WTC Environmental Health Center Pan-Cancer Database. Int. J. Environ. Res. Public Health 2021, 18, 1646. [Google Scholar] [CrossRef]
- Durmus, N.; Shao, Y.; Arslan, A.A.; Zhang, Y.; Pehlivan, S.; Fernandez-Beros, M.E.; Umana, L.; Corona, R.; Smyth-Giambanco, S.; Abbott, S.A.; et al. Characteristics of Cancer Patients in the World Trade Center Environmental Health Center. Int. J. Environ. Res. Public Health 2020, 17, 7190. [Google Scholar] [CrossRef]
- Li, J.; Brackbill, R.M.; Liao, T.S.; Qiao, B.; Cone, J.E.; Farfel, M.R.; Hadler, J.L.; Kahn, A.R.; Konty, K.J.; Stayner, L.T.; et al. Ten-year cancer incidence in rescue/recovery workers and civilians exposed to the September 11, 2001 terrorist attacks on the World Trade Center. Am. J. Ind. Med. 2016, 59, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.; Shao, Y.; Bhatt, D.H.; Stecker, M.M.; Berger, K.I.; Goldring, R.M.; Rosen, R.L.; Caplan-Shaw, C.; Kazeros, A.; Pradhan, D.; et al. Paresthesias Among Community Members Exposed to the World Trade Center Disaster. J. Occup. Environ. Med. 2017, 59, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.; Thawani, S.; Cotrina, M.L.; Shao, Y.; Wong, E.S.; Stecker, M.M.; Wang, B.; Allen, A.; Wilkenfeld, M.; Vinik, E.J.; et al. Case-Control Study of Paresthesia Among World Trade Center-Exposed Community Members. J. Occup. Environ. Med. 2020, 62, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Jordan, H.T.; Miller-Archie, S.A.; Cone, J.E.; Morabia, A.; Stellman, S.D. Heart disease among adults exposed to the September 11, 2001 World Trade Center disaster: Results from the World Trade Center Health Registry. Prev. Med. 2011, 53, 370–376. [Google Scholar] [CrossRef]
- Trasande, L.; Fiorino, E.K.; Attina, T.; Berger, K.; Goldring, R.; Chemtob, C.; Levy-Carrick, N.; Shao, Y.; Liu, M.; Urbina, E.; et al. Associations of World Trade Center exposures with pulmonary and cardiometabolic outcomes among children seeking care for health concerns. Sci. Total Environ. 2013, 444, 320–326. [Google Scholar] [CrossRef]
- Rosen, R.; Zhu, Z.; Shao, Y.; Liu, M.; Bao, J.; Levy-Carrick, N.; Reibman, J. Longitudinal Change of PTSD Symptoms in Community Members after the World Trade Center Destruction. Int. J. Environ. Res. Public Health 2019, 16, 1215. [Google Scholar] [CrossRef]
- Rosen, R.L.; Levy-Carrick, N.; Reibman, J.; Xu, N.; Shao, Y.; Liu, M.; Ferri, L.; Kazeros, A.; Caplan-Shaw, C.E.; Pradhan, D.R.; et al. Elevated C-reactive protein and posttraumatic stress pathology among survivors of the 9/11 World Trade Center attacks. J. Psychiatr. Res. 2017, 89, 14–21. [Google Scholar] [CrossRef]
- DiGrande, L.; Perrin, M.A.; Thorpe, L.E.; Thalji, L.; Murphy, J.; Wu, D.; Farfel, M.; Brackbill, R.M. Posttraumatic stress symptoms, PTSD, and risk factors among lower Manhattan residents 2–3 years after the September 11, 2001 terrorist attacks. J. Trauma. Stress 2008, 21, 264–273. [Google Scholar] [CrossRef]
- Liu, B.; Tarigan, L.H.; Bromet, E.J.; Kim, H. World Trade Center disaster exposure-related probable posttraumatic stress disorder among responders and civilians: A meta-analysis. PLoS ONE 2014, 9, e101491. [Google Scholar] [CrossRef]
- Boscarino, J.A.; Kirchner, H.L.; Hoffman, S.N.; Sartorius, J.; Adams, R.E. PTSD and alcohol use after the World Trade Center attacks: A longitudinal study. J. Trauma. Stress 2011, 24, 515–525. [Google Scholar] [CrossRef]
- North, C.S.; Adinoff, B.; Pollio, D.E.; Kinge, S.; Downs, D.L.; Pfefferbaum, B. Alcohol use disorders and drinking among survivors of the 9/11 attacks on the World Trade Center in New York City. Compr. Psychiatry 2013, 54, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Hudon, C.; Escudier, F.; De Roy, J.; Croteau, J.; Cross, N.; Dang-Vu, T.T.; Zomahoun, H.T.V.; Grenier, S.; Gagnon, J.F.; Parent, A.; et al. Behavioral and Psychological Symptoms that Predict Cognitive Decline or Impairment in Cognitively Normal Middle-Aged or Older Adults: A Meta-Analysis. Neuropsychol. Rev. 2020, 30, 558–579. [Google Scholar] [CrossRef] [PubMed]
- Jorm, A.F. Is depression a risk factor for dementia or cognitive decline? A review. Gerontology 2000, 46, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.; Reitz, C.; Honig, L.H.; Schupf, N.; Tang, M.X.; Manly, J.J.; Mayeux, R.; Devanand, D.; Luchsinger, J.A. Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol. 2013, 70, 374–382. [Google Scholar] [CrossRef]
- Gulpers, B.; Ramakers, I.; Hamel, R.; Kohler, S.; Oude Voshaar, R.; Verhey, F. Anxiety as a Predictor for Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis. Am. J. Geriatr. Psychiatry 2016, 24, 823–842. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Masurkar, A.V. The Relationship of Anxiety with Alzheimer’s Disease: A Narrative Review. Curr. Alzheimer Res. 2021, 18, 359–371. [Google Scholar] [CrossRef]
- Bhattarai, J.J.; Oehlert, M.E.; Multon, K.D.; Sumerall, S.W. Dementia and Cognitive Impairment Among U.S. Veterans With a History of MDD or PTSD: A Retrospective Cohort Study Based on Sex and Race. J. Aging Health 2018, 31, 1398–1422. [Google Scholar] [CrossRef]
- Cohen, B.E.; Neylan, T.C.; Yaffe, K.; Samuelson, K.W.; Li, Y.; Barnes, D.E. Posttraumatic stress disorder and cognitive function: Findings from the mind your heart study. J. Clin. Psychiatry 2013, 74, 1063–1070. [Google Scholar] [CrossRef]
- Qureshi, S.U.; Long, M.E.; Bradshaw, M.R.; Pyne, J.M.; Magruder, K.M.; Kimbrell, T.; Hudson, T.J.; Jawaid, A.; Schulz, P.E.; Kunik, M.E. Does PTSD Impair Cognition Beyond the Effect of Trauma? J. Neuropsychiatry Clin. Neurosci. 2011, 23, 16–28. [Google Scholar] [CrossRef]
- Schuitevoerder, S.; Rosen, J.W.; Twamley, E.W.; Ayers, C.R.; Sones, H.; Lohr, J.B.; Goetter, E.M.; Fonzo, G.A.; Holloway, K.J.; Thorp, S.R. A meta-analysis of cognitive functioning in older adults with PTSD. J. Anxiety Disord. 2013, 27, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Zeig-Owens, R.; Rabin, L.; Schwartz, T.; Webber, M.P.; Appel, D.; Prezant, D.J.; Hall, C.B. PTSD and Depressive Symptoms as Potential Mediators of the Association between World Trade Center Exposure and Subjective Cognitive Concerns in Rescue/Recovery Workers. Int. J. Environ. Res. Public Health 2020, 17, 5683. [Google Scholar] [CrossRef]
- Yehuda, R.; Tischler, L.; Golier, J.A.; Grossman, R.; Brand, S.R.; Kaufman, S.; Harvey, P.D. Longitudinal assessment of cognitive performance in Holocaust survivors with and without PTSD. Biol. Psychiatry 2006, 60, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Reibman, J.; Levy-Carrick, N.; Miles, T.; Flynn, K.; Hughes, C.; Crane, M.; Lucchini, R.G. Destruction of the World Trade Center Towers. Lessons Learned from an Environmental Health Disaster. Ann. Am. Thorac. Soc. 2016, 13, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, E.B.; Jones-Alexander, J.; Buckley, T.C.; Forneris, C.A. Psychometric properties of the PTSD checklist (PCL). Behav. Res. Ther. 1996, 34, 669–673. [Google Scholar] [CrossRef]
- Weathers, F.; Litz, B.; Herman, D.; Huska, J.; Keane, T. The PTSD checklist: Reliability, validity, and dagnostic utility. In Proceedings of the Annual Meeting of the International Society for Traumatic Stress Studies, San Antonio, TX, USA, 24 October 1993. [Google Scholar]
- Terhakopian, A.; Sinaii, N.; Engel, C.C.; Schnurr, P.P.; Hoge, C.W. Estimating population prevalence of posttraumatic stress disorder: An example using the PTSD checklist. J. Trauma. Stress 2008, 21, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9 Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ortiz, F.; Nunez, C.; Elashoff, D.; Woo, E.; Apostolova, L.G.; Wolf, S.; Casado, M.; Caceres, N.; Panchal, H.; et al. Use of the MoCA in Detecting Early Alzheimer’s Disease in a Spanish-Speaking Population with Varied Levels of Education. Dement. Geriatr. Cogn. Dis. Extra. 2015, 5, 85–95. [Google Scholar] [CrossRef]
- Perales-Puchalt, J.; Gauthreaux, K.; Shaw, A.; McGee, J.L.; Teylan, M.A.; Chan, K.C.G.; Rascovsky, K.; Kukull, W.A.; Vidoni, E.D. Risk of mild cognitive impairment among older adults in the United States by ethnoracial group. Int. Psychogeriatr. 2021, 33, 51–62. [Google Scholar] [CrossRef]
- Koster, A.; Penninx, B.W.; Bosma, H.; Kempen, G.I.; Newman, A.B.; Rubin, S.M.; Satterfield, S.; Atkinson, H.H.; Ayonayon, H.N.; Rosano, C.; et al. Socioeconomic differences in cognitive decline and the role of biomedical factors. Ann. Epidemiol. 2005, 15, 564–571. [Google Scholar] [CrossRef]
- Plassman, B.L.; Williams, J.W., Jr.; Burke, J.R.; Holsinger, T.; Benjamin, S. Systematic review: Factors associated with risk for and possible prevention of cogntive decline in later life. Ann. Intern. Med. 2010, 153, 182–193. [Google Scholar] [CrossRef]
- Lee, H.B.; Richardson, A.K.; Black, B.S.; Shore, A.D.; Kasper, J.D.; Rabins, P.V. Race and cognitive decline among community-dwelling elders with mild cognitive impairment: Findings from the Memory and Medical Care Study. Aging Ment. Health 2012, 16, 372–377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clouston, S.A.P.; Smith, D.M.; Mukherjee, S.; Zhang, Y.; Hou, W.; Link, B.G.; Richards, M. Education and Cognitive Decline: An Integrative Analysis of Global Longitudinal Studies of Cognitive Aging. J. Gerontol. B Psychol. Sci. Soc. Sci. 2020, 75, e151–e160. [Google Scholar] [CrossRef] [PubMed]
- Block, S.R.; Liberzon, I. Attentional processes in posttraumatic stress disorder and the associated changes in neural functioning. Exp. Neurol. 2016, 284, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M.; Cohen, M.D.; Chen, L.C. Health effects of World Trade Center (WTC) Dust: An unprecedented disaster’s inadequate risk management. Crit. Rev. Toxicol. 2015, 45, 492–530. [Google Scholar] [CrossRef]
- Kahn, L.G.; Han, X.; Koshy, T.T.; Shao, Y.; Chu, D.B.; Kannan, K.; Trasande, L. Adolescents exposed to the World Trade Center collapse have elevated serum dioxin and furan concentrations more than 12years later. Environ. Int. 2018, 111, 268–278. [Google Scholar] [CrossRef]
- Rosenkranz, M.A.; Dean, D.C., 3rd; Bendlin, B.B.; Jarjour, N.N.; Esnault, S.; Zetterberg, H.; Heslegrave, A.; Evans, M.D.; Davidson, R.J.; Busse, W.W. Neuroimaging and biomarker evidence of neurodegeneration in asthma. J. Allergy Clin. Immunol. 2021, 149, 589–598. [Google Scholar] [CrossRef]
- Weuve, J.; Bennett, E.E.; Ranker, L.; Gianattasio, K.Z.; Pedde, M.; Adar, S.D.; Yanosky, J.D.; Power, M.C. Exposure to Air Pollution in Relation to Risk of Dementia and Related Outcomes: An Updated Systematic Review of the Epidemiological Literature. Environ. Health Perspect. 2021, 129, 96001. [Google Scholar] [CrossRef]
- Chen, X.R.; Shao, Y.; Sadowski, M.J.; Alzheimer’s Disease Neuroimaging, I. Segmented Linear Mixed Model Analysis Reveals Association of the APOEvarepsilon4 Allele with Faster Rate of Alzheimer’s Disease Dementia Progression. J. Alzheimers Dis. 2021, 82, 921–937. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, Y.; Liu, M.; Fernandez-Beros, M.E.; Qian, M.; Reibman, J. Gene-Environment Interaction between the IL1RN Variants and Childhood Environmental Tobacco Smoke Exposure in Asthma Risk. Int. J. Environ. Res. Public Health 2020, 17, 2036. [Google Scholar] [CrossRef]
| Total (N = 480) | MoCA ≥ 26 (N = 199) | MoCA < 26 (N = 281) | p Value | |
|---|---|---|---|---|
| Age | 0.022 | |||
| Median | 56.4 | 54.6 | 56.9 | |
| Q1, Q3 | 48.9, 62.7 | 46.0, 62.2 | 50.3, 63.2 | |
| Gender, n (%) | 0.562 | |||
| Female | 259 | 111 (42.9) | 148 (57.1) | |
| Male | 221 | 88 (39.8) | 133 (60.2) | |
| Race/Ethnicity, n (%) | <0.001 | |||
| Asian | 20 | 9 (45.0) | 11 (55.0) | |
| Hispanic | 170 | 54 (31.8) | 116 (68.2) | |
| NH-Black | 91 | 27 (29.7) | 64 (70.3) | |
| NH-White | 187 | 104 (55.6) | 83 (44.4) | |
| Other | 5 | 2 (40.0) | 3 (60.0) | |
| BMI | 0.161 | |||
| Median | 28.10 | 27.50 | 28.50 | |
| Q1, Q3 | 24.6, 31.9 | 23.9, 31.4 | 24.9, 32.4 | |
| Smoking, n (%) | 0.285 | |||
| Current smoker | 53 | 20 (37.7) | 33 (62.3) | |
| Former smoker | 148 | 69 (46.6) | 79 (53.4) | |
| Never smoker | 278 | 109 (39.2) | 169 (60.8) | |
| Occupation, n (%) | 0.190 | |||
| Disability | 47 | 15 (31.9) | 32 (68.1) | |
| Not working | 99 | 39 (39.4) | 60 (60.6) | |
| Retired | 54 | 18 (33.3) | 36 (66.7) | |
| Working | 276 | 124 (44.9) | 152 (55.1) | |
| Language, n (%) | <0.001 | |||
| English | 398 | 184 (46.2) | 214 (53.8) | |
| Spanish | 80 | 14 (17.5) | 66 (82.5) | |
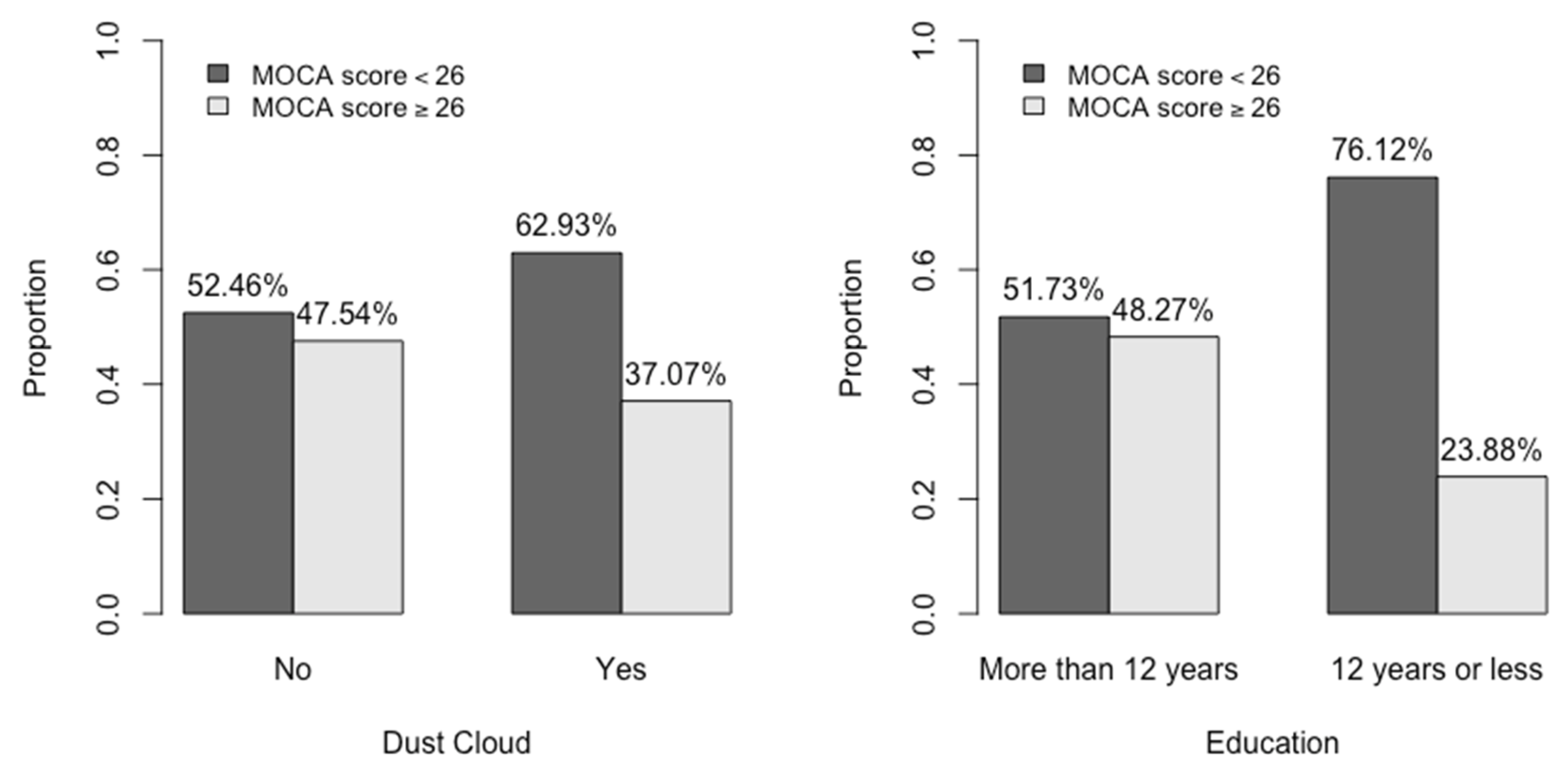
| Education, n (%) | <0.001 | |||
| >12 years | 346 | 167 (48.3) | 179 (51.7) | |
| ≤12 years | 134 | 32 (23.9) | 102 (76.1) | |
| Income, n (%) | <0.001 | |||
| ≤$30,000/year | 234 | 79 (33.8) | 155 (66.2) | |
| >$30,000/year | 215 | 108 (50.2) | 107 (49.8) | |
| PCL-17, n (%) | 0.346 | |||
| Negative | 117 | 53 (45.3) | 64 (54.7) | |
| Positive | 347 | 138 (39.8) | 209 (60.2) | |
| PCL-17 score | 0.013 | |||
| Median | 54.0 | 50.0 | 55.0 | |
| Q1, Q3 | 43.0, 64.0 | 41.0, 62.0 | 44.0, 64.0 | |
| PHQ-9, n (%) | 0.356 | |||
| None (0–4) | 35 | 13 (37.1) | 22 (62.9) | |
| Mild (5–9) | 104 | 48 (46.2) | 56 (53.9) | |
| Moderate (10–14) | 125 | 50 (40.0) | 75 (60.0) | |
| Mod. severe (15–19) | 123 | 55 (44.7) | 68 (55.3) | |
| Severe (20–27) | 85 | 28 (33.0) | 57 (67.1) | |
| PHQ-9 score | 0.160 | |||
| Median | 14.0 | 13.0 | 14.0 | |
| Q1, Q3 | 9.0, 18.0 | 9.0, 17.0 | 9.0, 19.0 | |
| Exposure, n (%) | <0.001 | |||
| Clean-up worker | 39 | 6 (15.4) | 33 (84.6) | |
| Other | 33 | 15 (45.5) | 18 (54.6) | |
| Resident | 89 | 54 (60.7) | 35 (39.3) | |
| Worker | 318 | 124 (39.0) | 194 (61.0) | |
| Dust Cloud, n (%) | 0.030 | |||
| No | 183 | 87 (47.6) | 96 (52.5) | |
| Yes | 294 | 109 (37.1) | 185 (62.9) |
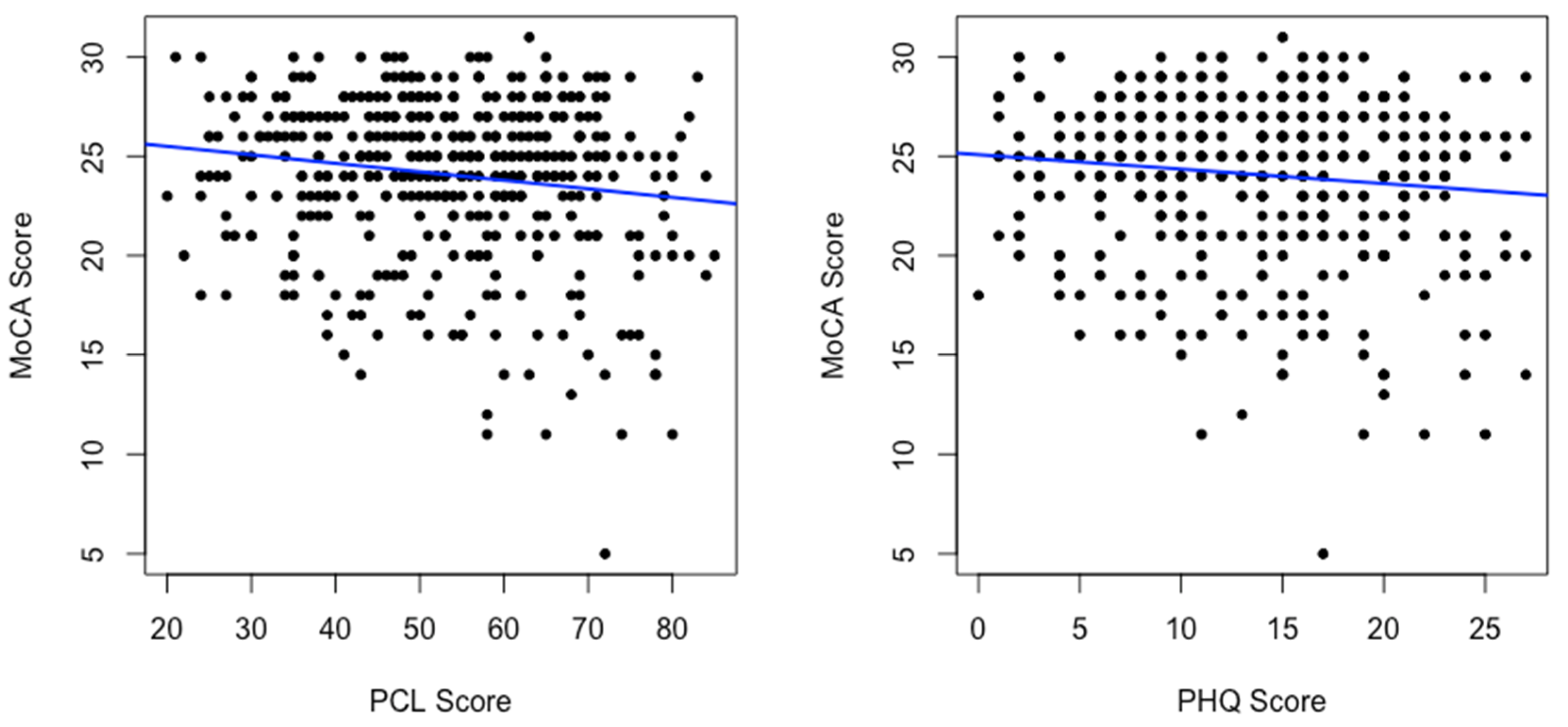
| Slope | p-Value | |
|---|---|---|
| PCL | −0.043 | 0.001 |
| PHQ-9 | −0.073 | 0.016 |
| Odds Ratio | 2.5% | 97.5% | p-Value | |
|---|---|---|---|---|
| Age | 1.04 | 1.02 | 1.06 | 0.001 |
| Race | ||||
| Asian | 2.38 | 0.83 | 6.88 | 0.11 |
| Hispanic | 1.80 | 1.01 | 3.19 | 0.045 |
| NH-Black | 3.09 | 1.68 | 5.68 | 0.000 |
| Other | 2.36 | 0.36 | 15.29 | 0.368 |
| NH-White (reference) | 1.00 | |||
| Language | ||||
| Spanish | 1.59 | 0.65 | 3.87 | 0.310 |
| Education | ||||
| ≤12 years | 2.01 | 1.12 | 3.63 | 0.020 |
| Income | ||||
| ≤$30,000/year | 1.76 | 1.12 | 2.77 | 0.014 |
| PHQ-9 | 0.98 | 0.93 | 1.04 | 0.51 |
| PCL | 1.02 | 1.0 | 1.04 | 0.133 |
| Exposure categories | ||||
| Clean-up worker | 3.02 | 0.78 | 11.74 | 0.110 |
| Other | 1.40 | 0.53 | 3.66 | 0.497 |
| Worker | 1.62 | 0.93 | 2.83 | 0.089 |
| Resident (Reference) | 1.00 | |||
| Dust Cloud exposure on 11 September 2011 | ||||
| Yes | 1.59 | 1.01 | 2.49 | 0.044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosen, R.; Shao, Y.; Zhang, Q.; Bao, J.; Zhang, Y.; Masurkar, A.; Wisniewski, T.; Urban, N.; Reibman, J. Cognitive Function among World Trade Center-Exposed Community Members with Mental Health Symptoms. Int. J. Environ. Res. Public Health 2022, 19, 3440. https://doi.org/10.3390/ijerph19063440
Rosen R, Shao Y, Zhang Q, Bao J, Zhang Y, Masurkar A, Wisniewski T, Urban N, Reibman J. Cognitive Function among World Trade Center-Exposed Community Members with Mental Health Symptoms. International Journal of Environmental Research and Public Health. 2022; 19(6):3440. https://doi.org/10.3390/ijerph19063440
Chicago/Turabian StyleRosen, Rebecca, Yongzhao Shao, Qiao Zhang, Jia Bao, Yian Zhang, Arjun Masurkar, Thomas Wisniewski, Nina Urban, and Joan Reibman. 2022. "Cognitive Function among World Trade Center-Exposed Community Members with Mental Health Symptoms" International Journal of Environmental Research and Public Health 19, no. 6: 3440. https://doi.org/10.3390/ijerph19063440
APA StyleRosen, R., Shao, Y., Zhang, Q., Bao, J., Zhang, Y., Masurkar, A., Wisniewski, T., Urban, N., & Reibman, J. (2022). Cognitive Function among World Trade Center-Exposed Community Members with Mental Health Symptoms. International Journal of Environmental Research and Public Health, 19(6), 3440. https://doi.org/10.3390/ijerph19063440







