Trends in Excess Winter Mortality (EWM) from 1900/01 to 2019/20—Evidence for a Complex System of Multiple Long-Term Trends
Abstract
1. Definitions
2. Introduction
3. Materials and Methods
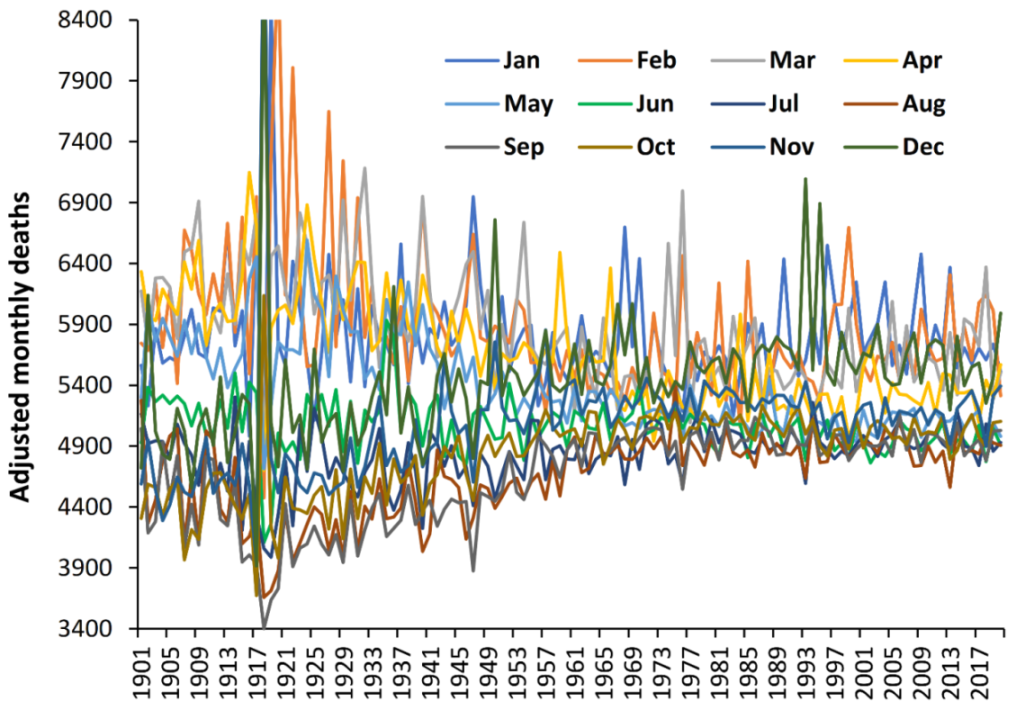
3.1. Monthly Deaths
3.2. Adjusting EWM for Differences between Countries
3.3. Influenza Vaccine Doses Distributed per 1000 Population
3.4. Influenza Vaccination Rates, Aged 65+
3.5. Data Manipulation
4. Results
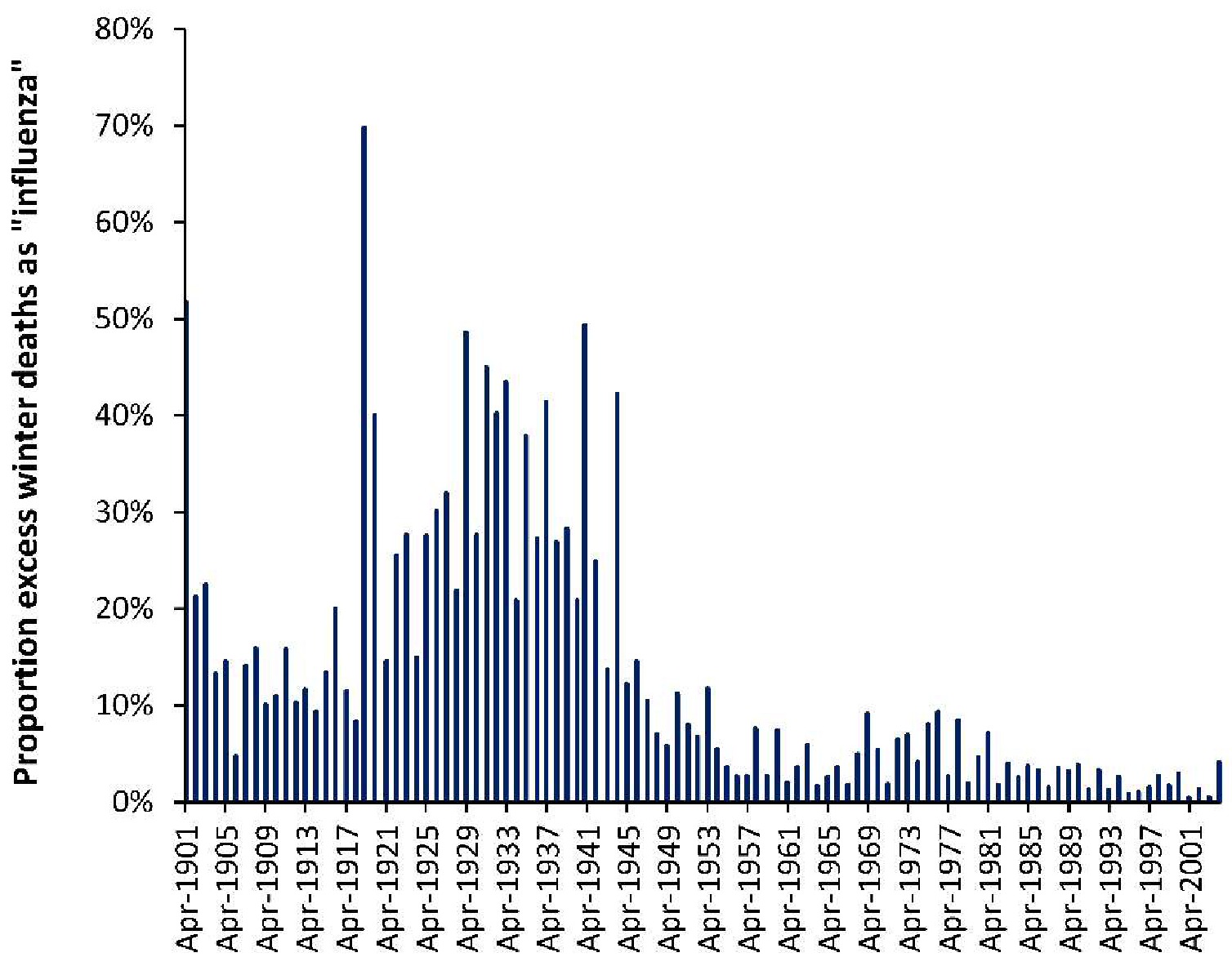
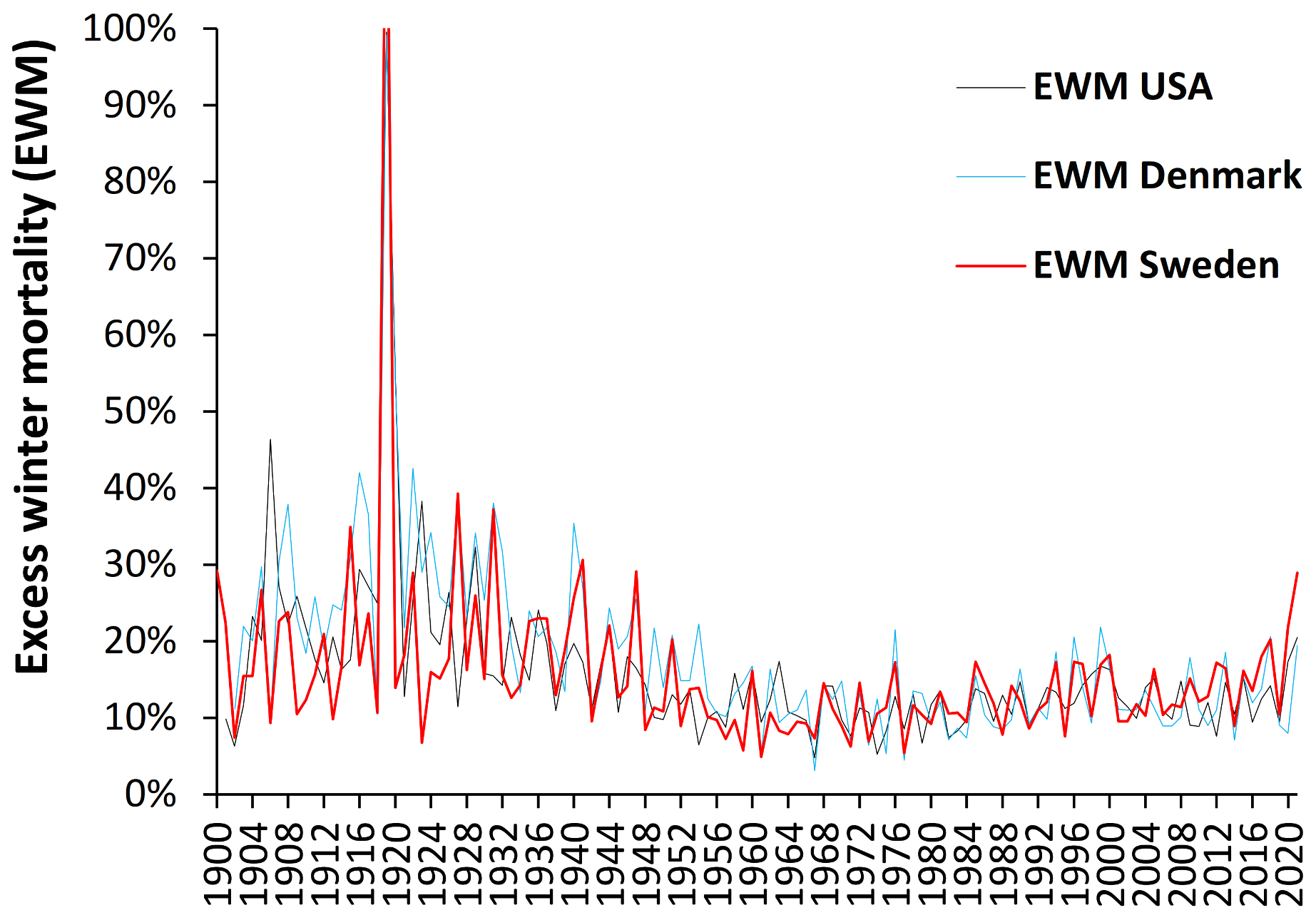
4.1. Long-Term Trends in EWM
- Both USA and Denmark show 100% EWM during the 1918/19 winter of the Spanish flu epidemic while Sweden had 131% EWM, i.e., EWM is directly influenced by, and sensitive to, influenza activity and virulence.
- While the EWM calculation for all three countries during the Spanish flu shows a maximum for the 4 months ending January 1919, the USA and Sweden shows maximum monthly deaths in October 1918, while maximum deaths in Denmark occurred in November 1918. In terms of the timing for higher Spanish flu deaths Denmark tends to be right skewed while the USA and Sweden are left skewed. This demonstrates the need for a rolling EWM calculation rather than a static one.
- In addition, EWM shows more complex trends, with a localized maximum around 1906–1907 (1900/01 to 1911/12) in Denmark and the USA, and a wider maximum centered around the time of the Spanish flu (1911/12 to 1930/31)—by coincidence Spanish flu occurred at a time of high baseline EWM. It should be noted that the emergence of Spanish flu precursor strains appears to have started somewhere around 1911 [50], and EWM appears to have correctly identified the timing of re-assortments culminating in the maximum at 1918 [50].
- After the Spanish flu, EWM then declined to reach a general minimum in the 1960s to 1980s which occurred in the very early days of the introduction of more widespread influenza vaccination.
- As an interesting observation there is no evidence that the four subsequent flu pandemics of the 1957–58 Asian flu, 1968–69 Hong Kong flu, 1977–79 Russian flu, or the 2009 Swine flu gave rise to unusually high EWM.
- There was another smaller peak for the three winters ending 1999/2000.
4.2. Month in Which EWM Reaches a Maximum
4.3. Winter Deaths Are Higher before the 1970s
4.4. Similar Trends Are Seen in World Countries since the 1960s
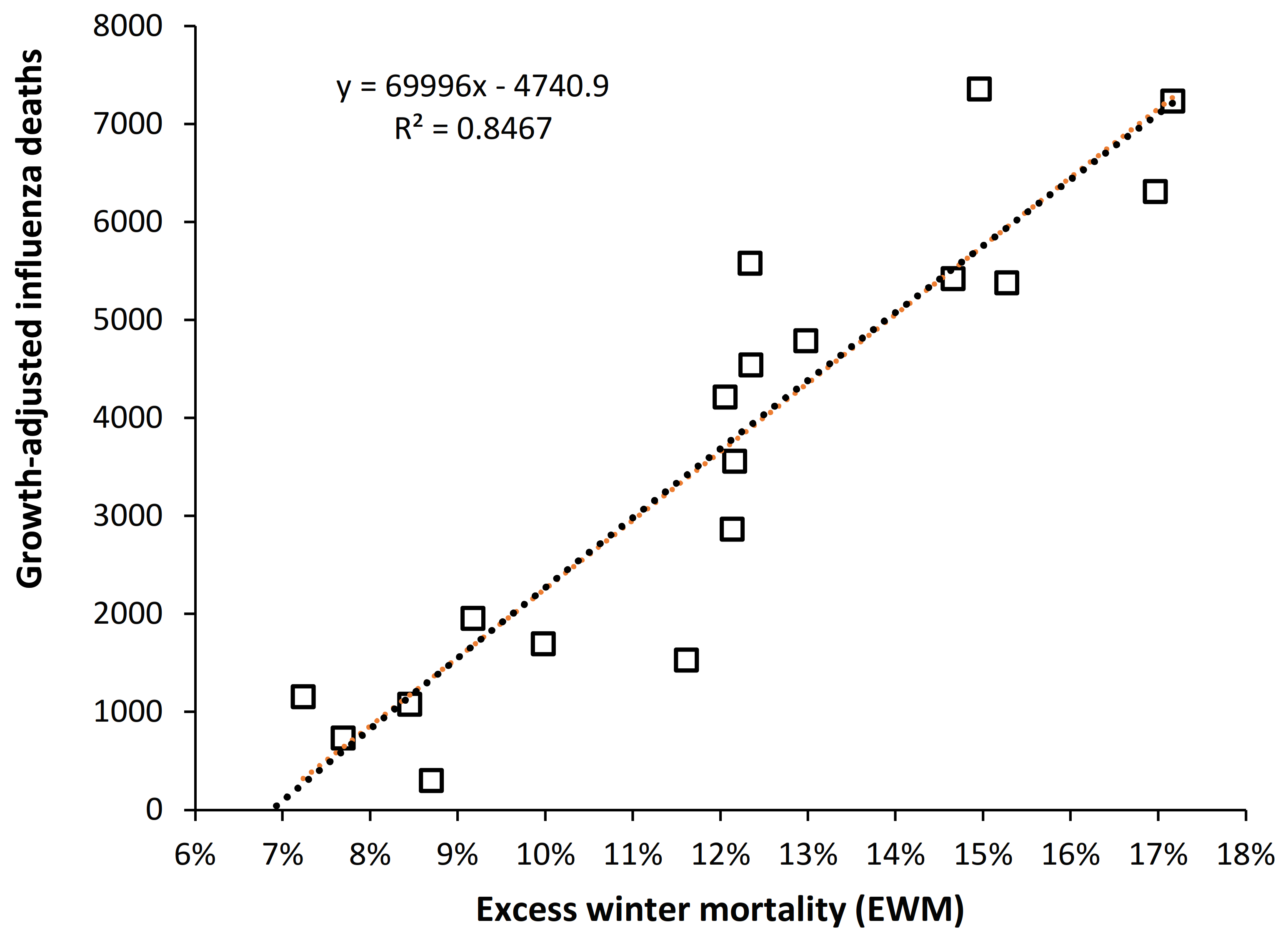
4.5. EWM Correlates Well with Calculated Influenza Deaths
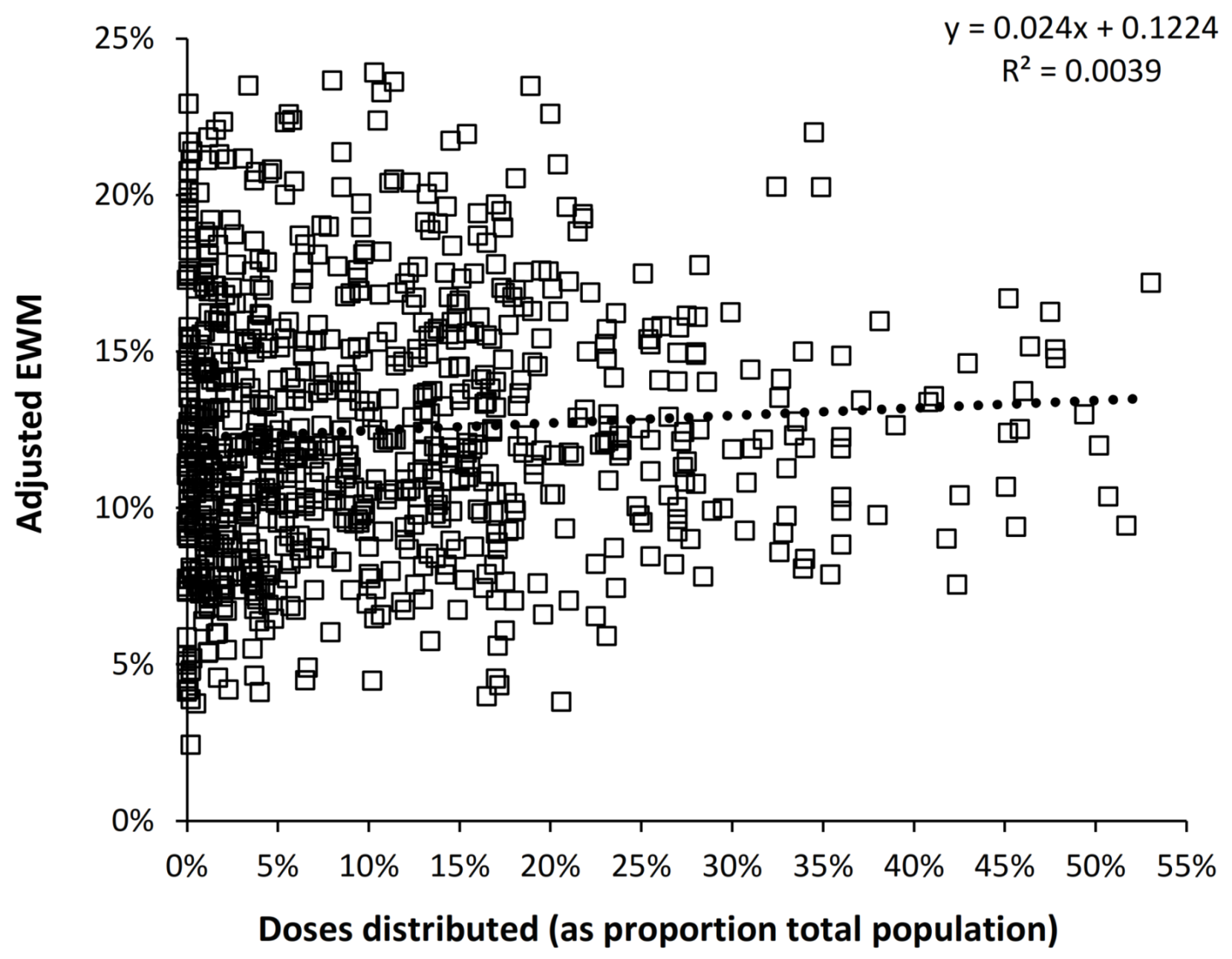
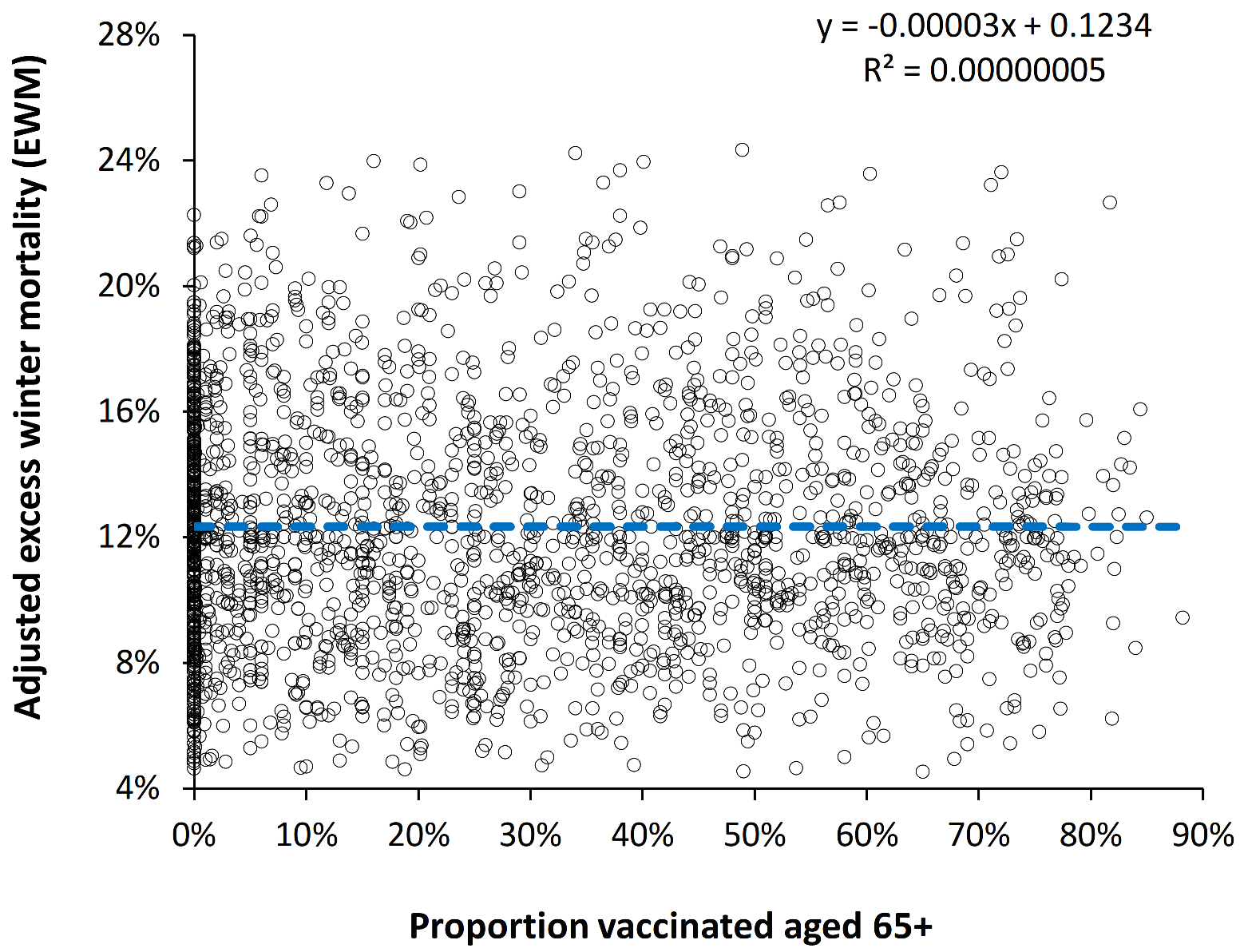
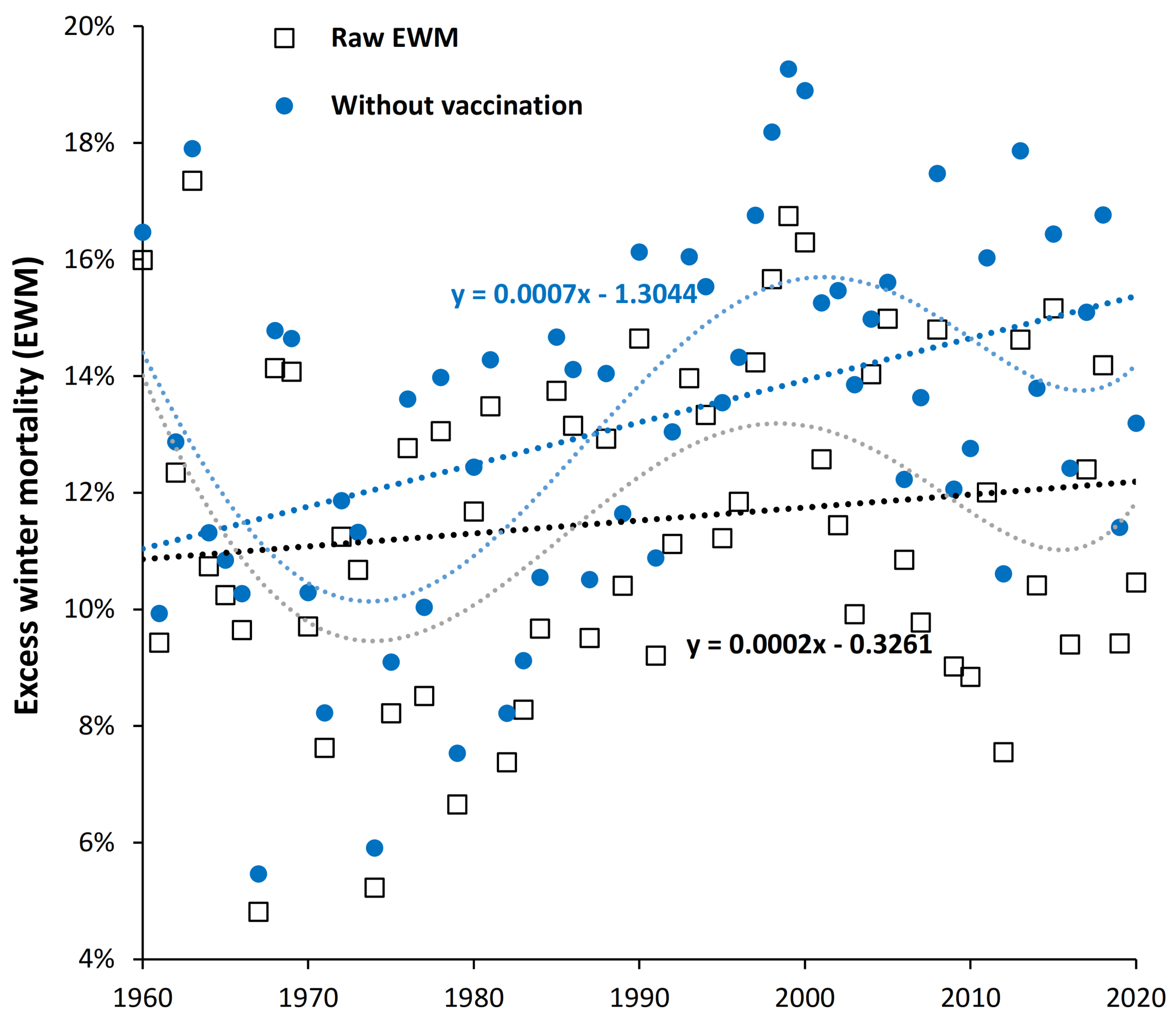
4.6. Is the Effect of Influenza Vaccination Detectable Using EWM?
4.7. Is Influenza Vaccination Masking a Trend to Poor Health in the USA?
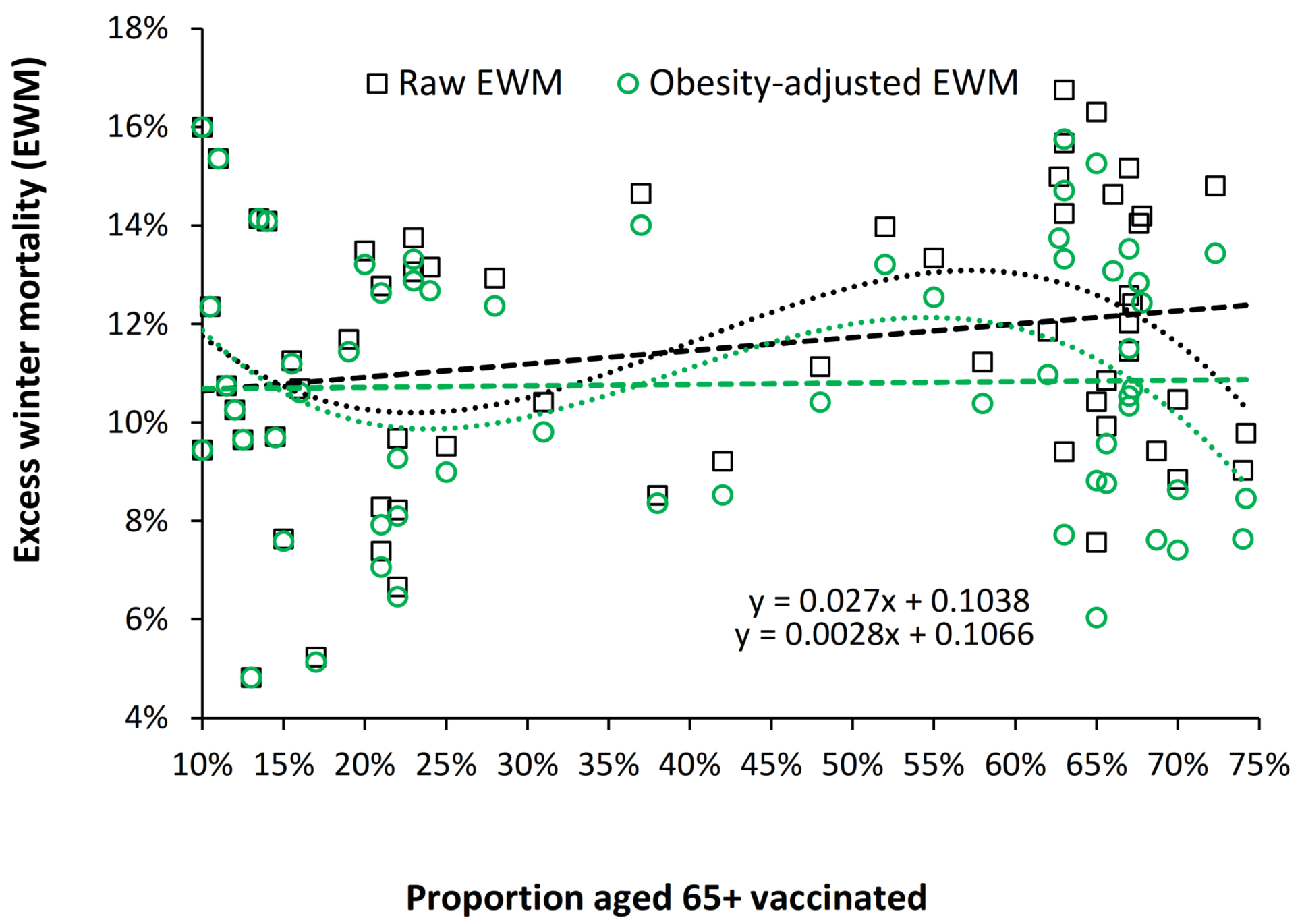
4.8. Obesity and Median EWM among American States
5. Discussion
5.1. Long-Term Trends in EWM
5.2. EWM Is Sensitive to Influenza Deaths
5.3. The EWM Adjustment Factor
5.4. Evidence for Seasonal Levels of Influenza Deaths during Subsequent Pandemics
5.5. What Constitutes a “Good” Estimate of Influenza Deaths?
- Influenza infection is low to near absent.
- Other winter pathogens are also less active
- The winter is exceptionally mild, i.e., warm
- Other factors are suppressing airborne pathogen spread
5.6. EWM and Influenza Vaccination—Competing Underlying Trends
5.7. Undulations in the Long-Term Trends
5.8. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Jones, R. Excess Winter Mortality (EWM) as a Dynamic Forensic Tool: Where, When, Which Conditions, Gender, Ethnicity and Age. Int. J. Environ. Res. Public Health 2021, 18, 2161. [Google Scholar] [CrossRef] [PubMed]
- Glezen, W.P. Emerging Infections: Pandemic Influenza. Epidemiol. Rev. 1996, 18, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, M.I.; Cox, N.J.; Fukuda, K. The Economic Impact of Pandemic Influenza in the United States: Priorities for Intervention. Emerg. Infect. Dis. 1999, 5, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, A.; Ciancio, B.C.; Chavarrias, V.L.; Mølbak, K.; Pebody, R.; Pedzinski, B.; Penttinen, P.; van der Sande, M.; Snacken, R.; Van Kerkhove, M.D. Influenza-related deaths-available methods for estimating numbers and detecting patterns for seasonal and pandemic influenza in Europe. Eurosurveillance 2012, 17, 20162. [Google Scholar] [CrossRef][Green Version]
- Muscatello, D.J.; Newall, A.T.; Dwyer, D.E.; MacIntyre, C.R. Mortality Attributable to Seasonal and Pandemic Influenza, Australia, 2003 to 2009, Using a Novel Time Series Smoothing Approach. PLoS ONE 2013, 8, e64734. [Google Scholar] [CrossRef]
- EuroMOMO. FluMOMO. FluMOMO—EUROMOMO. Available online: https://www.euromomo.eu/how-it-works/flumomo (accessed on 14 January 2022).
- Rolfes, M.A.; Foppa, I.M.; Garg, S.; Flannery, B.; Brammer, L.; Singleton, J.A.; Burns, E.; Jernigan, D.; Olsen, S.J.; Bresee, J.; et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir. Viruses 2018, 12, 132–137. [Google Scholar] [CrossRef]
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C. Global Seasonal Influenza-Associated Mortality Collaborator Network and GLaMOR Collaborating Teams. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 2019, 9, 020421. [Google Scholar] [CrossRef]
- Eurostat, Deaths (Total) by Month. Deaths (Total) by Month—Products Datasets—Eurostat (europa.eu). Available online: https://ec.europa.eu/eurostat/en/web/products-datasets/-/DEMO_MMONTH (accessed on 11 November 2021).
- UNData. Deaths by Month of Death. UNdata|Record View|Deaths by Month of Death. Available online: https://data.un.org/Data.aspx?d=POP&f=tableCode%3a65%3bcountryCode%3a804&c=2,3,6,8,10,12,13,14&s=_countryEnglishNameOrderBy:asc,refYear:desc,areaCode:asc&v=1 (accessed on 11 November 2021).
- Doshi, P. Trends in Recorded Influenza Mortality: United States, 1900–2004. Am. J. Public Health 2008, 98, 939–945. [Google Scholar] [CrossRef]
- CDC National Vital Statistics System. Monthly and 12 Month–Ending Number of Live Births, Deaths and Infant Deaths, United States. State and National Provisional Counts (cdc.gov). Available online: https://www.cdc.gov/nchs/nvss/vsrr/provisional-tables.htm (accessed on 11 November 2021).
- CDC National Vital Statistics System. Deaths by State. State and National Provisional Counts (cdc.gov). Available online: https://www.cdc.gov/nchs/nvss/vsrr/provisional-tables.htm (accessed on 19 February 2022).
- Statistics Denmark. Live Births and Deaths by Movement and Time. Available online: https://www.statbank.dk/20014 (accessed on 16 April 2021).
- Statistics Sweden. Deaths by Year and Month. Births and Deaths Per Month by Sex. Year 1851–2021. PxWeb (scb.se). Available online: https://www.statistikdatabasen.scb.se/pxweb/en/ssd/START__BE__BE0101__BE0101G/ManadFoddDod/ (accessed on 19 February 2022).
- Statistics Singapore. Deaths by Ethnic Group and Sex, Monthly. (DOS)|SingStat Table Builder—Deaths By Ethnic Group and Sex. Available online: https://tablebuilder.singstat.gov.sg/table/TS/M810121 (accessed on 14 January 2022).
- Statistics Estonia. Monthly Deaths. Deaths|Statistikaamet. Available online: https://www.stat.ee/en/find-statistics/statistics-theme/population/deaths (accessed on 12 December 2021).
- Fedson, D.S.; Hannoun, C.; Leese, J.; Sprenger, M.J.; Hampson, A.W.; Bro-Jørgensen, K.; Ahlbom, A.M.; Nøkleby, H.; Valle, M.; Olafsson, O.; et al. Influenza vaccination in 18 developed countries, 1980–1992. Vaccine 1995, 13, 623–627. [Google Scholar] [CrossRef]
- Fedson, D.S.; Hirota, Y.; Shin, H.-K.; Cambillard, P.-E.; Kiely, J.; Ambrosch, F.; Hannoun, C.; Leese, J.; Sprenger, M.J.; Hampson, A.W.; et al. Influenza vaccination in 22 developed countries: An update to 1995. Vaccine 1997, 15, 1506–1511. [Google Scholar] [CrossRef]
- Ambrosch, F.; Fedson, D.S. Influenza Vaccination in 29 Countries. PharmacoEconomics 1999, 16, 47–54. [Google Scholar] [CrossRef]
- Vanessen, G.; Palache, A.; Forleo, E.; Fedson, D. Influenza vaccination in 2000: Recommendations and vaccine use in 50 developed and rapidly developing countries. Vaccine 2003, 21, 1780–1785. [Google Scholar] [CrossRef]
- The Macroepidemiology of Influenza Vaccination (MIV) Study Group. The macroepidemiology of influenza vaccination in 56 countries, 1997–2003. Vaccine 2005, 23, 5133–5143. [Google Scholar] [CrossRef]
- Palache, A. Seasonal influenza vaccine provision in 157 countries (2004–2009) and the potential influence of national public health policies. Vaccine 2011, 29, 9459–9466. [Google Scholar] [CrossRef]
- Palache, A.; Oriol-Mathieu, V.; Abelin, A.; Music, T. Seasonal influenza vaccine dose distribution in 157 countries (2004–2011). Vaccine 2014, 32, 6369–6376. [Google Scholar] [CrossRef]
- Palache, A.; Oriol-Mathieu, V.; Fino, M.; Xydia-Charmanta, M. Seasonal influenza vaccine dose distribution in 195 countries (2004–2013): Little progress in estimated global vaccination coverage. Vaccine 2015, 33, 5598–5605. [Google Scholar] [CrossRef]
- CDC. Historical Reference of Seasonal Influenza Vaccine Doses Distributed. Historical Reference of Seasonal Influenza Vaccine Doses Distributed|CDC. Available online: https://www.cdc.gov/flu/prevent/vaccine-supply-historical.htm (accessed on 24 December 2021).
- Macrotrends. US Population Growth Rate 1950–2021. MacroTrends. Available online: https://www.macrotrends.net/countries/USA/us/population-growth-rate (accessed on 11 November 2021).
- Simonsen, L.; Reichert, T.A.; Viboud, C.; Blackwelder, W.C.; Taylor, R.J.; Miller, M.A. Impact of Influenza Vaccination on Seasonal Mortality in the US Elderly Population. Arch. Intern. Med. 2005, 165, 265–272. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Influenza Vaccination Coverage Trends, 1989 to 2008. Available online: https://www.cdc.gov/flu/pdf/professionals/nhis89_08fluvaxtrendtab.pdf (accessed on 8 February 2021).
- CDC. Influenza Vaccine Coverage, Adults Aged 65 Years and Older, United States. Influenza Vaccination Coverage, Adults 65 Years and Older, United States|FluVaxView|Seasonal Influenza (Flu)|CDC. Available online: https://www.cdc.gov/flu/fluvaxview/dashboard/vaccination-coverage-adults-65-over.htm (accessed on 11 November 2021).
- Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2018–2019 Influenza Season. FluVaxView|Seasonal Influenza (Flu)|CDC. Available online: https://www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm (accessed on 30 December 2021).
- CDC. Flu Vaccination Coverage, United States, 2019–2020 Influenza Season. FluVaxView|Seasonal Influenza (Flu)|CDC. Available online: https://www.cdc.gov/flu/fluvaxview/coverage-1920estimates.htm (accessed on 8 February 2021).
- OECD Data. Influenza Vaccination Rates (Full Indicator Data). Health Care Use—Influenza Vaccination Rates—OECD Data. Available online: https://data.oecd.org/healthcare/influenza-vaccination-rates.htm (accessed on 14 January 2022).
- Spruijt, I.T.; de Lange, M.M.; Dijkstra, F.; Donker, G.A.; van der Hoek, W. Long-term correlation between influenza vaccination coverage and incidence of influenza-like illness in 14 European Countries. PLoS ONE 2016, 11, e0163508. [Google Scholar] [CrossRef][Green Version]
- WHO. Influenza Vaccination Data Base. Influenza Vaccination Policy (who.int). Available online: https://immunizationdata.who.int/pages/indicators-by-category/influenza.html?ISO_3_CODE=&YEAR= (accessed on 14 January 2022).
- World Health Organisation. Influenza Vaccination Coverage, Elderly. European Health Information Gateway (who.int). 2020. Available online: https://gateway.euro.who.int/en/indicators/infl_8-influenza-vaccination-coverage-elderly/ (accessed on 8 February 2021).
- Public Health England. Green Book of Immunization. Chapter 19 Influenza. 2020; The Green Book of Immunisation—Chapter 19 Influenza (publishing.service.gov.uk). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/931139/Green_book_chapter_19_influenza_V7_OCT_2020.pdf (accessed on 12 December 2021).
- Public Health Wales. Annual Influenza Surveillance and Influenza Vaccination Uptake Reports: 2003–2020. Public Health Wales Health Protection Division. Available online: https://www.wales.nhs.uk/sites3/page.cfm?orgid=457&pid=55714 (accessed on 12 March 2021).
- Jorgensen, P.; Mereckiene, J.; Cotter, S.; Johansen, K.; Tsolova, S.; Brown, C. How close are countries of the WHO European Region to achieving the goal of vaccinating 75% of key risk groups against influenza? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine 2018, 36, 442–452. [Google Scholar] [CrossRef]
- ECDC. Seasonal Influenza Vaccination in Europe—Vaccination Recommendations and Coverage Rates for Eight Influenza Seasons (2007–2008 to 2014–2015). (europa.eu). 20 July 2017. Available online: https://www.ecdc.europa.eu/en/publications-data/seasonal-influenza-vaccination-europe-vaccination-recommendations-and-coverage-2007-2015 (accessed on 25 February 2022).
- Blank, P.; Schwenkglenks, M.; Szucs, T.D. The impact of European vaccination policies on seasonal influenza vaccination coverage rates in the elderly. Hum. Vaccines Immunother. 2012, 8, 328–335. [Google Scholar] [CrossRef]
- Blank, P.R.; Schwenkglenks, M.; Szucs, T.D. Influenza vaccination coverage rates in five European countries during season 2006/07 and trends over six consecutive seasons. BMC Public Health 2008, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, P.; Kilit, T.P.; Onbasi, K.; Ozatag, D.M.; Toka, O. Influenza vaccination prevalence among the elderly and individuals with chronic disease, and factors affecting vaccination uptake. Central Eur. J. Public Health 2019, 27, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Beard, F.; Hendry, A.; Macartney, K. Influenza vaccination uptake in our most vulnerable groups: How well are we protecting them in 2019? Commun. Dis. Intell. 2020, 2020, 44. [Google Scholar] [CrossRef] [PubMed]
- Newspoll Omnibus Survey. Summary Report Flu Vaccinations Department of Health Newspoll Ref: 140604 23 June 2014. (health.gov.au). Available online: https://www.health.gov.au/sites/default/files/report-newspoll-flu-vaccinations-survey-jun-2014.pdf (accessed on 12 December 2021).
- Zürcher, K.; Zwahlen, M.; Berlin, C.; Egger, M.; Fenner, L. Losing ground at the wrong time: Trends in self-reported influenza vaccination uptake in Switzerland, Swiss Health Survey 2007–2017. BMJ Open 2021, 11, e041354. [Google Scholar] [CrossRef] [PubMed]
- Singapore Ministry of Health. National Population Health Survey 2019. (moh.gov.sg). Available online: https://www.moh.gov.sg/docs/librariesprovider5/default-document-library/nphs-2019-survey-report.pdf (accessed on 17 February 2021).
- Kwon, D.S.; Kim, K.; Park, S.M. Factors associated with influenza vaccination coverage among the elderly in South Korea: The Fourth Korean National Health and Nutrition Examination Survey (KNHANES IV). BMJ Open 2016, 6, e012618. [Google Scholar] [CrossRef] [PubMed]
- González-Block, M. Ángel; Gutiérrez-Calderón, E.; Pelcastre-Villafuerte, B.E.; Arroyo-Laguna, J.; Comes, Y.; Crocco, P.; Fachel-Leal, A.; Noboa, L.; Riva-Knauth, D.; Rodríguez-Zea, B.; et al. Influenza vaccination hesitancy in five countries of South America. Confidence, complacency and convenience as determinants of immunization rates. PLoS ONE 2020, 15, e0243833. [Google Scholar] [CrossRef]
- Smith, G.J.; Bahl, J.; Vijaykrishna, D.; Zhang, J.; Poon, L.; Chen, H.; Webster, R.G.; Peiris, J.S.M.; Guan, Y. Dating the emergence of pandemic influenza viruses. Proc. Natl. Acad. Sci. USA 2009, 106, 11709–11712. [Google Scholar] [CrossRef] [PubMed]
- Jones, R. How many extra deaths have really occurred in the UK due to the COVID-19 outbreak? Part LVIX. German states most affected by COVID-19. Res. Gate 2022. [Google Scholar] [CrossRef]
- Nielsen, J.; Krause, T.G.; Mølbak, K. Influenza-associated mortality determined from all-cause mortality, Denmark 2010/11–2016/17: The FluMOMO model. Influenza Other Respir. Viruses 2018, 12, 591–604. [Google Scholar] [CrossRef]
- Schanzer, D.L.; Sevenhuysen, C.; Winchester, B.; Mersereau, T. Estimating Influenza Deaths in Canada, 1992–2009. PLoS ONE 2013, 8, e80481. [Google Scholar] [CrossRef]
- CDC. CDC Seasonal Flu Vaccine Effectiveness Studies. CDC Seasonal Flu Vaccine Effectiveness Studies|CDC. Available online: https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm (accessed on 25 January 2022).
- CDC. Adult Obesity Facts. Adult Obesity Facts|Overweight & Obesity|CDC. Available online: https://www.cdc.gov/obesity/data/adult.html (accessed on 12 February 2022).
- Achievements in Public Health, 1900–1999: Changes in the Public Health System. JAMA 2000, 283, 735–738. [CrossRef][Green Version]
- Woods, R. Mortality and sanitary conditions in the “Best governed city in the world”—Birmingham, 1870–1910. J. Hist. Geogr. 1978, 4, 35–56. [Google Scholar] [CrossRef]
- Daniel, T.M. The history of tuberculosis. Respir. Med. 2006, 100, 1862–1870. [Google Scholar] [CrossRef]
- Ledberg, A. A large decrease in the magnitude of seasonal fluctuations in mortality among elderly explains part of the increase in longevity in Sweden during 20th century. BMC Public Health 2020, 20, 1674. [Google Scholar] [CrossRef]
- Gan, G.; Pang, J. Obesity in Singapore: Prevention and control. Singap. Fam. Phys. 2012, 38, 8–13. [Google Scholar]
- Thomas, R.E. Are influenza-associated morbidity and mortality estimates for those ≥65 in statistical databases accurate, and an appropriate test of influenza vaccine effectiveness? Vaccine 2014, 32, 6884–6901. [Google Scholar] [CrossRef]
- Gagnon, A.; Miller, M.; Hallman, S.A.; Bourbeau, R.; Herring, D.A.; Earn, D.; Madrenas, J. Age-Specific Mortality During the 1918 Influenza Pandemic: Unravelling the Mystery of High Young Adult Mortality. PLoS ONE 2013, 8, e69586. [Google Scholar] [CrossRef]
- Grinbaum, E.; Ivanova, N.; Luzianina, T.; Nevedomskaia, G.; Kudriavtseva, V. Antigennaia i biologicheskaia kharakteristika virusov grippa A1, izolirovannykh v pandemiiu 1977 g [Antigenic and biological characteristics of influenza A1 viruses isolated in the 1977 pandemic]. Vopr Virusol. 1979, 3, 232–236. [Google Scholar]
- Rozo, M.; Gronvall, G.K. The Reemergent 1977 H1N1 Strain and the Gain-of-Function Debate. mBio 2015, 6, e01013-15. [Google Scholar] [CrossRef]
- Adalja, A.A.; Henderson, D. Original Antigenic Sin and Pandemic (H1N1) 2009. Emerg. Infect. Dis. 2010, 16, 1028. [Google Scholar] [CrossRef]
- Henry, C.; Palm, A.-K.E.; Krammer, F.; Wilson, P.C. From Original Antigenic Sin to the Universal Influenza Virus Vaccine. Trends Immunol. 2017, 39, 70–79. [Google Scholar] [CrossRef]
- Wu, A.; Peng, Y.; Du, X.; Shu, Y.; Jiang, T. Correlation of Influenza Virus Excess Mortality with Antigenic Variation: Application to Rapid Estimation of Influenza Mortality Burden. PLoS Comput. Biol. 2010, 6, e1000882. [Google Scholar] [CrossRef]
- Gupta, V.; Earl, D.J.; Deem, M.W. Quantifying influenza vaccine efficacy and antigenic distance. Vaccine 2006, 24, 3881–3888. [Google Scholar] [CrossRef]
- Sitaras, I. Antigenic Cartography: Overview and Current Developments. Methods Pharmacol. Toxicol. 2020, 2123, 61–68. [Google Scholar] [CrossRef]
- Anderson, C.S.; McCall, P.R.; Stern, H.A.; Yang, H.; Topham, D.J. Antigenic cartography of H1N1 influenza viruses using sequence-based antigenic distance calculation. BMC Bioinform. 2018, 19, 51. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, T.; Wan, X.-F. Antigenic distance measurements for seasonal influenza vaccine selection. Vaccine 2012, 30, 448–453. [Google Scholar] [CrossRef]
- Kawakami, C.; Yamayoshi, S.; Akimoto, M.; Nakamura, K.; Miura, H.; Fujisaki, S.; Pattinson, D.J.; Shimizu, K.; Ozawa, H.; Momoki, T.; et al. Genetic and antigenic characterisation of influenza A(H3N2) viruses isolated in Yokohama during the 2016/17 and 2017/18 influenza seasons. Eurosurveillance 2019, 24, 1800467. [Google Scholar] [CrossRef]
- CDC. Summary of the 2017–2018 Influenza Season. Summary of the 2017–2018 Influenza Season|CDC. Available online: https://www.cdc.gov/flu/about/season/flu-season-2017-2018.htm#:~:text=The%202017-2018%20influenza%20season%20was%20a%20high%20severity,geographically%20widespread%20influenza%20activity%20for%20an%20extended%20period. (accessed on 27 November 2021).
- Thompson, W.W.; Moore, M.R.; Weintraub, E.; Cheng, P.-Y.; Jin, X.; Bridges, C.B.; Bresee, J.S.; Shay, D. Estimating Influenza-Associated Deaths in the United States. Am. J. Public Health 2009, 99, S225–S230. [Google Scholar] [CrossRef]
- CDC. Summary of the 2011–2012 Influenza Season. Summary of the 2011–2012 Influenza Season|CDC. Available online: https://www.cdc.gov/flu/pastseasons/1112season.htm (accessed on 15 December 2021).
- CDC. Summary of the 2014–2015 Influenza Season. Summary of the 2014–2015 Influenza Season|CDC. Available online: https://www.cdc.gov/flu/pastseasons/1415season.htm (accessed on 15 December 2021).
- CDC. Summary of the 2013–2014 Influenza Season. Summary of the 2013–2014 Influenza Season|CDC. Available online: https://www.cdc.gov/flu/pastseasons/1314season.htm#:~:text=During%20the%202013-2014%20season%2C%20CDC%20received%20reports%20of,were%20in%20people%2018%20to%2064%20years%20old. (accessed on 15 December 2021).
- CDC. Disease Burden of Flu. Disease Burden of Flu|CDC. Available online: https://www.cdc.gov/flu/about/burden/index.html?web=1&wdLOR=c51D04AA5-C27A-4E2B-8344-9780EA08A310 (accessed on 15 December 2021).
- CDC. Estimates of Deaths Associated with Seasonal Influenza—United States, 1976–2007. Morb. Mortal. Wkly. Rep. 2010, 59, 1057–1062. [Google Scholar]
- CDC. How Many Adults Die from Flu Each Year?. Frequently Asked Questions about Estimated Flu Burden|CDC. Available online: https://www.cdc.gov/flu/about/burden/faq.htm (accessed on 15 December 2021).
- WHO. Introduction of Seasonal Influenza Vaccine. Introduction of Seasonal Influenza Vaccine (who.int). Available online: https://immunizationdata.who.int/pages/vaccine-intro-by-antigen/influenza.html (accessed on 16 January 2022).
- Walzer, P.; Estève, C.; Barben, J.; Menu, D.; Cuenot, C.; Manckoundia, P.; Putot, A. Impact of Influenza Vaccination on Mortality in the Oldest Old: A Propensity Score-Matched Cohort Study. Vaccines 2020, 8, 356. [Google Scholar] [CrossRef]
- Rizzo, C.; Viboud, C.; Montomoli, E.; Simonsen, L.; Miller, M.A. Influenza-related mortality in the Italian elderly: No decline associated with increasing vaccination coverage. Vaccine 2006, 24, 6468–6475. [Google Scholar] [CrossRef] [PubMed]
- Head, A.; Fleming, K.; Kypridemos, C.; Schofield, P.; Pearson-Stuttard, J.; O’Flaherty, M. Inequalities in incident and prevalent multimorbidity in England, 2004–2019: A population-based, descriptive study. Lancet Health Longev. 2021, 2, e489–e497. [Google Scholar] [CrossRef]
- Lebenbaum, M.; Zaric, G.S.; Thind, A.; Sarma, S. Trends in obesity and multimorbidity in Canada. Prev. Med. 2018, 116, 173–179. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Kuchel, G.; Zhou, X.; Swain, S.L.; Ehaynes, L. T-Cell Immunity to Influenza in Older Adults: A Pathophysiological Framework for Development of More Effective Vaccines. Front. Immunol. 2016, 7, 41. [Google Scholar] [CrossRef]
- Gutiérrez-González, E.; Cantero-Escribano, J.M.; Redondo-Bravo, L.; Juan-Sanz, I.S.; Robustillo-Rodela, A.; Cendejas-Bueno, E. Influenza Working Group Effect of vaccination, comorbidities and age on mortality and severe disease associated with influenza during the season 2016–2017 in a Spanish tertiary hospital. J. Infect. Public Health 2019, 12, 486–491. [Google Scholar] [CrossRef]
- Preval, N.; Keall, M.; Telfar-Barnard, L.; Grimes, A.; Howden-Chapman, P. Impact of improved insulation and heating on mortality risk of older cohort members with prior cardiovascular or respiratory hospitalisations. BMJ Open 2017, 7, e018079. [Google Scholar] [CrossRef]
- WHO. Housing and Health Guidelines. Chapter 4: Low Indoor Temperatures and Insulation. 2018; Low Indoor Temperatures and Insulation—WHO Housing and Health Guidelines—NCBI Bookshelf (nih.gov). Available online: https://www.ncbi.nlm.nih.gov/books/NBK535294/ (accessed on 15 December 2021).
- Huebner, G.M.; Hamilton, I.; Chalabi, Z.; Shipworth, D.; Oreszczyn, T. Comparison of indoor temperatures of homes with recommended temperatures and effects of disability and age: An observational, cross-sectional study. BMJ Open 2018, 8, e021085. [Google Scholar] [CrossRef]
- Saeki, K.; Obayashi, K.; Iwamoto, J.; Tone, N.; Okamoto, N.; Tomioka, K.; Kurumatani, N. Stronger association of indoor temperature than outdoor temperature with blood pressure in colder months. J. Hypertens. 2014, 32, 1582–1589. [Google Scholar] [CrossRef]
- NICE. Excess Winter Deaths and Illness and the Health Risks Associated with Cold Homes. NICE Guideline [NG6], 5 March 2015. Overview|Excess Winter Deaths and Illness and the Health Risks Associated with Cold Homes|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/NG6 (accessed on 29 December 2021).
- Hajat, S. Evidence Review and Economic Analysis of Excess Winter Deaths. 2015. Available online: https://www.nice.org.uk/guidance/ng6/evidence/evidence-review-2-interventions-and-economic-studies-pdf-544621934 (accessed on 29 December 2021).
- Fyfe, C.; Telfar, L.; Barnard; Howden-Chapman, P.; Douwes, J. Association between home insulation and hospital admission rates: Retrospective cohort study using linked data from a national intervention programme. BMJ 2020, 371, m4571. [Google Scholar] [CrossRef]
- Oktora, M.P.; Denig, P.; Bos, J.H.J.; Schuiling-Veninga, C.C.M.; Hak, E. Trends in polypharmacy and dispensed drugs among adults in the Netherlands as compared to the United States. PLoS ONE 2019, 14, e0214240. [Google Scholar] [CrossRef]
- Agarwal, D.; Schmader, K.E.; Kossenkov, A.V.; Doyle, S.; Kurupati, R.; Ertl, H.C.J. Immune response to influenza vaccination in the elderly is altered by chronic medication use. Immun. Ageing 2018, 15, 19. [Google Scholar] [CrossRef]
- Iloanusi, S.; Mgbere, O.; Essien, E.J. Polypharmacy among COVID-19 patients: A systematic review. J. Am. Pharm. Assoc. 2021, 61, e14–e25. [Google Scholar] [CrossRef]
- Frazier, S.C. Health Outcomes and Polypharmacy in Elderly Individuals. J. Gerontol. Nurs. 2005, 31, 4–9. [Google Scholar] [CrossRef]
- Lin, C.-J.; Chang, Y.-C.; Tsou, M.-T.; Chan, H.-L.; Chen, Y.-J.; Hwang, L.-C. Factors associated with hospitalization for community-acquired pneumonia in home health care patients in Taiwan. Aging Clin. Exp. Res. 2019, 32, 149–155. [Google Scholar] [CrossRef]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef]
- Frasca, D.; Ferracci, F.; Diaz, A.; Romero, M.; Lechner, S.; Blomberg, B.B. Obesity decreases B cell responses in young and elderly individuals. Obesity 2016, 24, 615–625. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Blomberg, B.B. Phenotypic and Functional Characterization of Double Negative B Cells in the Blood of Individuals With Obesity. Front. Immunol. 2021, 12, 98. [Google Scholar] [CrossRef]
- Frasca, D.; Reidy, L.; Romero, M.; Diaz, A.; Cray, C.; Kahl, K.; Blomberg, B.B. The majority of SARS-CoV-2-specific antibodies in COVID-19 patients with obesity are autoimmune and not neutralizing. Int. J. Obes. 2021, 46, 427–432. [Google Scholar] [CrossRef]
- Livio, L.; Radaelli, M. Influenza and obesity: Its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020, 57, 759–764. [Google Scholar]
- CDC. Current Cigarette Smoking among Adults in the United States. Current Cigarette Smoking Among Adults in the United States|CDC. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm (accessed on 24 December 2021).
- Chavez, J.; Hai, R. Effects of Cigarette Smoking on Influenza Virus/Host Interplay. Pathogens 2021, 10, 1636. [Google Scholar] [CrossRef]
- Godoy, P.; Castilla, J.; Soldevila, N.; Mayoral, J.M.; Toledo, D.; Martín, V.; Astray, J.; Egurrola, M.; Morales-Suarez-Varela, M.; Domínguez, A. Smoking may increase the risk of influenza hospitalization and reduce influenza vaccine effectiveness in the elderly. Eur. J. Public Health 2017, 28, 150–155. [Google Scholar] [CrossRef]
- Corrada, M.M.; Brookmeyer, R.; Paganini-Hill, A.; Berlau, D.; Kawas, C.H. Dementia incidence continues to increase with age in the oldest old: The 90+ study. Ann. Neurol. 2010, 67, 114–121. [Google Scholar] [CrossRef]
- Brandeis, G.H.; Berlowitz, D.; Coughlin, N. Mortality Associated with an Influenza Outbreak on a Dementia Care Unit. Alzheimer Dis. Assoc. Disord. 1998, 12, 140–145. [Google Scholar] [CrossRef]
- Pawelec, G.; McElhaney, J. Recent advances in influenza vaccines. F1000Research 2020, 9, 305. [Google Scholar] [CrossRef]
- Tsilibary, E.-P.; Charonis, S.A.; Georgopoulos, A.P. Vaccines for Influenza. Vaccines 2021, 9, 47. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Imperatore, G.; Geiss, L.S.; Wang, J.; Saydah, S.H.; Cowie, C.C.; Gregg, E.W. Secular Changes in the Age-Specific Prevalence of Diabetes Among U.S. Adults: 1988–2010. Diabetes Care 2013, 36, 2690–2696. [Google Scholar] [CrossRef]
- Valdez, R.; Narayan, K.M.; Geiss, L.S.; Engelgau, M.M. Impact of diabetes mellitus on mortality associated with pneumonia and influenza among non-Hispanic black and white US adults. Am. J. Public Health 1999, 89, 1715–1721. [Google Scholar] [CrossRef]
- Wang, C.-S.; Wang, S.-T.; Lai, C.-T.; Lin, L.-J.; Chou, P. Impact of influenza vaccination on major cause-specific mortality. Vaccine 2007, 25, 1196–1203. [Google Scholar] [CrossRef]
- Schade, C.P.; McCombs, M.A. Influenza immunization and mortality among diabetic Medicare beneficiaries in West Virginia. West Va. Med. J. 2003, 96, 444–448. [Google Scholar]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and Cancer Risk: A Potentially Modifiable Relationship. Am. J. Prev. Med. 2014, 46, S7–S15. [Google Scholar] [CrossRef]
- Carreira, H.; Strongman, H.; Peppa, M.; McDonald, H.I.; Dos-Santos-Silva, I.; Stanway, S.; Smeeth, L.; Bhaskaran, K. Prevalence of COVID-19-related risk factors and risk of severe influenza outcomes in cancer survivors: A matched cohort study using linked English electronic health records data. eClinicalMedicine 2020, 29–30, 100656. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.A.; Karim, N.A. Influenza Vaccination in Cancer Patients Undergoing Systemic Therapy. Clin. Med. Insights Oncol. 2014, 8, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kaufman, J.S.; Olaniyan, T.; Pinault, L.; Tjepkema, M.; Chen, L.; van Donkelaar, A.; Martin, R.V.; Hystad, P.; Chen, C.; et al. Changes in exposure to ambient fine particulate matter after relocating and long term survival in Canada: Quasi-experimental study. BMJ 2021, 375, 2368. [Google Scholar] [CrossRef] [PubMed]
- Glencross, D.A.; Ho, T.-R.; Camiña, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air pollution and its effects on the immune system. Free Radic. Biol. Med. 2020, 151, 56–68. [Google Scholar] [CrossRef]
- Lindner-Cendrowska, K.; Bröde, P. Impact of biometeorological conditions and air pollution on influenza-like illnesses incidence in Warsaw. Int. J. Biometeorol. 2021, 65, 929–944. [Google Scholar] [CrossRef]
- Lu, B.; Wang, Y.; Zhu, Z.; Zhang, Z.; Dong, T.; Li, F.; Gao, Y.; Du, X.; Qu, Z. Epidemiological and genetic characteristics of influenza virus and the effects of air pollution on laboratory-confirmed influenza cases in Hulunbuir, China, from 2010 to 2019. Epidemiol. Infect. 2020, 148, e159. [Google Scholar] [CrossRef]
- Wong, C.M.; Yang, L.; Thach, T.Q.; Chau, P.Y.K.; Chan, K.P.; Thomas, G.N.; Lam, T.H.; Wong, T.W.; Hedley, A.J.; Peiris, J.M. Modification by Influenza on Health Effects of Air Pollution in Hong Kong. Environ. Health Perspect. 2009, 117, 248–253. [Google Scholar] [CrossRef]
- Liu, K.; Yang, B.-Y.; Guo, Y.; Bloom, M.S.; Dharmage, S.; Knibbs, L.D.; Heinrich, J.; Leskinen, A.; Lin, S.; Morawska, L.; et al. The role of influenza vaccination in mitigating the adverse impact of ambient air pollution on lung function in children: New insights from the Seven Northeastern Cities Study in China. Environ. Res. 2020, 187, 109624. [Google Scholar] [CrossRef]
- ONS. Deaths by Single Year of Age Tables, UK—2020—GOV.UK (www.gov.uk). Available online: https://www.gov.uk/government/statistics/deaths-by-single-year-of-age-tables-uk-2020 (accessed on 21 February 2022).
- Yin, Q.; Wang, J.; Ren, Z.; Li, J.; Guo, Y. Mapping the increased minimum mortality temperatures in the context of global climate change. Nat. Commun. 2019, 10, 4640. [Google Scholar] [CrossRef]
- Allen, M.J.; Sheridan, S.C. Mortality risks during extreme temperature events (ETEs) using a distributed lag non-linear model. Int. J. Biometeorol. 2015, 62, 57–67. [Google Scholar] [CrossRef]
- Office for National Statistics. Excess Winter Mortality in England and Wales: 2012 to 2013 (Provisional) and 2011 to 2012 (Final). 26 November 2013. (publishing.service.gov.uk). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/229819/Excess_winter_mortality_2012.pdf#:~:text=Excess%20winter%20mortality%202012-13%20Excess%20all-cause%20mortality%20has,Diseases%20Department%20%28RDD%29%20within%20Public%20Health%20England%20%28PHE%29 (accessed on 15 December 2021).
- Murtas, R.; Russo, A.G. Effects of pollution, low temperature and influenza syndrome on the excess mortality risk in winter 2016–2017. BMC Public Health 2019, 19, 1445. [Google Scholar] [CrossRef]
- Rosano, A.; Bella, A.; Gesualdo, F.; Acampora, A.; Pezzotti, P.; Marchetti, S.; Ricciardi, W.; Rizzo, C. Investigating the impact of influenza on excess mortality in all ages in Italy during recent seasons (2013/14–2016/17 seasons). Int. J. Infect. Dis. 2019, 88, 127–134. [Google Scholar] [CrossRef]
- Thurner, S.; Klimek, P.; Hanel, R. Introduction to the Theory of Complex Systems; Oxford University Press (OUP): Oxford, UK, 2018. [Google Scholar]



| Factors Increasing EWM with Time | Factors Reducing EWM with Time |
|---|---|
| Multimorbidity—Levels of basic and complex multimorbidity have been increasing over time [84,85]. Multimorbidity is associated with diminished response to influenza vaccination [86] and risk of influenza mortality [87]. | Home insulation—this is a major contributor to reductions in winter hospitalization and mortality in some countries [88,89,90,91,92,93,94]. The greatest benefit will occur in countries mid-way between the poles and equator which tend to have housing suited to summer rather than winter [1]. |
| Polypharmacy—Polypharmacy in the Netherlands and USA had more than doubled in the interval 1999 to 2014 [95]. Certain pharmaceuticals and polypharmacy alter the immune response to influenza vaccination [96]. Polypharmacy has been found to be a risk factor for hospitalization, and death from COVID-19 [97] and for pneumonia admissions for nursing home residents [98,99]. | Increased access to health care (critical care, antibiotics, antivirals, etc.) and wider public health measures [1]—this will mainly apply to the less developed countries. |
| Obesity—has been increasing over time [100]. It creates systemic inflammation, reduces B-cell function [101,102], generates auto-immune antibodies during infection [103,104], and interferes with influenza vaccination efficiency [103,104]. As demonstrated in Figure S1 and Figure A5 the effect of the trends in elderly obesity alone has the potential to explain a moderate proportion of the gap between the lines in Figure 7. | Reduced smoking prevalence—smoking leads to inflammation and is a risk factor in influenza mortality [105,106,107]. |
| Alzheimers and dementia—Incidence increases exponentially with age [108] and are a significant risk factor for influenza mortality [109]. | Improvements in influenza vaccine technology such as cell versus egg grown vaccines, adjuvants, etc. [110,111]. |
| Diabetes—Incidence increases with age [112] and is a significant risk factor in influenza mortality [113]. In the USA persons aged 65+ experienced the greatest increase in the incidence of diabetes since 1988 [112]. However, mortality is reduced by influenza vaccination [114,115]. | Influenza vaccination in the elderly—increased vaccination will lead to lower influenza deaths [82], especially in years with a high VE. |
| Cancer—Cancer incidence increases with age [116] with incidence especially high in the US [116]. Cancer survivors are at far higher risk of influenza mortality [117]. Mortality is reduced by influenza vaccination [118] | |
| Air pollution (especially in large cities with population growth)—Air pollution is well recognized for its ability to increase systemic inflammation [119], alter aspects of immune function [120], increase incidence of ILI [121], and increase the proportion of persons infected with influenza [122]. It also interferes with influenza vaccination efficiency and is a risk factor for influenza mortality [123]. In children, air pollution has an adverse effect on lung function which is moderated by influenza vaccination [124]. | |
| Longeivity or increasing age at death—EWM increases with age at death, however, difficult to assess over many decades as chronological age is not a good measure of biological or epigenetic age [1]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, R.P.; Ponomarenko, A. Trends in Excess Winter Mortality (EWM) from 1900/01 to 2019/20—Evidence for a Complex System of Multiple Long-Term Trends. Int. J. Environ. Res. Public Health 2022, 19, 3407. https://doi.org/10.3390/ijerph19063407
Jones RP, Ponomarenko A. Trends in Excess Winter Mortality (EWM) from 1900/01 to 2019/20—Evidence for a Complex System of Multiple Long-Term Trends. International Journal of Environmental Research and Public Health. 2022; 19(6):3407. https://doi.org/10.3390/ijerph19063407
Chicago/Turabian StyleJones, Rodney P., and Andriy Ponomarenko. 2022. "Trends in Excess Winter Mortality (EWM) from 1900/01 to 2019/20—Evidence for a Complex System of Multiple Long-Term Trends" International Journal of Environmental Research and Public Health 19, no. 6: 3407. https://doi.org/10.3390/ijerph19063407
APA StyleJones, R. P., & Ponomarenko, A. (2022). Trends in Excess Winter Mortality (EWM) from 1900/01 to 2019/20—Evidence for a Complex System of Multiple Long-Term Trends. International Journal of Environmental Research and Public Health, 19(6), 3407. https://doi.org/10.3390/ijerph19063407







