Factors Associated with an Outbreak of COVID-19 in Oilfield Workers, Kazakhstan, 2020
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Specific Setting
2.3. Study Participants
2.4. Sources and Data Variables
2.5. Sample Size
2.6. Statistical Methods
3. Results
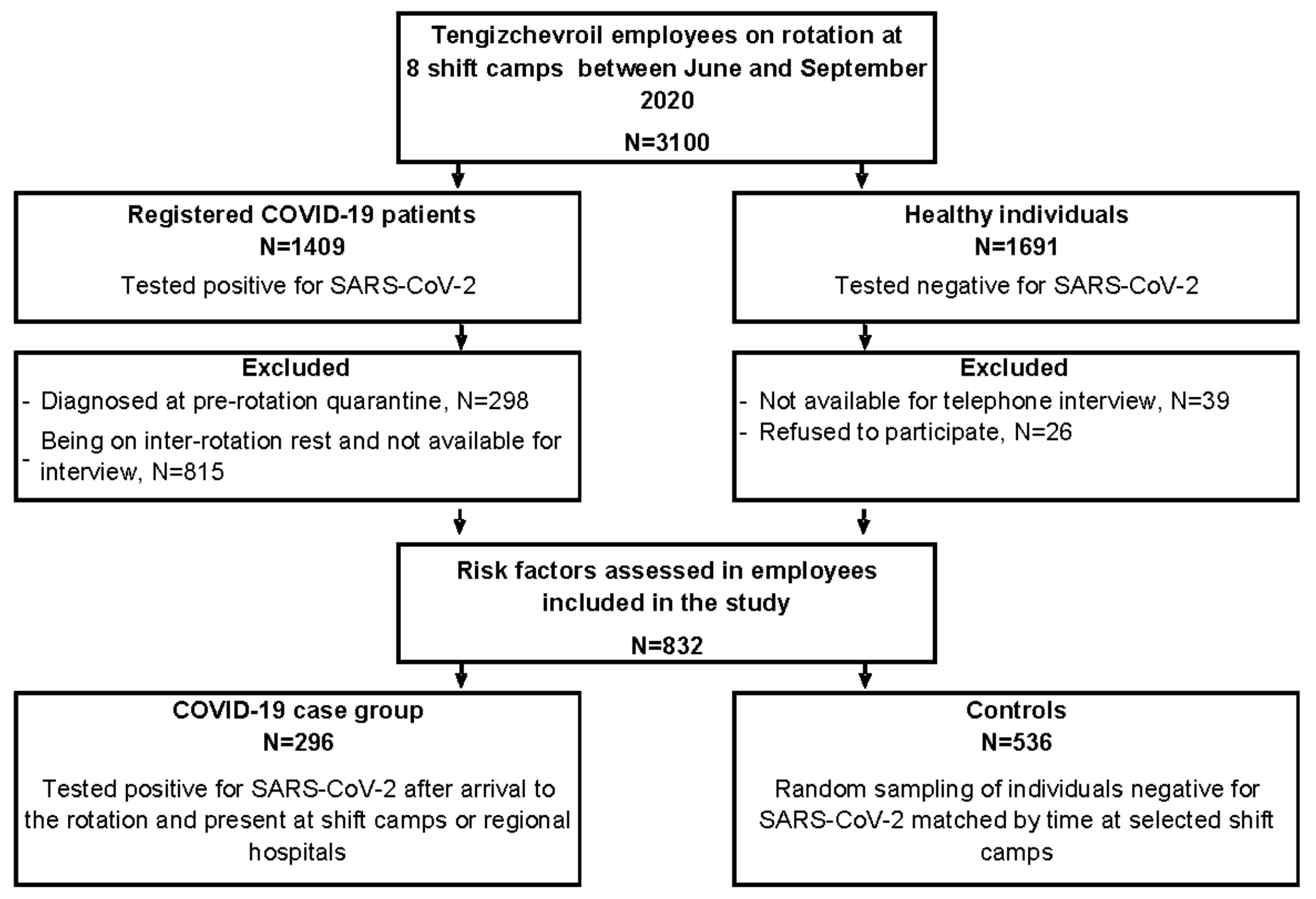
3.1. Recruitment/Response of Participants
3.2. Baseline Characteristics of Case Patients and Controls
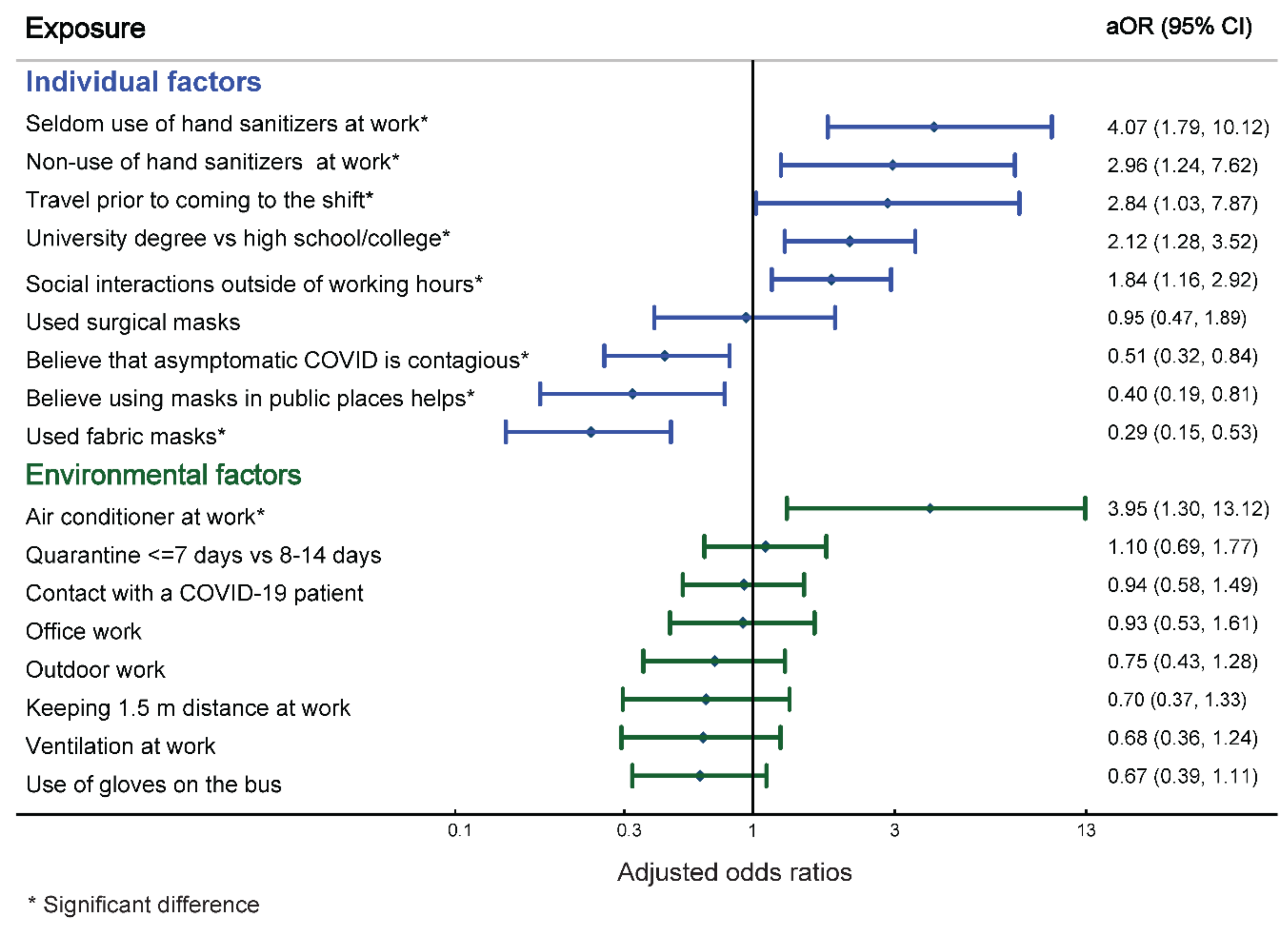
3.3. Bivariate Analysis
3.4. Multivariable Analysis
3.5. Stratified Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristics | Stratum-Specific Odds Ratio (95% CI) * by Shift Camp | BD § p-value | LR ¶ p-value | |||||
|---|---|---|---|---|---|---|---|---|
| New Tengiz | Denkholm | Shanyrak | SK † | Bolashak | Other ‡ | |||
| Male sex | 1.9 (1.0–3.7) | 0.4 (0.1–1.4) | 1.9 (1.0–3.9) | 2.2 (0.4–56.7) | 0.4 (0.2–1.0) | 0.6 (0.2–1.7) | 0.217 | 0.223 |
| Age group (≥ 36 years/ <36 years) | 0.7 (0.3–1.3) | 1.1 (0.6–2.0) | 0.5 (0.3–0.9) | 1.3 (0.5–3.6) | 1.6 (0.7–3.7) | 0.9 (0.4–2.1) | 0.188 | 0.160 |
| University degree vs. high school/college | 2.1 (1.0–4.2) | 2.0 (1.0–4.0) | 2.2 (1.2–4.1) | 0.9 (0.3–2.6) | 0.6 (0.2–1.5) | 0.5 (0.2–1.2) | 0.014 | 0.082 |
| Overweight (25.0–29.9 kg/m2) | 1.2 (0.6–2.4) | 1.2 (0.6–2.4) | 1.0 (0.5–2.0) | 1.3 (0.4–4.4) | 0.8 (0.3–2.1) | 0.8 (0.3–2.3) | 0.972 | 0.958 |
| Obesity (≥30 kg/m2)/ normal weight (<24.9) | 1.3 (0.4–3.9) | 3.4 (1.3–9.2) | 0.8 (0.3–1.9) | 1.3 (0.2–6.1) | 2.5 (0.6–11.7) | 0.7 (0.2–2.6) | 0.197 | 0.112 |
| Travel prior to coming to the shift | 1.8 (0.6–5.2) | - | - | - | 1.4 (<0.1,56.9) | 0.7 (<0.1,9.2) | - | - |
| Social interaction outside of working hours | 4.2 (1.9–9.3) | 2.5 (1.3–4.9) | 2.2 (1.2–4.2) | 0.8 (0.3–2.3) | 1.4 (0.6–3.3) | 2.0 (0.8–4.7) | 0.158 | 0.034 |
| Fever # | 0.6 (0.3–1.4) | 0.3 (0.1–0.5) | 1.8 (0.8–4.9) | 0.5 (0.2–1.5) | 0.4 (0.2–1.0) | 0.6 (0.2–1.7) | <0.001 | 0.028 |
| Shortness of breath # | 0.8 (0.4–1.5) | 0.6 (0.3–1.2) | 3.2 (1.7–6.0) | 0.4 (0.1–1.0) | 0.2 (0.1–0.4) | 1.3 (0.5–3.2) | <0.001 | <0.001 |
| Cough # | 1.0 (0.5–1.9) | 0.4 (0.2–0.7) | 2.1 (1.1–3.9) | 0.5 (0.2–1.4) | 0.2 (0.1–0.6) | 1.0 (0.4–2.4) | <0.001 | 0.001 |
| Loss of smell or taste # | 1.4 (0.8–2.7) | 1.2 (0.6–2.3) | 3.5 (1.8–6.8) | 0.3 (0.1–0.9) | 0.4 (0.2–0.8) | 0.8 (0.3–1.9) | <0.001 | 0.001 |
| Consider COVID-19 to be a serious issue | 1.0 (0.4–2.2) | 2.1 (1.1–4.2) | 0.8 (0.4–1.8) | 2.6 (0.7–8.5) | 1.8 (0.7–5.2) | 1.6 (0.5–5.9) | 0.396 | 0.507 |
| Believe that asymptomatic COVID-19 is contagious | 0.5 (0.3–1.0) | 0.3 (0.1–0.5) | 1.2 (0.6–2.4) | 0.6 (0.2–1.7) | 0.2 (0.1–0.5) | 0.7 (0.3–1.8) | 0.007 | 0.005 |
| Believe masks should be used outdoors | 0.3 (0.1–0.6) | 0.4 (0.2–0.8) | 0.5 (0.2–0.9) | - | 1.3 (0.4–4.0) | 0.9 (0.4–2.5) | 0.037 | - |
| Believe masks should be used in public places | 0.1 (<0.1–0.7) | 0.5 (0.2–1.1) | 1.8 (0.7–5.1) | - | 0.4 (0.1–1.7) | 0.1 (<0.1–0.6) | 0.009 | - |
| Believe masks should be used in a dormitory | 0.1 (<0.1–0.4) | 0.8 (0.4–1.7) | 1.8 (0.7–5.1) | 2.0 (0.3–50.9) | 0.7 (0.2–2.7) | 0.6 (0.1–2.1) | 0.014 | 0.005 |
| Used surgical masks | 4.8 (2.5–9.5) | 0.1 (0.1–1.0) | 0.8 (0.3–2.0) | 2.0 (0.3–50.9) | 0.4 (0.1–1.2) | 1.4 (0.3–12.2) | <0.001 | 0.001 |
| Used respirators | 15.3 (2.6–396.8) | 1.0 (0.2–3.4) | 0.9 (0.4–1.9) | 0.8 (0.1–3.5) | 0.3 (0.1–1.1) | 0.9 (0.2–3.6) | 0.007 | 0.094 |
| Used fabric masks | 0.1 (0.1–0.2) | 0.7 (<0.1,6.2) | 0.6 (0.1–3.3) | - | 3.4 (1.2–10.1) | 1.4 (0.2–8.5) | <0.001 | - |
| 3–4 masks vs. ≥ 5 changed per day | 1.2 (0.5–2.8) | 2.0 (1.0–4.1) | 1.5 (0.7–3.2) | 0.6 (0.2–2.5) | 0.2 (0.1–0.7) | 0.6 (0.2–1.6) | 0.013 | 0.017 |
| 2 masks vs. ≥ 5 changed per day | 9.1 (2.6–44.8) | 1.9 (0.7–5.2) | 0.7 (0.2–2.0) | 0.9 (0.3–3.5) | 8.3 (1.4–219.6) | 1.2 (0.2–5.8) | 0.013 | 0.001 |
| 1 mask vs. ≥ 5 changed per day | 2.9 (0.5–17.3) | 8.2 (0.9–243.8) | 1.8 (0.5–6.6) | 0.5 (<0.1–4.5) | 1.2 (0.1–12.1) | – | 0.349 | - |
| Seldom use of hand sanitizers at the work vs. always | - | 11.6 (5.1–28.5) | 1.2 (0.3–5.3) | 0.8 (0.2–3.5) | 2.3 (0.3–21.7) | 0.8 (0.1–8.5) | 0.118 | - |
| Non-use of hand sanitizers at the work vs. always | - | 7.6 (2.7–22.8) | 1.4 (0.4–6.0) | 0.4 (0.1–1.9) | 0.5 (0.1–5.0) | 0.7 (0.1–6.8) | <0.001 | - |
| Effect modification: | risk factor | protective factor | non-significant factor | |||||
| No effect modification: | ||||||||
| Characteristics | Stratum-Specific Odds Ratio (95% CI)* by Shift Camp | BD § p-value | LR ¶ p-value | |||||
|---|---|---|---|---|---|---|---|---|
| New Tengiz | Denkholm | Shanyrak | SK † | Bolashak | Other ‡ | |||
| Pre-shift quarantine ≤ 7 vs. 8–14 days | 4.0 (1.8–9.0) | 1.4 (0.6–3.4) | 0.3 (0.2–0.7) | 0.1 (<0.1–0.6) | 0.6 (0.2–1.7) | 2.0 (0.7–6.8) | <0.001 | 0.001 |
| Trained on COVID-19 prevention measures | 0.1 (<0.1–0.3) | 0.8 (0.3–1.9) | 0.4 (0.1–1.0) | 1.6 (0.4–12.1) | 0.7 (0.1–3.2) | 0.7 (0.2–2.3) | 0.025 | - |
| Contact with a COVID-19 patient at work (n = 756) | 2.9 (1.4–5.9) | 1.2 (0.5–2.6) | 0.4 (0.2–0.7) | 4.8 (0.5–34.7) | 1.7 (0.7–4.3) | 1.0 (0.4–2.5) | <0.001 | 0.019 |
| Exposed to a COVID-19 patient with <1.5 m | 0.4 (0.1–1.4) | 9.4 (1.3–271.0) | 5.4 (1.7–21.1) | - | 1.7 (0.1–58.7) | 0.5 (0.1–3.5) | 0.009 | - |
| Exposed to COVID-19 patient >15 min in the room | 0.9 (0.2–4.0) | - | 2.2 (0.5–16.6) | - | - | 0.7 (0.1–4.9) | 0.105 | - |
| Sharing toilet on the floor of the dormitory | 3.5 (0.9–14.7) | 0.8 (0.4–1.4) | 1.7 (0.9–3.4) | 2.7 (0.9–10.5) | 1.5 (0.2–8.9) | 1.5 (0.6–3.6) | 0.182 | - |
| Individual toilet in the room | 2.9 (0.7–12.6) | 1.2 (0.6–2.2) | 0.6 (0.3–1.1) | 0.5 (0.1–1.3) | 0.2 (<0.1–2.2) | 0.6 (0.2–1.4) | 0.304 | - |
| Living with 1–4 neighbors vs. alone | 0.2 (0.1–0.3) | 0.3 (0.1–0.6) | 0.4 (0.1–1.1) | 1.1 (0.4–3.1) | 1.0 (0.4–2.3) | 0.5 (0.2–1.1) | 0.008 | 0.033 |
| Working in the infirmary/clinic | - | - | - | - | - | 4.4 (0.9–34.9) | - | - |
| Transport work | 1.1 (0.3–3.8) | - | 1.2 (0.3–4.5) | 2.6 (0.7–8.5) | 1.4 (0.6–3.3) | 0.7 (0.1–4.0) | 0.076 | - |
| Working in an office | 4.8 (2.0–11.7) | 1.4 (0.6–3.3) | 1.3 (0.7–2.4) | 0.5 (<0.1–2.7) | 0.5 (0.2–1.3) | 0.8 (0.3–1.9) | 0.006 | - |
| Working outdoors | 3.8 (1.5–9.8) | 0.3 (0.2–0.6) | 1.2 (0.6–2.5) | 0.9 (0.3–2.4) | 0.5 (0.1–1.5) | 0.8 (0.3–2.0) | <0.001 | 0.717 |
| Working in a kitchen | 0.3 (0.1–0.5) | 5.6 (0.6–163.3) | 0.2 (0.1–0.6) | 1.6 (0.1–14.5) | 2.5 (0.6–13.7) | 2.1 (0.3–18.6) | <0.001 | - |
| Working in a storeroom | - | 0.5 (<0.1–4.0) | 0.6 (<0.1–7.7) | - | - | - | 0.344 | - |
| Other work stations ‡ | 0.8 (0.4–1.6) | 2.0 (0.9–4.8) | 1.8 (0.8–4.2) | 0.9 (0.2–3.1) | 2.0 (0.4–11.2) | 2.2 (0.6–9.6) | 0.442 | 0.049 |
| Maintaining 1.5 m distance at work | 0.1 (<0.1–0.3) | 0.4 (0.2–0.9) | 0.4 (0.1–1.0) | 0.2 (0.1–0.7) | 2.1 (0.4–17.0) | 1.3 (0.3–5.5) | 0.008 | - |
| Air conditioner at work | 10.(1.6–286.0) | - | 0.9 (0.2–3.8) | - | - | 1.4 (<0.1–54.7) | 0.050 | - |
| Ventilation system at work | 0.3 (0.1–0.8) | 0.4 (0.1–1.8) | 0.6 (0.3–1.2) | - | 0.6 (0.1–2.4) | 1.0 (0.2–5.3) | 0.489 | - |
| Availability of sanitizers at work | 0.2 (<0.1–1.4) | 10.0 (4.7–22.6) | 3.0 (0.9–14.5) | 1.0 (0.3–4.1) | 1.0 (0.2–9.2) | 3.5 (0.5–96.0) | <0.001 | - |
| Use of gloves in the dormitory corridors | 1.1 (0.5–2.5) | 0.2 (0.1–0.5) | 1.7 (0.7–4.1) | 0.1 (<0.1–0.7) | 0.4 (0.1–1.1) | 2.0 (0.6–6.8) | <0.001 | - |
| Used gloves on a bus | 0.5 (0.2–1.4) | 0.2 (0.1–0.4) | 3.3 (1.5–7.9) | 0.3 (0.1–0.9) | 0.3 (0.1–0.8) | 1.4 (0.4–4.7) | <0.001 | 0.018 |
| Used gloves at work | 0.2 (0.1–0.4) | 13.1 (6.0–31.3) | 0.8 (0.4–1.4) | 0.4 (0.1–1.1) | 2.0 (0.7–6.2) | 2.4 (1.0–6.1) | <0.001 | 0.004 |
| Effect modification: | risk factor | protective factor | non-significant factor | |||||
| No effect modification: | ||||||||
References
- World Health Organization. Data as Received by WHO from National Authorities by 10:00 CEST. Coronavirus Disease (COVID-19), Weekly Operational Update Issue No 60. 28 July 2021. Available online: https://www.who.int/docs/default-source/coronaviruse/weekly-updates/wou_2021_28-june_cleared-.pdf (accessed on 29 June 2021).
- Environmental and Modelling Group (EMG). SARS-COV-2: Transmission Routes and Environments. 2020. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/933225/S0824SARS-CoV-2_Transmission_routes_and_environments.pdf (accessed on 29 June 2021).
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef] [PubMed]
- Milasi, S.; Bisello, M.; Hurley, J.; Sostero, M.; Fernández-Macías, E. The Potential for Teleworking in Europe and the Risk of a New Digital Divide. VoxEU.org. Available online: https://voxeu.org/article/potential-teleworking-europe-and-risk-new-digital-divide (accessed on 14 August 2020).
- Hickman, A.; Saad, L. Reviewing Remote Work in the U.S. under COVID-19. Gallap. Available online: https://news.gallup.com/poll/311375/reviewing-remote-work-covid.aspx (accessed on 22 May 2020).
- Mhango, M.; Dzobo, M.; Chitungo, I.; Dzinamarira, T. COVID-19 Risk Factors Among Health Workers: A Rapid Review. Safety and Health at Work. Saf. Health Work 2020, 11, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Waltenburg, M.A.; Victoroff, T.; Rose, C.E.; Butterfield, M.; Jervis, R.H.; Fedak, K.M.; Gabel, J.A.; Feldpausch, A.; Dunne, E.M.; Austin, C.; et al. Update: COVID-19 Among Workers in Meat and Poultry Processing Facilities—United States, April–May 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 887–892. [Google Scholar] [CrossRef]
- Belhadi, A.; Kamble, S.; Jabbour, C.J.C.; Gunasekaran, A.; Ndubisi, N.O.; Venkatesh, M. Manufacturing and service supply chain resilience to the COVID-19 outbreak: Lessons learned from the automobile and airline industries. Technol. Soc. Chang. 2021, 163, 120447. [Google Scholar] [CrossRef] [PubMed]
- Guenther TaC-S, M.; Indenbirken, D.; Robitailles, A.; Tenhaken, P.; Exner, M.; Ottinger, M.; Fischer, N.; Grundhoff, A.; Brinkmann, M. Investigation of a Superspreading Event Preceding the Largest Meat Processing Plant-Related SARS-Coronavirus 2 Outbreak in Germany. 2020. Available online: https://ssrn.com/abstract=3654517 (accessed on 17 July 2020).
- Maidstone, R.; Anderson, S.G.; Ray, D.W.; Rutter, M.K.; Durrington, H.J.; Blaikley, J.F. Shift work is associated with positive COVID-19 status in hospitalised patients. Thorax 2021, 76, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Meyerowitz, E.A.; Richterman, A.; Gandhi, R.T.; Sax, P.E. Transmission of SARS-CoV-2: A Review of Viral, Host, and Environmental Factors. Ann. Intern. Med. 2021, 174, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Environmental and Modelling Group (EMG). Transmission Group: COVID-19 Risk by Occupation and Workplace. 11 February 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/933225/S0824_SARS-CoV-2_Transmission_routes_and_environments.pdf (accessed on 29 June 2021).
- “Kazinform” International News Agency JSC. Coronavirus Situation in Kazakhstan. Available online: https://www.coronavirus2020.kz// (accessed on 12 July 2020).
- Putz, C. COVID-19 Cases at Kazakhstan’s Tengiz Oil Field Top 1000. The Diplomat. Available online: https://thediplomat.com/2020/06/covid-19-cases-at-kazakhstans-tengiz-oil-field-top-1000/ (accessed on 4 June 2020).
- Smyth, L. Turning Point in a Turnaround for Tengizchevroil LLP. Engineer Live. Available online: https://www.engineerlive.com/content/turning-point-turnaround-tengizchevroil-llp (accessed on 9 February 2021).
- Taber, K.S. The Use of Cronbach’s Alpha When Developing and Reporting Research Instruments in Science Education. Res. Sci. Educ. 2018, 48, 1273–1296. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Levin, B.; Paik, M.C. Statistical Methods for Rates and Proportions; Formulas 3.18 &3.19; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Fox, J. Applied Regression Analysis and Generalized Linear Models, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2016. [Google Scholar]
- Fox, J.; Monette, G. Generalized collinearity diagnostics. JASA 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Lu, J.; Gu, J.; Li, K.; Xu, C.; Su, W.; Lai, Z.; Zhou, D.; Yu, C.; Xu, B.; Yang, Z. COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 2020, 26, 1628–1631. [Google Scholar] [CrossRef]
- Chinazzi, M.; Davis, J.T.; Ajelli, M.; Gioannini, C.; Litvinova, M.; Merler, S.; Piontti, Y.; Pastore, A.; Mu, K.; Rossi, L.; et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science 2020, 368, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Bielecki, M.; Patel, D.; Hinkelbein, J.; Komorowski, M.; Kester, J.; Ebrahim, S.; Rodriguez-Morales, A.J.; Memish, Z.A.; Schlagenhauf, P. Air travel and COVID-19 prevention in the pandemic and peri-pandemic period: A narrative review. Travel Med. Infect. Dis. 2021, 39, 101915. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Khosrawipour, V.; Kocbach, P.; Mikolajczyk, A.; Ichii, H.; Zacharski, M.; Bania, J.; Khosrawipour, T. The association between international and domestic air traffic and the coronavirus (COVID-19) outbreak. J. Microbiol. Immunol. Infect. 2020, 53, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Wolford, H.; Paul, P.; Diaz, P.S.; Chen, T.-H.; Brown, C.M.; Cetron, M.S.; Alvarado-Ramy, F. Reducing travel-related SARS-CoV-2 transmission with layered mitigation measures: Symptom monitoring, quarantine, and testing. BMC Med. 2021, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Options to Reduce Quarantine for Contacts of Persons with SARS-CoV-2 Infection Using Symptom Monitoring and Diagnostic Testing. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-options-to-reduce-quarantine.html (accessed on 15 January 2021).
- Porter, K.A.; Ramaswamy, M.; Koloski, T.; Castrodale, L.; McLaughlin, J. COVID-19 Among Workers in the Seafood Processing Industry: Implications for Prevention Measures—Alaska, March–October 2020. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Callahan, C.; Lee, R.; Lee, G.; Zulauf, K.E.; Kirby, J.E.; Arnaout, R. Nasal-Swab Testing Misses Patients with Low SARS-CoV-2 Viral Loads. Medrxiv Prepr. Serv. Health Sci. 2020, 2020, 20128736. [Google Scholar]
- Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction–Based SARS-CoV-2 Tests by Time Since Exposure. Ann. Intern. Med. 2020, 173, 262–267. [CrossRef]
- Ma, Q.; Liu, J.; Liu, Q.; Kang, L.; Liu, R.; Jing, W.; Wu, Y.; Liu, M. Global Percentage of Asymptomatic SARS-CoV-2 Infections Among the Tested Population and Individuals With Confirmed COVID-19 Diagnosis: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2137257. [Google Scholar] [CrossRef]
- Kronbichler, A.; Kresse, D.; Yoon, S.; Lee, K.H.; Effenberger, M.; Shin, J.I. Asymptomatic patients as a source of COVID-19 infections: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 98, 180–186. [Google Scholar] [CrossRef]
- Ogilvie, B.H.; Solis-Leal, A.; Lopez, J.B.; Poole, B.D.; Robison, R.A.; Berges, B.K. Alcohol-free hand sanitizer and other quaternary ammonium disinfectants quickly and effectively inactivate SARS-CoV-2. J. Hosp. Infect. 2021, 108, 142–145. [Google Scholar] [CrossRef]
- Gupta, M.K.; Lipner, S.R. Hand hygiene in preventing COVID-19 transmission. Cutis 2020, 105, 233–234. [Google Scholar]
- Tan, L.; Ma, B.; Lai, X.; Han, L.; Cao, P.; Zhang, J.; Fu, J.; Zhou, Q.; Wei, S.; Wang, Z.; et al. Air and surface contamination by SARS-CoV-2 virus in a tertiary hospital in Wuhan, China. Int. J. Infect. Dis. 2020, 99, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen-Camacho, M.E.; Velazquez-Alva, M.C.; Zepeda-Zepeda, M.A.; Cabrer-Rosales, M.F.; Lazarevich, I.; Castaño-Seiquer, A. Effect of Income Level and Perception of Susceptibility and Severity of COVID-19 on Stay-at-Home Preventive Behavior in a Group of Older Adults in Mexico City. Int. J. Environ. Res. Public Health 2020, 17, 7418. [Google Scholar] [CrossRef] [PubMed]
- Chadeau-Hyam, M.; Bodinier, B.; Elliott, J.; Whitaker, M.D.; Tzoulaki, I.; Vermeulen, R.; Kelly-Irving, M.; Delpierre, C.; Elliott, P. Risk factors for positive and negative COVID-19 tests: A cautious and in-depth analysis of UK biobank data. Int. J. Epidemiol. 2020, 49, 1454–1467. [Google Scholar] [CrossRef] [PubMed]
- Upshaw, T.L.; Brown, C.; Smith, R.; Perri, M.; Ziegler, C.; Pinto, A.D. Social determinants of COVID-19 incidence and outcomes: A rapid review. PLoS ONE 2021, 16, e0248336. [Google Scholar] [CrossRef] [PubMed]
- Drummond, C.; Fischhoff, B. Individuals with greater science literacy and education have more polarized beliefs on controversial science topics. Proc. Natl. Acad. Sci. USA 2017, 114, 9587–9592. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, C.V.; Martin, L.M.; Kim, S.S.; Kirmse, B.M.; Haynie, L.; McGraw, S.; Byers, P.; Taylor, K.G.; Patel, M.M.; Flannery, B.; et al. Factors Associated with Positive SARS-CoV-2 Test Results in Outpatient Health Facilities and Emergency Departments Among Children and Adolescents Aged <18 Years—Mississippi, September–November 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1925–1929. [Google Scholar] [CrossRef]
- Oksanen, A.; Kaakinen, M.; Latikka, R.; Savolainen, I.; Savela, N.; Koivula, A. Regulation and Trust: 3-Month Follow-up Study on COVID-19 Mortality in 25 European Countries. JMIR Public Health Surveill. 2020, 6, e19218. [Google Scholar] [CrossRef]
- Sami, S.; Vuong, N.; Miller, H.; Priestley, R.; Payne, M.; Licata-Portentoso, G.; Drobeniuc, J.; Petersen, L.R. SARS-CoV-2 Infection and Mitigation Efforts among Office Workers, Washington, DC, USA. Emerg. Infect. Dis. 2021, 27, 669–672. [Google Scholar] [CrossRef]
- Chou, W.-P.; Wang, P.-W.; Chen, S.-L.; Chang, Y.-P.; Wu, C.-F.; Lu, W.-H.; Yen, C.-F. Voluntary Reduction of Social Interaction during the COVID-19 Pandemic in Taiwan: Related Factors and Association with Perceived Social Support. Int. J. Environ. Res. Public Health 2020, 17, 8039. [Google Scholar] [CrossRef]
- Nardell, E.A. Air Disinfection for Airborne Infection Control with a Focus on COVID-19: Why Germicidal UV is Essential. Photochem. Photobiol. 2021, 97, 493–497. [Google Scholar] [CrossRef]
- Barbosa, M.H.; Graziano, K.U. Influence of wearing time on efficacy of disposable surgical masks as microbial barrier. Braz. J. Microbiol. 2006, 37, 216–217. [Google Scholar] [CrossRef] [Green Version]
- Howard, J.; Huang, A.; Li, Z.; Tufekci, Z.; Zdimal, V.; van der Westhuizen, H.-M.; von Delft, A.; Price, A.; Fridman, L.; Tang, L.-H.; et al. An evidence review of face masks against COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2014564118. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.R.; Qureshi, Z.U.; Temple, R.J.; Larwood, J.P.J.; Greenhalgh, T.; Bourouiba, L. Two metres or one: What is the evidence for physical distancing in COVID-19? BMJ 2020, 370, m3223. [Google Scholar] [CrossRef] [PubMed]
- Günther, T.; Czech-Sioli, M.; Indenbirken, D.; Robitaille, A.; Tenhaken, P.; Exner, M.; Ottinger, M.; Fischer, N.; Grundhoff, A.; Brinkmann, M.M. SARS-CoV-2 outbreak investigation in a German meat processing plant. EMBO Mol. Med. 2020, 12, e13296. [Google Scholar] [CrossRef]
- Siobhan, R. The Swiss Cheese Model of Pandemic Defense. New York Times. Available online: https://www.nytimes.com/2020/12/05/health/coronavirus-swiss-cheese-infection-mackay.html (accessed on 5 December 2020).
- Hyland-Wood, B.; Gardner, J.; Leask, J.; Ecker, U.K.H. Toward effective government communication strategies in the era of COVID-19. Humanit. Soc. Sci. Commun. 2021, 8, 30. [Google Scholar] [CrossRef]
| COVID-19 Cases, no. (%) N = 296 | Controls, no. (%) N = 536 | p-Value | |
|---|---|---|---|
| Sex | |||
| Male | 232 (78.4%) | 395 (73.7%) | 0.133 |
| Female | 64 (21.6%) | 141 (26.3%) | |
| Age, years | |||
| 20–29 | 80 (27.0%) | 139 (25.9%) | 0.333 |
| 30–39 | 119 (40.2%) | 189 (35.3%) | |
| 40–49 | 64 (21.6%) | 133 (24.8%) | |
| 50–59 | 32 (10.8%) | 68 (12.7%) | |
| 60+ | 1 (0.3%) | 7 (1.3%) | |
| Education | |||
| University | 125 (42.2%) | 175 (32.6%) | 0.003 * |
| Vocational | 162 (54.7%) | 323 (60.3%) | |
| High school | 9 (3.0%) | 38 (7.1%) | |
| Body mass index, kg/m2 | |||
| Normal weight (< 24.9) | 115 (38.9%) | 228 (42.5%) | 0.178 |
| Overweight (25.0–29.9) | 115 (38.9%) | 205 (38.2%) | |
| Obese > 30 | 50 (16.9%) | 66 (12.3%) | |
| Missing | 16 (5.4%) | 37 (6.9%) | |
| Place of residence | |||
| West Kazakhstan | 239 (80.7%) | 406 (75.7%) | 0.598 |
| South Kazakhstan | 35 (11.8%) | 86 (16.0%) | |
| North Kazakhstan | 6 (2.0%) | 9 (1.7%) | |
| East Kazakhstan | 4 (1.4%) | 7 (1.3%) | |
| Central Kazakhstan | 1 (0.3%) | 3 (0.6%) | |
| Other countries | 11 (3.7%) | 25 (4.7%) |
| Characteristics | Cases, no, (%), N = 296 | Controls, no, (%), N = 536 | cOR (95% CI) * | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 232 (78.4%) | 395 (73.7%) | 1.3 (0.9–1.8) | 0.134 |
| Female | 64 (21.6%) | 141 (26.3%) | 1 | |
| Age group | ||||
| Less than 36 years | 160 (54.1%) | 274 (51.1%) | 1 | |
| 36 years or more | 136 (45.9%) | 262 (48.9%) | 0.9 (0.7–1.2) | 0.417 |
| Education | ||||
| University degree | 125 (42.2%) | 175 (32.6%) | 1.5 (1.1–2) | 0.006 † |
| Vocational or high school degree | 171 (57.8%) | 361 (67.4%) | 1 | |
| Body mass index (kg/m2) | ||||
| Normal weight (≤ 24.9) | 115 (38.9%) | 228 (42.5%) | 1 | |
| Overweight (25.0–29.9) | 115 (38.9%) | 205 (38.2%) | 1.1 (0.8–1.5) | 0.515 |
| Obese ≥ 30 | 50 (16.9%) | 66 (12.3%) | 1.5 (1–2.3) | 0.063 |
| Travel before coming to shift | ||||
| Yes | 17 (5.7%) | 12 (2.2%) | 2.6 (1.2–5.8) | 0.008 † |
| No | 279 (94.3%) | 524 (97.8%) | 1 | |
| Social interactions outside of working hours | ||||
| Yes | 138 (46.6%) | 159 (29.7%) | 2.1 (1.5–2.8) | < 0.001 † |
| No | 158 (53.4%) | 377 (70.3%) | 1 | |
| Knowledge of symptoms: | ||||
| Fever | ||||
| Yes | 218 (73.6%) | 442 (82.5%) | 0.6 (0.4–0.8) | 0.003 † |
| No | 78 (26.4%) | 94 (17.5%) | 1 | |
| Cough | ||||
| Yes | 149 (50.3%) | 309 (57.6%) | 0.7 (0.6–1) | 0.042 † |
| No | 147 (49.7%) | 227 (42.4%) | 1 | |
| Loss of smell or taste | ||||
| Yes | 154 (52.0%) | 251 (46.8%) | 1.2 (0.9–1.6) | 0.151 |
| No | 142 (48.0%) | 285 (53.2%) | 1 | |
| Shortness of breath | ||||
| Yes | 110 (37.2%) | 217 (40.5%) | 0.9 (0.6–1.2) | 0.347 |
| No | 186 (62.8%) | 319 (59.5%) | 1 | |
| Consider COVID-19 to be a nonserious issue | ||||
| Not serious or unsure | 68 (23.0%) | 96 (17.9%) | 1.4 (1–2) | 0.040 † |
| Serious | 216 (73.0%) | 440 (82.1%) | 1 | |
| Believe asymptomatic COVID-19 is contagious | ||||
| Yes | 160 (54.1%) | 372 (69.4%) | 0.5 (0.4–0.7) | < 0.001 † |
| No | 136 (45.9%) | 164 (30.6%) | 1 | |
| Believe they should use masks outdoors | ||||
| Yes | 211 (71.3%) | 447 (83.4%) | 0.5 (0.4–0.7) | < 0.001 † |
| No | 85 (28.7%) | 89 (16.6%) | 1 | |
| Believe they should use masks in public places | ||||
| Yes | 255 (86.1%) | 494 (92.2%) | 0.5 (0.3–0.8) | 0.006 † |
| No | 41 (13.9%) | 42 (7.8%) | 1 | |
| Believe they should use masks in the dormitory | ||||
| Yes | 253 (85.5%) | 477 (89.0%) | 0.7 (0.5–1.1) | 0.138 |
| No | 43 (14.5%) | 59 (11.0%) | 1 | |
| Use of N95 respirators | ||||
| Yes | 34 (11.5%) | 57 (10.6%) | 1.1 (0.7–1.7) | 0.706 |
| No | 262 (88.5%) | 479 (89.4%) | 1 | |
| Use of fabric facemask | ||||
| Yes | 42 (14.2%) | 128 (23.9%) | 0.5 (0.4–0.8) | 0.001 † |
| No | 254 (85.8%) | 408 (76.1%) | 1 | |
| Use of surgical facemask | ||||
| Yes | 246 (83.1%) | 410 (76.5%) | 1.5 (1.1–2.2) | 0.025 † |
| No | 50 (16.9%) | 126 (23.5%) | 1 | |
| Number of masks changed per day | ||||
| 5 or more | 159 (53.7%) | 304 (56.7%) | 1 | |
| 3–4 | 72 (24.3%) | 150 (28.0%) | 1 (0.7–1.4) | 0.911 |
| 2 | 43 (14.5%) | 61 (11.4%) | 1.3 (0.9–2.1) | 0.178 |
| 1 | 16 (5.4%) | 19 (3.5%) | 1.6 (0.8–3.2) | 0.174 |
| Use of sanitizer at the work | ||||
| Always | 26 (8.8%) | 100 (18.7%) | 1 | |
| Seldom | 159 (53.7%) | 209 (39.0%) | 2.9 (1.8–4.8) | < 0.001 † |
| Never | 111 (37.5%) | 227 (42.4%) | 1.9 (1.2–3.1) | 0.010 † |
| Characteristics | Cases, no, (%), N = 296 | Controls, no, (%), N = 536 | cOR (95% CI) * | p-Value |
|---|---|---|---|---|
| Pre-shift quarantine, N = 589 | ||||
| Less than 8 days | 95 (32.1%) | 161 (30.0%) | 1.4 (1–2) | 0.049 † |
| 8 or more days | 98 (33.1%) | 235 (43.8%) | 1 | |
| Trained on COVID-19 prevention measures | ||||
| Yes | 248 (83.8%) | 486 (90.7%) | 0.5 (0.3–0.8) | 0.003 † |
| No | 48 (16.2%) | 50 (9.3%) | 1 | |
| Contact with a COVID-19 patient at work | ||||
| Yes | 86 (29.1%) | 146 (27.2%) | 1.4 (1–2) | 0.028 † |
| No | 152 (51.4%) | 372 (69.4%) | 1 | |
| Exposed to a COVID-19 patient with < 1.5 m | ||||
| Yes | 56 (65.1%) | 83 (56.8%) | 2.2 (1.2–4.2) | 0.016 † |
| No | 17 (19.8%) | 55 (37.7%) | 1 | |
| Exposed to COVID-19 patient for > 15 min in the room | ||||
| Yes | 66 (76.7%) | 99 (67.8%) | 2.2 (1–5) | 0.042 † |
| No | 10 (11.6%) | 33 (22.6%) | 1 | |
| Living in the dormitory | ||||
| 1–4 roommates | 93 (31.4%) | 280 (52.2%) | 0.4 (0.3–0.5) | < 0.001 † |
| Alone | 201 (67.9%) | 240 (44.8%) | 1 | |
| Sharing toilet on the floor of the dormitory | ||||
| Yes | 127 (42.9%) | 194 (36.2%) | 1.3 (1–1.8) | 0.057 |
| No | 169 (57.1%) | 342 (63.8%) | 1 | |
| Individual toilet in the room | ||||
| Yes | 171 (57.8%) | 350 (65.3%) | 0.7 (0.5–1) | 0.032 † |
| No | 125 (42.2%) | 186 (34.7%) | 1 | |
| Working in the infirmary/clinic | ||||
| Yes | 11 (3.7%) | 2 (0.4%) | 9.7 (2.5–68.8) | < 0.001 † |
| No | 285 (96.3%) | 534 (99.6%) | 1 | |
| Transport work | ||||
| Yes | 36 (12.2%) | 43 (8.0%) | 1.6 (1–2.5) | 0.051 † |
| No | 260 (87.8%) | 493 (92.0%) | 1 | |
| Working in an office | ||||
| Yes | 88 (29.7%) | 115 (21.5%) | 1.5 (1.1–2.1) | 0.008 † |
| No | 208 (70.3%) | 421 (78.5%) | 1 | |
| Working outdoors | ||||
| Yes | 86 (29.1%) | 196 (36.6%) | 0.7 (0.5–1) | 0.028 † |
| No | 210 (70.9%) | 340 (63.4%) | 1 | |
| Working in a kitchen | ||||
| Yes | 29 (9.8%) | 96 (17.9%) | 0.5 (0.3–0.8) | 0.002 † |
| No | 267 (90.2%) | 440 (82.1%) | 1 | |
| Working in a storeroom | ||||
| Yes | 3 (1.0%) | 10 (1.9%) | 0.6 (0.1–1.9) | 0.343 |
| No | 293 (99.0%) | 526 (98.1%) | 1 | |
| Other work stations ‡ | ||||
| Yes | 56 (18.9%) | 82 (15.3%) | 1.3 (0.9–1.9) | 0.179 |
| No | 240 (81.1%) | 454 (84.7%) | 1 | |
| Maintaining 1.5 m distance at work | ||||
| Yes | 221 (74.7%) | 453 (84.5%) | 0.4 (0.3–0.7) | < 0.001 † |
| No | 61 (20.6%) | 55 (10.3%) | 1 | |
| Air conditioner at work | ||||
| Yes | 17 (5.7%) | 7 (1.3%) | 4.5 (1.9–12) | < 0.001 † |
| No | 279 (94.3%) | 529 (98.7%) | 1 | |
| Ventilation system at work | ||||
| Yes | 32 (10.8%) | 103 (19.2%) | 0.5 (0.3–0.8) | 0.002 † |
| No | 264 (89.2%) | 433 (80.8%) | 1 | |
| Availability of hand sanitizers at work | ||||
| Yes | 268 (90.5%) | 405 (75.6%) | 3.2 (2.0–5.1) | < 0.001 † |
| No | 24 (8.1%) | 115 (21.5%) | 1 | |
| Use of gloves in the dormitory corridors | ||||
| Yes | 64 (21.6%) | 169 (31.5%) | 0.6 (0.4–0.8) | 0.002 † |
| No | 232 (78.4%) | 367 (68.5%) | 1 | |
| Use of gloves on the bus | ||||
| Yes | 70 (23.6%) | 191 (35.6%) | 0.6 (0.4–0.8) | < 0.001 † |
| No | 226 (76.4%) | 345 (64.4%) | 1 | |
| Use of gloves at work | ||||
| Yes | 204 (68.9%) | 343 (64.0%) | 1.3 (1–1.8) | 0.083 |
| No | 89 (30.1%) | 193 (36.0%) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabirova, D.; Taubayeva, R.; Maratova, A.; Henderson, A.; Nassyrova, S.; Kalkanbayeva, M.; Alaverdyan, S.; Smagul, M.; Levy, S.; Yesmagambetova, A.; et al. Factors Associated with an Outbreak of COVID-19 in Oilfield Workers, Kazakhstan, 2020. Int. J. Environ. Res. Public Health 2022, 19, 3291. https://doi.org/10.3390/ijerph19063291
Nabirova D, Taubayeva R, Maratova A, Henderson A, Nassyrova S, Kalkanbayeva M, Alaverdyan S, Smagul M, Levy S, Yesmagambetova A, et al. Factors Associated with an Outbreak of COVID-19 in Oilfield Workers, Kazakhstan, 2020. International Journal of Environmental Research and Public Health. 2022; 19(6):3291. https://doi.org/10.3390/ijerph19063291
Chicago/Turabian StyleNabirova, Dilyara, Ryszhan Taubayeva, Ainur Maratova, Alden Henderson, Sayagul Nassyrova, Marhzan Kalkanbayeva, Sevak Alaverdyan, Manar Smagul, Scott Levy, Aizhan Yesmagambetova, and et al. 2022. "Factors Associated with an Outbreak of COVID-19 in Oilfield Workers, Kazakhstan, 2020" International Journal of Environmental Research and Public Health 19, no. 6: 3291. https://doi.org/10.3390/ijerph19063291
APA StyleNabirova, D., Taubayeva, R., Maratova, A., Henderson, A., Nassyrova, S., Kalkanbayeva, M., Alaverdyan, S., Smagul, M., Levy, S., Yesmagambetova, A., & Singer, D. (2022). Factors Associated with an Outbreak of COVID-19 in Oilfield Workers, Kazakhstan, 2020. International Journal of Environmental Research and Public Health, 19(6), 3291. https://doi.org/10.3390/ijerph19063291





