The Aging Process of Cadmium in Paddy Soils under Intermittent Irrigation with Acid Water: A Short-Term Simulation Experiment
Abstract
:1. Introduction
2. Material and Methods
2.1. Soil and Analysis
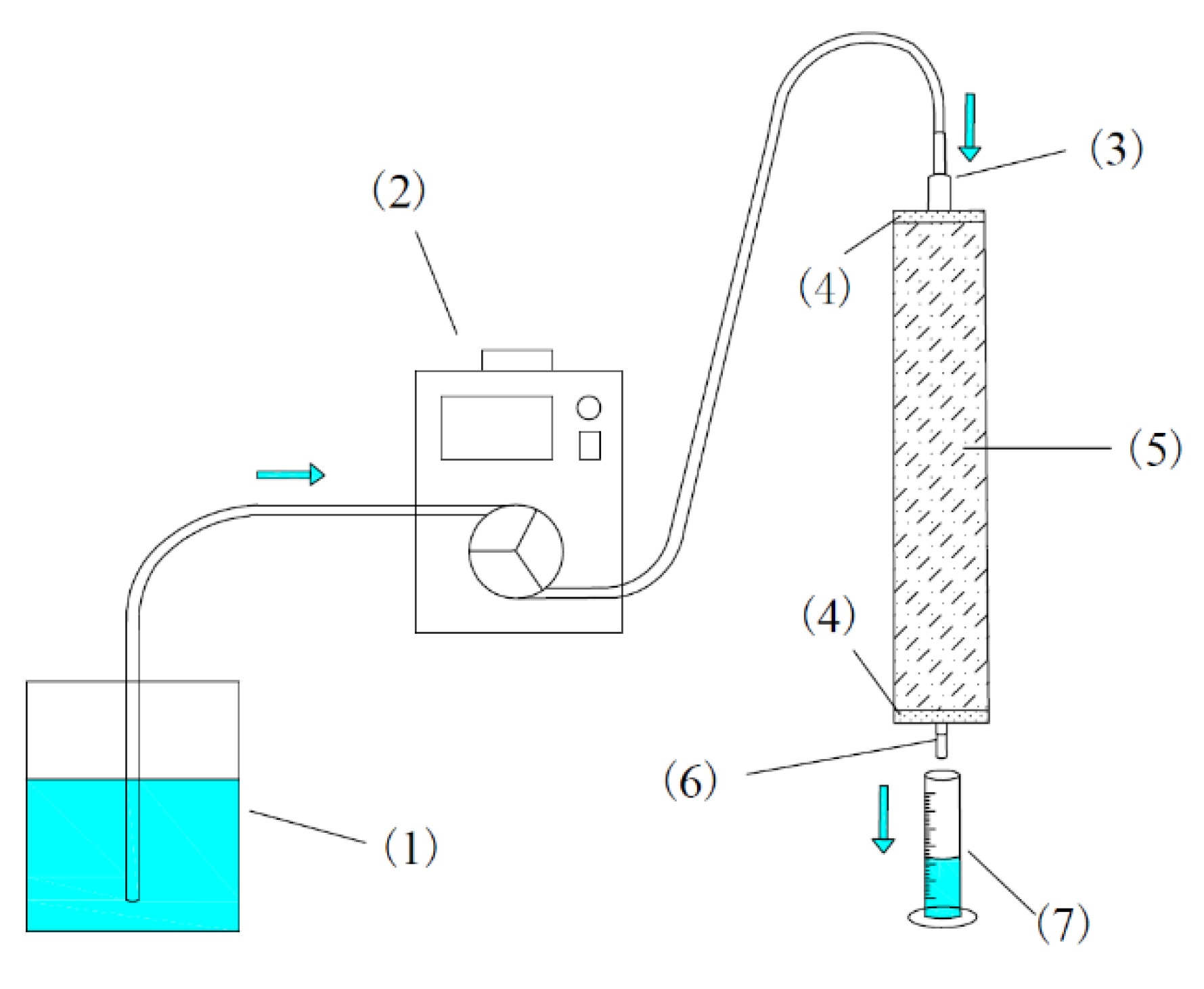
2.2. Experimental Scheme
2.3. Sequential Extraction Procedure of Cd
2.4. Quality Control and Data Analysis
3. Results and Discussion
3.1. Soil Analysis
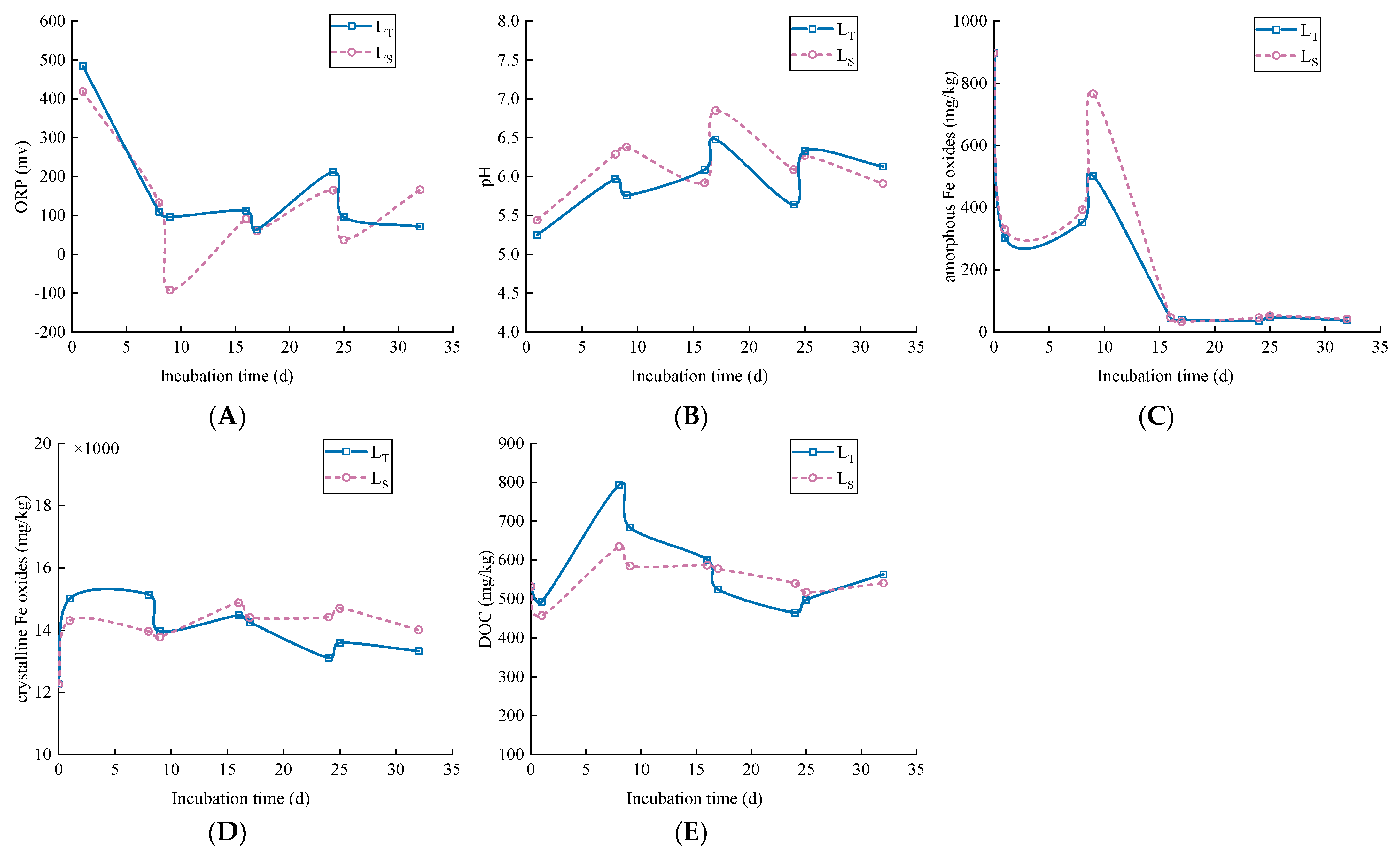
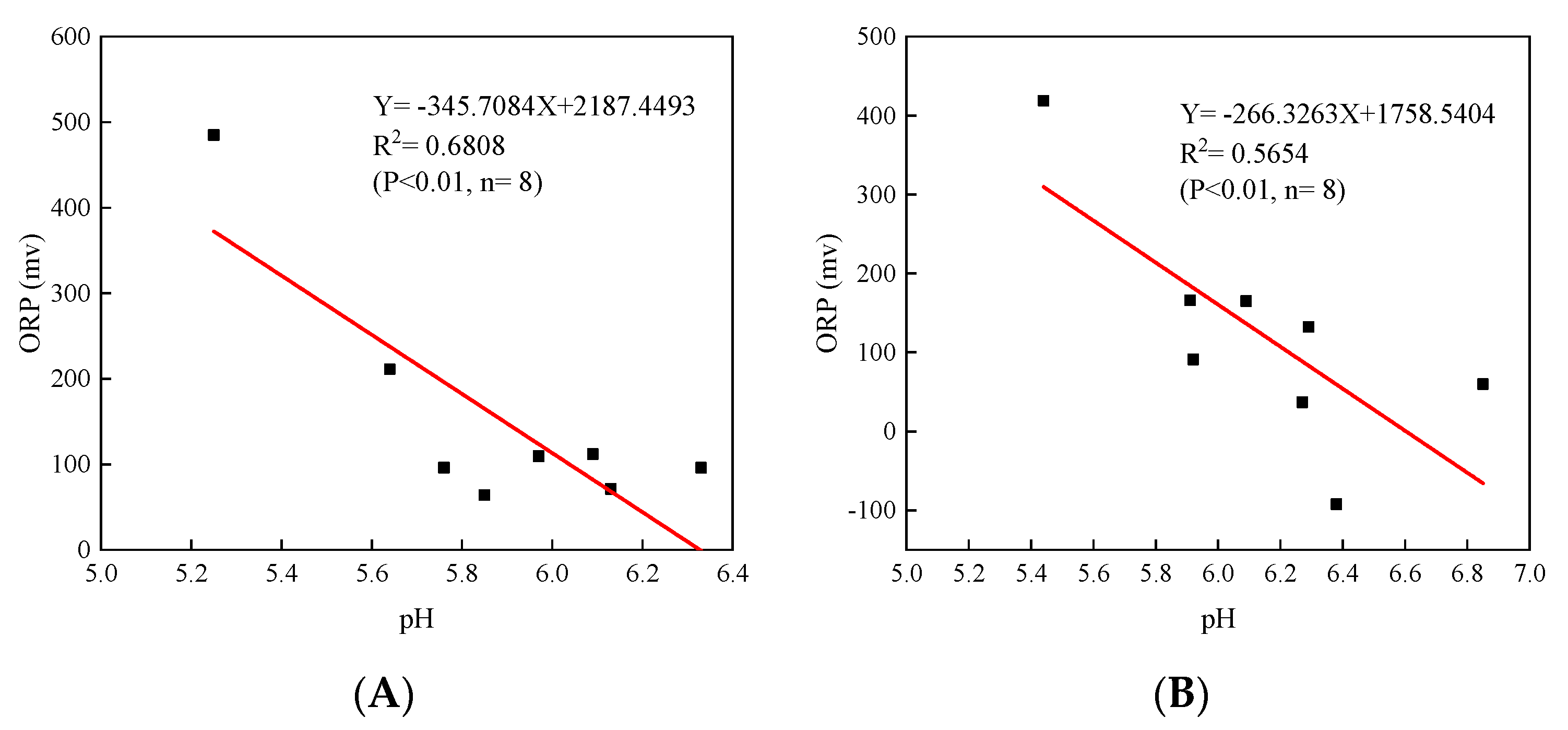
3.2. Soil pH, ORP, Fe and DOC
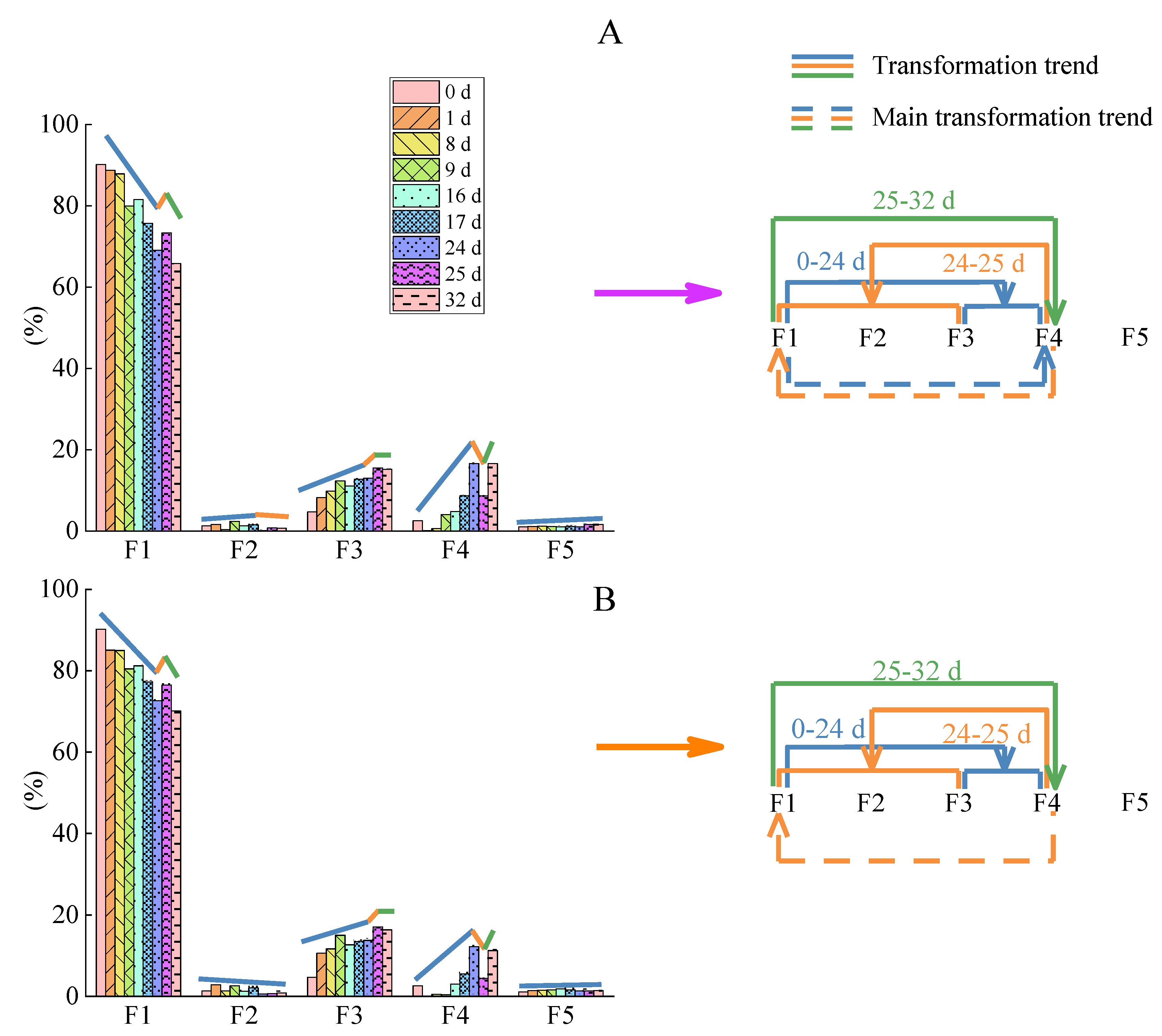
3.3. Influences of Intermittent Irrigation on Cd Fractionation
3.4. Influences of Intermittent Irrigation on Cd Stabilization Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baba, H.; Tsuneyama, K.; Yazaki, M.; Nagata, K.; Minamisaka, T.; Tsuda, T.; Nomoto, K.; Hayashi, S.; Miwa, S.; Nakajima, T.; et al. The liver in itai-itai disease (chronic cadmium poisoning): Pathological features and metallothionein expression. Mod. Pathol. 2013, 26, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.W.; Liu, G.J.; Zhou, C.C.; Sun, R.Y. Chemical speciation and risk assessment of cadmium in soils around a typical coal mining area of China. Ecotoxicol. Environ. Saf. 2018, 160, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Durand, C.; Sauthier, N.; Schwoebel, V. Assessment of exposure to soils contaminated with lead, cadmium, and arsenic near a zinc smelter, Cassiopee Study, France, 2008. Environ. Monit. Assess. 2015, 187, 352. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.W.; Han, J.G.; Chen, Y.; Zhu, Y.L.; Li, P.P. Hormetic effects of Cd on alkaline phosphatase in soils across particle-size fractions in a typical coastal wetland. Sci. Total Environ. 2018, 613, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Fulda, B.; Voegelin, A.; Kretzschmar, R. Redox-Controlled Changes in Cadmium Solubility and Solid-Phase Speciation in a Paddy Soil as Affected by Reducible Sulfate and Copper. Environ. Sci. Technol. 2013, 47, 12775–12783. [Google Scholar] [CrossRef] [PubMed]
- Hensawang, S.; Chanpiwat, P. Health impact assessment of arsenic and cadmium intake via rice consumption in Bangkok, Thailand. Environ. Monit. Assess. 2017, 189, 599. [Google Scholar] [CrossRef]
- Chakraborty, P. Speciation of Co, Ni and Cu in the coastal and estuarine sediments: Some fundamental characteristics. J. Geochem. Explor. 2012, 115, 13–23. [Google Scholar] [CrossRef]
- Jiang, M.; Zeng, G.M.; Zhang, C.; Ma, X.Y.; Chen, M.; Zhang, J.C.; Lu, L.H.; Yu, Q.; Hu, L.P.; Liu, L.F. Assessment of Heavy Metal Contamination in the Surrounding Soils and Surface Sediments in Xiawangang River, Qingshuitang District. PLoS ONE 2013, 8, e71176. [Google Scholar] [CrossRef] [Green Version]
- Ratie, G.; Chrastny, V.; Guinoiseau, D.; Marsac, R.; Vankova, Z.; Komarek, M. Cadmium Isotope Fractionation during Complexation with Humic Acid. Environ. Sci. Technol. 2021, 55, 7430–7444. [Google Scholar] [CrossRef]
- Tang, W.Z.; Xia, Q.; Shan, B.Q.; Ng, J.C. Relationship of bioaccessibility and fractionation of cadmium in long-term spiked soils for health risk assessment based on four in vitro gastrointestinal simulation models. Sci. Total Environ. 2018, 631–632, 1582–1589. [Google Scholar] [CrossRef]
- Mann, S.S.; Ritchie, G.S.P. Changes in the forms of cadmium with time in some western-Australian soils. Aust. J. Soil Res. 1994, 32, 241–250. [Google Scholar] [CrossRef]
- He, S.R.; Lu, Q.; Li, W.Y.; Ren, Z.L.; Zhou, Z.; Feng, X.; Zhang, Y.L.; Li, Y.T. Factors controlling cadmium and lead activities in different parent material-derived soils from the Pearl River Basin. Chemosphere 2017, 182, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Rinklebe, J.; Du Laing, G.; Selim, H.M. Factors Controlling the Dynamics of Trace Metals in Frequently Flooded Soils; CRC Press—Taylor & Francis Group: Boca Raton, FL, USA, 2011; pp. 245–270. [Google Scholar]
- Rupp, H.; Rinklebe, J.; Bolze, S.; Meissner, R. A scale-dependent approach to study pollution control processes in wetland soils using three different techniques. Ecol. Eng. 2010, 36, 1439–1447. [Google Scholar] [CrossRef]
- Yu, H.Y.; Liu, C.P.; Zhu, J.S.; Li, F.B.; Deng, D.M.; Wang, Q.; Liu, C.S. Cadmium availability in rice paddy fields from a mining area: The effects of soil properties highlighting iron fractions and pH value. Environ. Pollut. 2016, 209, 38–45. [Google Scholar] [CrossRef]
- Yu, Y.; Wan, Y.N.; Camara, A.Y.; Li, H.F. Effects of the addition and aging of humic acid-based amendments on the solubility of Cd in soil solution and its accumulation in rice. Chemosphere 2018, 196, 303–310. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhang, W.W.; Yang, X.P.; Wang, P.; McGrath, S.P.; Zhao, F.J. Effective methods to reduce cadmium accumulation in rice grain. Chemosphere 2018, 207, 699–707. [Google Scholar] [CrossRef]
- Zhu, H.H.; Chen, C.; Xu, C.; Zhu, Q.H.; Huang, D.Y. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef]
- Ardestani, M.M.; van Gestel, C.A.M. Sorption and pH determine the long-term partitioning of cadmium in natural soils. Environ. Sci. Pollut. Res. 2016, 23, 18492–18501. [Google Scholar] [CrossRef]
- Wan, Y.N.; Huang, Q.Q.; Camara, A.Y.; Wang, Q.; Li, H.F. Water management impacts on the solubility of Cd, Pb, As, and Cr and their uptake by rice in two contaminated paddy soils. Chemosphere 2019, 228, 360–369. [Google Scholar] [CrossRef]
- Suda, A.; Makino, T. Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on arsenic and cadmium: A review. Geoderma 2016, 270, 68–75. [Google Scholar] [CrossRef]
- de Livera, J.; McLaughlin, M.J.; Hettiarachchi, G.M.; Kirby, J.K.; Beak, D.G. Cadmium solubility in paddy soils: Effects of soil oxidation, metal sulfides and competitive ions. Sci. Total Environ. 2011, 409, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Furuya, M.; Yamaguchi, N.; Makino, T. Zerovalent Iron with High Sulfur Content Enhances the Formation of Cadmium Sulfide in Reduced Paddy Soils. Soil Sci. Soc. Am. J. 2016, 80, 55–63. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.M.; Gu, Y.; Kopittke, P.M.; Zhao, F.J.; Wang, P. Iron-Manganese (Oxyhydro)oxides, Rather than Oxidation of Sulfides, Determine Mobilization of Cd during Soil Drainage in Paddy Soil Systems. Environ. Sci. Technol. 2019, 53, 2500–2508. [Google Scholar] [CrossRef]
- Khaokaew, S.; Chaney, R.L.; Landrot, G.; Ginder-Vogel, M.; Sparks, D.L. Speciation and Release Kinetics of Cadmium in an Alkaline Paddy Soil under Various Flooding Periods and Draining Conditions. Environ. Sci. Technol. 2011, 45, 4249–4255. [Google Scholar] [CrossRef]
- Li, S.S.; Lei, X.Q.; Qin, L.Y.; Sun, X.Y.; Wang, L.F.; Zhao, S.W.; Wang, M.; Chen, S.B. Fe(III) reduction due to low pe plus pH contributes to reducing Cd transfer within a soil-rice system. J. Hazard. Mater. 2021, 415, 125668. [Google Scholar] [CrossRef]
- Rousk, J.; Jones, D.L. Loss of low molecular weight dissolved organic carbon (DOC) and nitrogen (DON) in H2O and 0.5 M K2SO4 soil extracts. Soil Biol. Biochem. 2010, 42, 2331–2335. [Google Scholar] [CrossRef]
- Han, X.Q.; Xiao, X.Y.; Guo, Z.H.; Xie, Y.H.; Zhu, H.W.; Peng, C.; Liang, Y.Q. Release of cadmium in contaminated paddy soil amended with NPK fertilizer and lime under water management. Ecotoxicol. Environ. Saf. 2018, 159, 38–45. [Google Scholar] [CrossRef]
- Sun, Y.C.; Chi, P.H.; Shiue, M.Y. Comparison of different digestion methods for total decomposition of siliceous and organic environmental samples. Anal. Sci. 2001, 17, 1395–1399. [Google Scholar] [CrossRef] [Green Version]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace-metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Han, Y.S.; Park, J.H.; Kim, S.J.; Jeong, H.Y.; Ahn, J.S. Redox transformation of soil minerals and arsenic in arsenic-contaminated soil under cycling redox conditions. J. Hazard. Mater. 2019, 378, 120745. [Google Scholar] [CrossRef]
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.W.; Yu, C. The leaching characteristics of Cd, Zn, and As from submerged paddy soil and the effect of limestone treatment. Paddy Water Environ. 2015, 13, 61–69. [Google Scholar] [CrossRef]
- Abgottspon, F.; Bigalke, M.; Wilcke, W. Fast colloidal and dissolved release of trace elements in a carbonatic soil after experimental flooding. Geoderma 2015, 259, 156–163. [Google Scholar] [CrossRef]
- Chohji, T.; Nakagawa, C.; Hirai, E. Analysis of neutralization of acidic precipitation with soil. Kag. Kog. Ronbunshu 1993, 19, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Maria-Cervantes, A.; Conesa, H.M.; Gonzalez-Alcaraz, M.N.; Alvarez-Rogel, J. Rhizosphere and flooding regime as key factors for the mobilisation of arsenic and potentially harmful metals in basic, mining-polluted salt marsh soils. Appl. Geochem. 2010, 25, 1722–1733. [Google Scholar] [CrossRef]
- Grybos, M.; Davranche, M.; Gruau, G.; Petitjean, P.; Pedrot, M. Increasing pH drives organic matter solubilization from wetland soils under reducing conditions. Geoderma 2009, 154, 13–19. [Google Scholar] [CrossRef]
- Patrick, W.H.; Jugsujinda, A. Sequential reduction and oxidation of inorganic nitrogen, manganese, and iron in flooded soil. Soil Sci. Soc. Am. J. 1992, 56, 1071–1073. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Rinklebe, J.; Frohne, T.; White, J.R.; DeLaune, R.D. Redox effects on release kinetics of arsenic, cadmium, cobalt, and vanadium in Wax Lake Deltaic freshwater marsh soils. Chemosphere 2016, 150, 740–748. [Google Scholar] [CrossRef]
- Yan, F.; Schubert, S.; Mengel, K. Soil pH increase due to biological decarboxylation of organic anions. Soil Biol. Biochem. 1996, 28, 617–624. [Google Scholar] [CrossRef]
- Wang, X.Q.; Yu, H.Y.; Li, F.B.; Liu, T.X.; Wu, W.J.; Liu, C.P.; Liu, C.S.; Zhang, X.Q. Enhanced immobilization of arsenic and cadmium in a paddy soil by combined applications of woody peat and Fe(NO3)3: Possible mechanisms and environmental implications. Sci. Total Environ. 2019, 649, 535–543. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, X.; Chi, W.T.; Wang, P.; Hu, S.W.; Li, F.B.; Li, X.M.; Liu, T.X.; Sun, Y.; Qin, H.L. Modelling evaluation of key cadmium transformation processes in acid paddy soil under alternating redox conditions. Chem. Geol. 2021, 581, 120409. [Google Scholar] [CrossRef]
- Chaves, M.R.M.; Valsaraj, K.T.; Gambrell, R.P.; Delaune, R.D.; Buchler, P.M. Mercury Uptake by Modified Mackinawite. Soil Sediment Contam. 2013, 22, 95–104. [Google Scholar] [CrossRef]
- Ding, C.F.; Du, S.Y.; Ma, Y.B.; Li, X.G.; Zhang, T.L.; Wang, X.X. Changes in the pH of paddy soils after flooding and drainage: Modeling and validation. Geoderma 2019, 337, 511–513. [Google Scholar] [CrossRef]
- Huang, G.X.; Chen, Z.Y.; Wang, J.; Hou, Q.X.; Zhang, Y. Impact of temperature on the aging mechanisms of arsenic in soils: Fractionation and bioaccessibility. Environ. Sci. Pollut. Res. 2016, 23, 4594–4601. [Google Scholar] [CrossRef]
- Tang, X.Y.; Zhu, Y.G.; Cui, Y.S.; Duan, J.; Tang, L. The effect of ageing on the bioaccessibility and fractionation of cadmium in some typical soils of China. Environ. Int. 2006, 32, 682–689. [Google Scholar] [CrossRef]
- Vicente-Beckett, V.A.; McCauley, G.J.T.; Duivenvoorden, L.J. Metal speciation in sediments and soils associated with acid- mine drainage in Mount Morgan (Queensland, Australia). J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2016, 51, 121–134. [Google Scholar] [CrossRef]
- Zhao, X.M.; Dong, D.M.; Hua, X.Y.; Dong, S.F. Investigation of the transport and fate of Pb, Cd, Cr(VI) and As(V) in soil zones derived from moderately contaminated farmland in Northeast, China. J. Hazard. Mater. 2009, 170, 570–577. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, J.F.; Gao, K.; Lu, G.N.; Ye, H.; Wen, Z.N.; Yi, X.Y.; Dang, Z. Spatial and temporal variations of Cu and Cd mobility and their controlling factors in pore water of contaminated paddy soil under acid mine drainage: A laboratory column study. Sci. Total Environ. 2021, 792, 148523. [Google Scholar] [CrossRef]
- Huang, G.X.; Chen, Z.Y.; Zhang, Y.; Liu, F.; Wang, J.; Hou, Q.X. Changes of arsenic fractionation and bioaccessibility in wastewater-irrigated soils as a function of aging: Influence of redox condition and arsenic load. Geoderma 2016, 280, 1–7. [Google Scholar] [CrossRef]
- Antoniadis, V.; Robinson, J.S.; Alloway, B.J. Effects of short-term pH fluctuations on cadmium, nickel, lead, and zinc availability to ryegrass in a sewage sludge-amended field. Chemosphere 2008, 71, 759–764. [Google Scholar] [CrossRef]
- Basta, N.T.; Ryan, J.A.; Chaney, R.L. Trace element chemistry in residual-treated soil: Key concepts and metal bioavailability. J. Environ. Qual. 2005, 34, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morillo, J.; Usero, J.; Gracia, I. Partitioning of metals in sediments from the Odiel River (Spain). Environ. Int. 2002, 28, 263–271. [Google Scholar] [CrossRef]
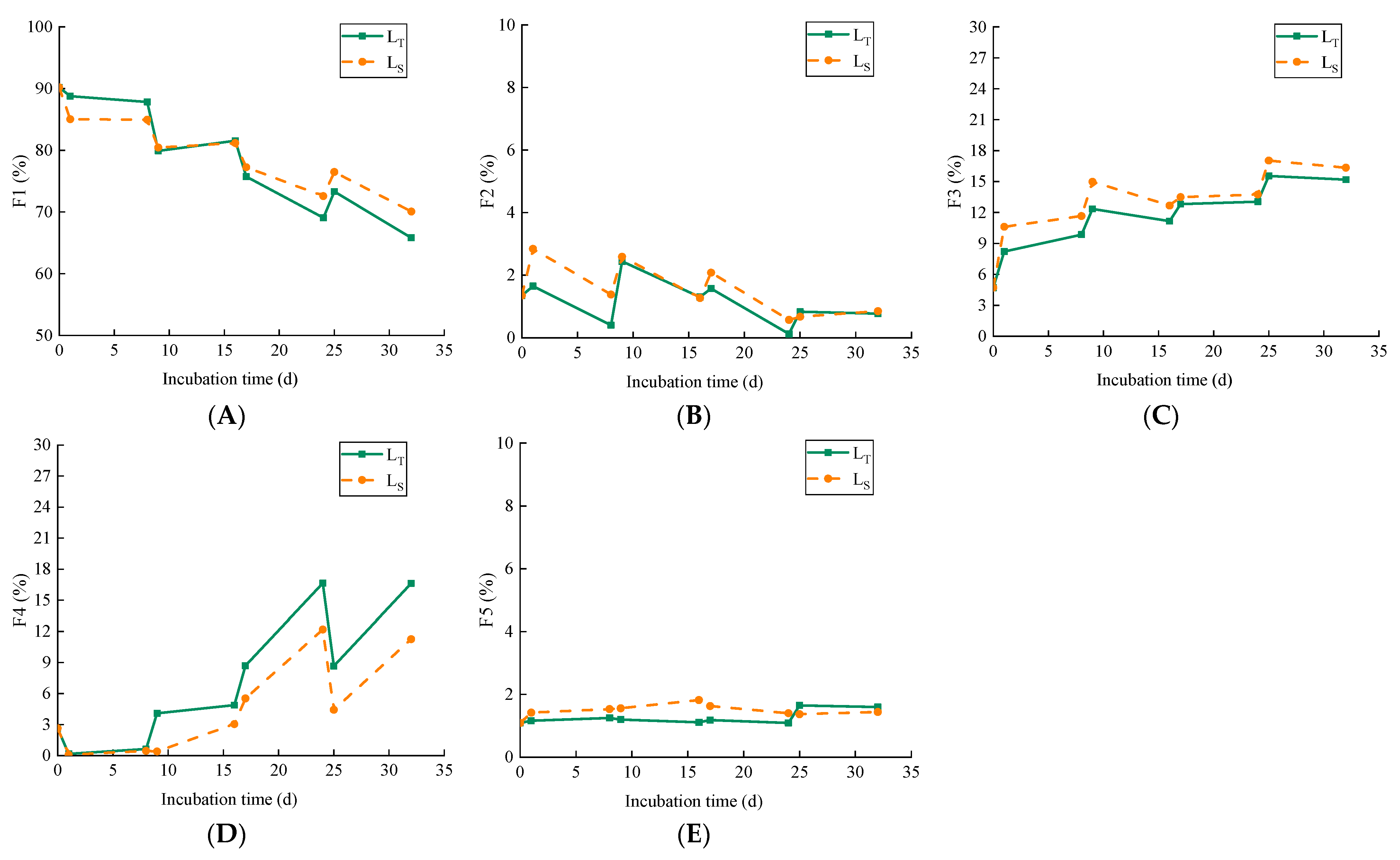
| Items | Content | Unit | Items | Content | Unit |
|---|---|---|---|---|---|
| pH | 5.47 | Amorphous Fe oxides | 0.51 | (mg/kg) | |
| Clay | 10.91 | (%) | Total Cd | 0.15 | (mg/kg) |
| Silt | 69.16 | (%) | Exchangeable Cd | 0.012 | (mg/kg) |
| Sand | 19.93 | (%) | Carbonates bound Cd | 0.014 | (mg/kg) |
| Specific area | 0.74 | (m2/g) | Fe and Mn oxides bound Cd | 0.031 | (mg/kg) |
| DOC | 25.39 | (mg/L) | Organic matter and secondary sulfide bound Cd | 0.075 | (mg/kg) |
| Free Fe oxides | 18.45 | (mg/kg) | Residual Cd | 0.022 | (mg/kg) |
| Extraction Steps | Extracted Fractionation | Extracting Solution | Extraction Conditions |
|---|---|---|---|
| 1 | F1 | 40 mL 1 mol/L MgCl2 | Shake 4 h, room temperature |
| 2 | F2 | 40 mL 1 mol/L NaOAc | Shake 16 h, room temperature |
| 3 | F3 | 40 mL 0.25 mol/L NH2OH·HCl | Shake 22 h, room temperature |
| 4 | F4 | 15 mL 30% H2O2 +9 mL HNO3 | Shake 2 h, 83 °C ± 2 °C in the water bath |
| 9 mL 30% H2O2 | Shake 2 h, 83 °C ± 2 °C in the water bath | ||
| 7.5 mL 9.6 mol/L NH4OAc + 20% HNO3 | Shake 30 min, room temperature | ||
| 5 | F5 | aqua regia | Digestion, 240 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Pei, L.; Huang, G.; Hou, Q.; Zhang, M.; Song, J.; Gan, L.; Wu, H. The Aging Process of Cadmium in Paddy Soils under Intermittent Irrigation with Acid Water: A Short-Term Simulation Experiment. Int. J. Environ. Res. Public Health 2022, 19, 3339. https://doi.org/10.3390/ijerph19063339
Han D, Pei L, Huang G, Hou Q, Zhang M, Song J, Gan L, Wu H. The Aging Process of Cadmium in Paddy Soils under Intermittent Irrigation with Acid Water: A Short-Term Simulation Experiment. International Journal of Environmental Research and Public Health. 2022; 19(6):3339. https://doi.org/10.3390/ijerph19063339
Chicago/Turabian StyleHan, Dongya, Lixin Pei, Guanxing Huang, Qinxuan Hou, Meng Zhang, Jiangmin Song, Lin Gan, and Heqiu Wu. 2022. "The Aging Process of Cadmium in Paddy Soils under Intermittent Irrigation with Acid Water: A Short-Term Simulation Experiment" International Journal of Environmental Research and Public Health 19, no. 6: 3339. https://doi.org/10.3390/ijerph19063339
APA StyleHan, D., Pei, L., Huang, G., Hou, Q., Zhang, M., Song, J., Gan, L., & Wu, H. (2022). The Aging Process of Cadmium in Paddy Soils under Intermittent Irrigation with Acid Water: A Short-Term Simulation Experiment. International Journal of Environmental Research and Public Health, 19(6), 3339. https://doi.org/10.3390/ijerph19063339






