Risk of Nonalcoholic Fatty Liver Disease Is Associated with Urinary Phthalate Metabolites Levels in Adults with Subclinical Hypothyroidism: Korean National Environmental Health Survey (KoNEHS) 2012–2014
Abstract
:1. Introduction
2. Materials and Methods
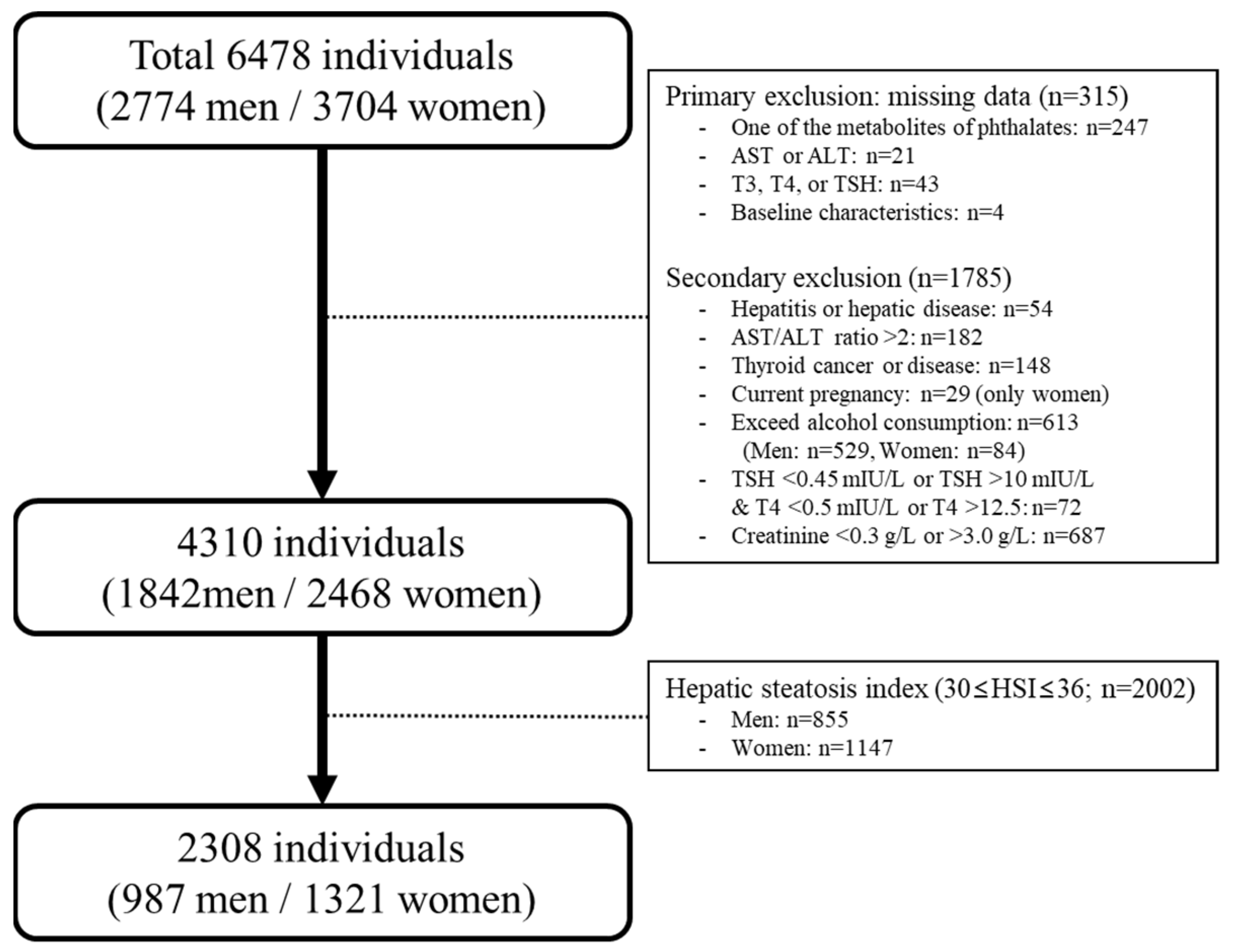
2.1. Study Population
2.2. Data Collection and Diagnosis
2.3. Measurement of Phthalate Metabolites in Urine
2.4. Definition of Nonalcoholic Fatty Liver Disease
2.5. Definition of Subclinical Hypothyroidism
2.6. Statistical Analysis
3. Results
3.1. General Characteristics of Study Participants
3.2. Prevalence of NAFLD in Participants with Euthyroidism and with SCH
3.3. The Concentrations of Phthalate Metabolites in Urine
3.4. The Association between Urinary Phthalate Metabolites and NAFLD after Stratified by TSH Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hauser, R.; Calafat, A.M. Phthalates and human health. Occup. Environ. Med. 2005, 62, 806–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calafat, A.M.; Ye, X.; Silva, M.J.; Kuklenyik, Z.; Needham, L.L. Human exposure assessment to environmental chemicals using biomonitoring. Int. J. Androl. 2006, 29, 166–171; discussion 181–165. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Bolt, H.M.; Preuss, R.; Angerer, J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch. Toxicol. 2005, 79, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Barr, D.B.; Reidy, J.A.; Kato, K.; Malek, N.A.; Hodge, C.C.; Hurtz, D., 3rd; Calafat, A.M.; Needham, L.L.; Brock, J.W. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch. Toxicol. 2003, 77, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Bolt, H.M.; Angerer, J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch. Toxicol. 2004, 78, 123–130. [Google Scholar] [CrossRef]
- Frederiksen, H.; Skakkebaek, N.E.; Andersson, A.M. Metabolism of phthalates in humans. Mol. Nutr. Food Res. 2007, 51, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Stepanova, M.; Younossi, Y.; Golabi, P.; Mishra, A.; Rafiq, N.; Henry, L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020, 69, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motamed, N.; Rabiee, B.; Poustchi, H.; Dehestani, B.; Hemasi, G.R.; Khonsari, M.R.; Maadi, M.; Saeedian, F.S.; Zamani, F. Non-alcoholic fatty liver disease (NAFLD) and 10-year risk of cardiovascular diseases. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Jung, H.S.; Yun, K.E.; Cho, J.; Cho, Y.K.; Ryu, S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am. J. Gastroenterol. 2013, 108, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, M.; Cheng, M.; Fan, L.; Wang, X.; Xu, T.; Wang, B.; Chen, W. Associations between urinary phthalate metabolite concentrations and markers of liver injury in the US adult population. Environ. Int. 2021, 155, 106608. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Kim, T.; Hong, Y.P. Urinary Phthalate Levels Associated with the Risk of Nonalcoholic Fatty Liver Disease in Adults: The Korean National Environmental Health Survey (KoNEHS) 2012–2014. Int. J. Environ. Res. Public Health 2021, 18, 6035. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Fan, J.; Ye, J.; Rao, X.; Li, Y. Phthalates exposure is associated with non-alcoholic fatty liver disease among US adults. Ecotoxicol. Environ. Saf. 2021, 224, 112665. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, N.; Milanovic, M.; Sudji, J.; Bosic Zivanovic, D.; Stojanoski, S.; Vukovic, B.; Milic, N.; Medic Stojanoska, M. Could phthalates exposure contribute to the development of metabolic syndrome and liver disease in humans? Environ. Sci. Pollut. Res. Int. 2020, 27, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Haddow, J.E.; Thyroid Study, G. Subclinical hypothyroidism and pregnancy outcomes. Obstet. Gynecol. 2005, 106, 198; author reply 198–199. [Google Scholar] [CrossRef] [PubMed]
- Surks, M.I.; Ortiz, E.; Daniels, G.H.; Sawin, C.T.; Col, N.F.; Cobin, R.H.; Franklyn, J.A.; Hershman, J.M.; Burman, K.D.; Denke, M.A.; et al. Subclinical thyroid disease: Scientific review and guidelines for diagnosis and management. JAMA 2004, 291, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, G.E.; Kim, D.; Kim, W.; Yim, J.Y.; Park, M.J.; Kim, Y.J.; Yoon, J.H.; Lee, H.S. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J. Hepatol. 2012, 57, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Pagadala, M.R.; Zein, C.O.; Dasarathy, S.; Yerian, L.M.; Lopez, R.; McCullough, A.J. Prevalence of hypothyroidism in nonalcoholic fatty liver disease. Dig. Dis. Sci. 2012, 57, 528–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshraghian, A.; Dabbaghmanesh, M.H.; Eshraghian, H.; Fattahi, M.R.; Omrani, G.R. Nonalcoholic fatty liver disease in a cluster of Iranian population: Thyroid status and metabolic risk factors. Arch. Iran. Med. 2013, 16, 584–589. [Google Scholar] [PubMed]
- Mazo, D.F.; Lima, V.M.; Stefano, J.T.; Rabelo, F.; Faintuch, J.; Oliveira, C.P. Gluco-lipidic indices in treated hypothyroidism associated with nonalcoholic fatty liver disease. Arq. Gastroenterol. 2011, 48, 186–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Zhang, W.; Rui, B.B.; Yang, S.M.; Xu, W.P.; Wei, W. Di(2-ethylhexyl) phthalate exacerbates non-alcoholic fatty liver in rats and its potential mechanisms. Environ. Toxicol. Pharmacol. 2016, 42, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, X.Y.; Zhang, W.W.; Chen, H.; Xu, W.P.; Wei, W. The effects of di 2-ethyl hexyl phthalate (DEHP) on cellular lipid accumulation in HepG2 cells and its potential mechanisms in the molecular level. Toxicol. Mech. Methods 2017, 27, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Huff, M.; da Silveira, W.A.; Carnevali, O.; Renaud, L.; Hardiman, G. Systems Analysis of the Liver Transcriptome in Adult Male Zebrafish Exposed to the Plasticizer (2-Ethylhexyl) Phthalate (DEHP). Sci. Rep. 2018, 8, 2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feige, J.N.; Gerber, A.; Casals-Casas, C.; Yang, Q.; Winkler, C.; Bedu, E.; Bueno, M.; Gelman, L.; Auwerx, J.; Gonzalez, F.J.; et al. The pollutant diethylhexyl phthalate regulates hepatic energy metabolism via species-specific PPARalpha-dependent mechanisms. Environ. Health Perspect. 2010, 118, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; He, Z.; Li, Y.; Jiang, X.; Yu, H.; Tan, Q. Mono-2-ethylhexyl phthalate induces the expression of genes involved in fatty acid synthesis in HepG2 cells. Environ. Toxicol. Pharmacol. 2019, 69, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, S.; Yang, Z.; Li, Z.; Gong, X.; Zhang, Q.; Dong, W.; Dong, C. Disturbance of di-(2-ethylhexyl) phthalate in hepatic lipid metabolism in rats fed with high fat diet. Food Chem. Toxicol. 2020, 146, 111848. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Qi, W.; Xu, Q.; Zhao, T.; Li, X.; Yin, J.; Zhang, R.; Huo, C.; Zhou, L.; Ye, L. Relationships between di-(2-ethylhexyl) phthalate exposure and lipid metabolism in adolescents: Human data and experimental rat model analyses. Environ. Pollut. 2021, 286, 117570. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T. Environmental chemicals as thyroid hormone analogues: New studies indicate that thyroid hormone receptors are targets of industrial chemicals? Mol. Cell. Endocrinol. 2005, 242, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, L.; Wei, L.; Li, L. DEHP reduces thyroid hormones via interacting with hormone synthesis-related proteins, deiodinases, transthyretin, receptors, and hepatic enzymes in rats. Environ. Sci. Pollut. Res. Int. 2015, 22, 12711–12719. [Google Scholar] [CrossRef] [PubMed]
- Korean National Institute of Environmental Research. Guidelines for Using Raw Materials for Korean National Environmental Health Survey—The Second Stage (‘12–‘14); Korean National Institute of Environmental Research: Incheon, Korean, 2015. [Google Scholar]
- Kang, Y.; Park, J.; Yoon, K. Association between urinary phthalate metabolites and obesity in adult Korean population: Korean National Environmental Health Survey (KoNEHS), 2012–2014. Ann. Occup. Environ. Med. 2019, 31, e23. [Google Scholar] [CrossRef] [PubMed]
- Korean National Institute of Environmental Research. Analysis manual of environmental hazardous substances in biological samples (organic compounds). In Guidelines for Using Raw Materials for Korean National Environmental Health Survey—the Second Stage (‘12–‘14); Korean National Institute of Environmental Research: Incheon, Korean, 2015. [Google Scholar]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbuhler, K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Thayer, K.A.; Heindel, J.J.; Bucher, J.R.; Gallo, M.A. Role of environmental chemicals in diabetes and obesity: A National Toxicology Program workshop review. Environ. Health Perspect. 2012, 120, 779–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.J.; Lee, E.J.; Jeong, S.H.; Hong, Y.P.; Ahn, S.; Yang, Y.J. Perinatal exposure to di-(2-ethylhexyl) phthalate induces hepatic lipid accumulation mediated by diacylglycerol acyltransferase 1. Hum. Exp. Toxicol. 2021, 9603271211003314. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Ha, M.; Yang, M.; Yue, P.; Xie, Z.; Liu, C. Di2-ethylhexyl phthalate disrupts thyroid hormone homeostasis through activating the Ras/Akt/TRHr pathway and inducing hepatic enzymes. Sci. Rep. 2017, 7, 40153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howarth, J.A.; Price, S.C.; Dobrota, M.; Kentish, P.A.; Hinton, R.H. Effects on male rats of di-(2-ethylhexyl) phthalate and di-n-hexylphthalate administered alone or in combination. Toxicol. Lett. 2001, 121, 35–43. [Google Scholar] [CrossRef]
- Huang, P.C.; Kuo, P.L.; Guo, Y.L.; Liao, P.C.; Lee, C.C. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum. Reprod. 2007, 22, 2715–2722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeker, J.D.; Calafat, A.M.; Hauser, R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ. Health Perspect. 2007, 115, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Choi, W.; Hwang, M.; Lee, Y.; Kim, S.; Yu, S.; Lee, I.; Paek, D.; Choi, K. Associations between urinary phthalate metabolites and bisphenol A levels, and serum thyroid hormones among the Korean adult population—Korean National Environmental Health Survey (KoNEHS) 2012–2014. Sci. Total Environ. 2017, 584–585, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, A.; Franz, C.; Breous, E.; Loos, U. Modulation of iodide uptake by dialkyl phthalate plasticisers in FRTL-5 rat thyroid follicular cells. Mol. Cell. Endocrinol. 2005, 244, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Ahn, M.Y.; Kim, H.J.; Kim, I.Y.; Han, S.Y.; Kang, T.S.; Hong, J.H.; Park, K.L.; Lee, B.M.; Kim, H.S. Effect of di(n-butyl) phthalate on testicular oxidative damage and antioxidant enzymes in hyperthyroid rats. Environ. Toxicol. 2007, 22, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.P. Hypothyroidism and thyroiditis. Williams Textb. Endocrinol. 2003, 12, 423–455. [Google Scholar]
- He, W.; An, X.; Li, L.; Shao, X.; Li, Q.; Yao, Q.; Zhang, J.A. Relationship between Hypothyroidism and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Front. Endocrinol. 2017, 8, 335. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Y.; Brent, G.A. Thyroid hormone crosstalk with nuclear receptor signaling in metabolic regulation. Trends Endocrinol. Metab. 2010, 21, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, D.S. Serum thyroid-stimulating hormone measurement for assessment of thyroid function and disease. Endocrinol. Metab. Clin. N. Am. 2001, 30, 245–264. [Google Scholar] [CrossRef]
| Total (n = 2308) | Non-NAFLD (n = 1202) | NAFLD (n = 1106) | p-Value | |
|---|---|---|---|---|
| Age, y | 43.98 ± 0.53 | 41.60 ± 0.65 | 46.81 ± 0.69 | <0.001 |
| Gender (men, %) | 49.23 ± 1.33 | 45.14 ± 1.91 | 54.12 ± 1.93 | 0.002 |
| BMI (kg/m2) | 24.19 ± 13.08 | 20.81 ± 0.07 | 28.23 ± 0.11 | <0.001 |
| Drinking Status (%) | 0.016 | |||
| Never | 31.60 ± 1.22 | 28.51 ± 1.50 | 35.29 ± 2.01 | |
| Former | 4.04 ± 0.53 | 4.54 ± 0.80 | 3.45 ± 0.65 | |
| Current | 64.35 ± 1.27 | 66.94 ± 1.63 | 61.24 ± 2.10 | |
| Smoking Status (%) | 0.002 | |||
| Never | 64.76 ± 1.41 | 67.95 ± 1.84 | 60.95 ± 1.96 | |
| Former | 13.70 ± 0.92 | 13.84 ± 1.27 | 13.53 ± 1.31 | |
| Current | 21.53 ± 1.26 | 18.20 ± 1.56 | 25.50 ± 1.90 | |
| Physical activity (%) | 0.280 | |||
| No | 64.30 ± 1.40 | 62.72 ± 1.97 | 66.19 ± 1.93 | |
| Moderate | 21.05 ± 1.13 | 22.23 ± 1.56 | 19.65 ± 1.67 | |
| Vigorous | 14.63 ± 1.01 | 15.04 ± 1.41 | 14.15 ± 1.30 | |
| Monthly household income (%) | 0.001 | |||
| ≤1.5 million KRW | 21.03 ± 1.58 | 17.62 ± 1.63 | 25.10 ± 2.13 | |
| 1.5–3 million KRW | 34.54 ± 1.66 | 34.81 ± 2.07 | 34.22 ± 2.19 | |
| 3–4.5 million KRW | 18.67 ± 1.33 | 19.23 ± 1.75 | 18.00 ± 1.72 | |
| >4.5 million KRW | 25.74 ± 1.86 | 28.33 ± 2.25 | 22.66 ± 2.21 | |
| Education (%) | <0.001 | |||
| <Middle school | 20.37 ± 1.26 | 16.05 ± 1.32 | 25.53 ± 1.83 | |
| Middle-High school | 40.65 ± 1.53 | 41.63 ± 1.96 | 39.48 ± 2.10 | |
| ≥College/University | 38.97 ± 1.68 | 42.31 ± 2.16 | 34.97 ± 2.20 | |
| Marital status (%) | <0.001 | |||
| Single | 23.82 ± 1.55 | 30.06 ± 2.14 | 16.36 ± 1.84 | |
| Married | 68.07 ± 1.65 | 63.12 ± 2.23 | 73.99 ± 2.04 | |
| Divorced/Separated | 8.09 ± 0.80 | 6.80 ± 0.89 | 9.64 ± 1.19 | |
| Comorbidities | ||||
| Hypertension | 14.19 ± 0.94 | 6.74 ± 0.77 | 23.10 ± 1.65 | <0.001 |
| Hyperlipidemia | 7.46 ± 0.67 | 1.32 ± 0.28 | 14.81 ± 1.35 | <0.001 |
| Diabetes mellitus | 30.82 ± 1.16 | 15.22 ± 1.27 | 49.47 ± 1.87 | <0.001 |
| Total (n = 2308) | Euthyroidism (n = 2125) | Subclinical Hypothyroidism (n = 183) | p-Value | |
|---|---|---|---|---|
| Age, y | 43.98 ± 0.53 | 43.59 ± 0.54 | 49.36 ± 1.84 | 0.002 |
| Gender (men, %) | 49.23 ± 1.33 | 50.04 ± 1.37 | 31.58 ± 4.80 | <0.001 |
| BMI (kg/m2) | 24.19 ± 13.08 | 24.16 ± 0.13 | 24.64 ± 0.40 | 0.240 |
| Drinking Status (%) | 0.009 | |||
| Never | 31.60 ± 1.22 | 30.74 ± 1.27 | 43.65 ± 4.92 | |
| Former | 4.04 ± 0.53 | 4.06 ± 0.56 | 3.77 ± 1.99 | |
| Current | 64.35 ± 1.27 | 65.18 ± 1.31 | 52.56 ± 4.68 | |
| Smoking Status (%) | <0.001 | |||
| Never | 67.76 ± 1.41 | 63.33 ± 1.48 | 84.84 ± 3.07 | |
| Former | 13.70 ± 0.92 | 13.80 ± 0.97 | 12.30 ± 2.91 | |
| Current | 21.53 ± 1.26 | 22.85 ± 1.34 | 2.85 ± 1.23 | |
| Physical activity (%) | 0.540 | |||
| No | 64.30 ± 1.40 | 64.47 ± 1.47 | 61.88 ± 4.55 | |
| Moderate | 21.05 ± 1.13 | 21.00 ± 1.18 | 21.74 ± 4.20 | |
| Vigorous | 14.63 ± 1.01 | 14.51 ± 1.05 | 16.36 ± 3.39 | |
| Monthly household income (%) | 0.253 | |||
| ≤1.5 million KRW | 21.03 ± 1.58 | 20.73 ± 1.60 | 25.28 ± 3.96 | |
| 1.5–3 million KRW | 34.54 ± 1.66 | 34.47 ± 1.69 | 35.48 ± 4.65 | |
| 3–4.5 million KRW | 18.67 ± 1.33 | 18.91 ± 1.36 | 15.20 ± 3.69 | |
| >4.5 million KRW | 25.74 ± 1.86 | 25.87 ± 1.90 | 24.01 ± 4.35 | |
| Education (%) | 0.041 | |||
| <Middle school | 20.37 ± 1.26 | 19.77 ± 1.29 | 28.75 ± 4.40 | |
| Middle-High school | 40.65 ± 1.53 | 40.71 ± 1.60 | 39.77 ± 4.67 | |
| ≥College/University | 38.97 ± 1.68 | 39.50 ± 1.72 | 31.47 ± 5.05 | |
| Marital status (%) | 0.038 | |||
| Single | 23.82 ± 1.55 | 24.55 ± 1.56 | 13.51 ± 5.05 | |
| Married | 68.07 ± 1.65 | 67.46 ± 1.66 | 76.65 ± 5.23 | |
| Divorced/Separated | 8.09 ± 0.80 | 7.97 ± 0.82 | 9.83 ± 2.61 | |
| Comorbidities | ||||
| Hypertension | 14.19 ± 0.94 | 13.99 ± 0.99 | 17.12 ± 3.33 | 0.372 |
| Hyperlipidemia | 7.46 ± 0.67 | 30.57 ± 1.20 | 34.45 ± 4.65 | 0.422 |
| Diabetes mellitus | 30.82 ± 1.16 | 7.18 ± 0.67 | 11.49 ± 2.80 | 0.121 |
| Euthyroidism (n = 2125) | Subclinical Hypothyroidism (n = 183) | p-Value | ||
|---|---|---|---|---|
| NAFLD | Actual number | 1007 | 99 | 0.321 |
| Weighted frequency (95% CI) | 45.23 (42.37–48.11) | 50.19 (40.72–59.63) |
| Concentrations (GM [95% CI]) | ||||
|---|---|---|---|---|
| Total (n = 2308) | Non-NAFLD (n = 1202) | NAFLD (n = 1106) | p-Value | |
| Euthyroidism (n = 2125) | ||||
| MEHHP | 3.02 (2.97–3.06) | 2.99 (2.92–3.05) | 3.05 (2.98–3.12) | 0.070 |
| MEOHP | 2.66 (2.61–2.71) | 2.67 (2.61–2.74) | 2.65 (2.58–2.72) | 0.957 |
| MECPP | 3.15 (3.10–3.19) | 3.14 (3.08–3.20) | 3.16 (3.10–3.22) | 0.353 |
| MnBP | 3.32 (3.26–3.39) | 3.36 (3.28–3.43) | 3.28 (3.20–3.37) | 0.210 |
| MBzP | 1.13 (1.05–1.21) | 1.11 (1.02–1.20) | 1.15 (1.04–1.26) | 0.209 |
| Subclinical hypothyroidism (n = 183) | ||||
| MEHHP | 3.10 (2.98–3.23) | 2.93 (2.74–3.11) | 3.28 (3.11–3.44) | 0.001 |
| MEOHP | 2.76 (2.64–2.89) | 2.62 (2.44–2.80) | 2.91 (2.72–3.09) | 0.028 |
| MECPP | 3.22 (3.11–3.33) | 3.11 (2.94–3.27) | 3.33 (3.18–3.49) | 0.011 |
| MnBP | 3.35 (3.22–3.48) | 3.35 (3.17–3.53) | 3.35 (3.18–3.52) | 0.971 |
| MBzP | 1.02 (0.84–1.20) | 0.85 (0.59–1.12) | 1.18 (0.95–1.40) | 0.037 |
| Euthyroidism (n = 2125) | Subclinical Hypothyroidism (n = 183) | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude Analysis | Multivariate Analysis | Crude Analysis | Multivariate Analysis | |||||
| OR (95% CI) | p for Trend | OR (95% CI) | p for Trend | OR (95% CI) | p for Trend | OR (95% CI) | p for Trend | |
| MEHHP | ||||||||
| Quartile 1 | 1 | 0.072 | 1 | 0.841 | 1 | 0.003 | 1 | 0.006 |
| Quartile 2 | 1.19 (0.87–1.64) | 1.09 (0.74–1.06) | 1.00 (0.34–2.96) | 2.18 (0.59–8.02) | ||||
| Quartile 3 | 1.37 (1.02–1.85) | 1.21 (0.81–1.80) | 2.78 (0.90–8.52) | 3.22 (0.68–15.13) | ||||
| Quartile 4 | 1.31 (0.93–1.83) | 1.01 (0.65–1.56) | 3.87 (1.40–10.70) | 13.59 (2.13–86.44) | ||||
| MEOHP | ||||||||
| Quartile 1 | 1 | 0.956 | 1 | 0.097 | 1 | 0.038 | 1 | 0.033 |
| Quartile 2 | 0.94 (0.69–1.29) | 0.77 (0.53–1.10) | 0.89 (0.28–2.85) | 1.46 (0.34–6.20) | ||||
| Quartile 3 | 1.08 (0.78–1.49) | 0.76 (0.50–1.16) | 1.90 (0.63–5.72) | 1.72 (0.41–7.15) | ||||
| Quartile 4 | 0.96 (0.69–1.34) | 0.66 (0.41–1.04) | 2.55 (0.91–7.10) | 8.55 (1.20–60.53) | ||||
| MECPP | ||||||||
| Quartile 1 | 1 | 0.360 | 1 | 0.421 | 1 | 0.020 | 1 | 0.024 |
| Quartile 2 | 0.94 (0.69–1.27) | 0.72 (0.51–1.01) | 1.18 (0.36–3.80) | 2.57 (0.76–8.60) | ||||
| Quartile 3 | 1.22 (0.91–1.62) | 0.86 (0.58–1.29) | 1.76 (0.56–5.54) | 1.38 (0.30–6.22) | ||||
| Quartile 4 | 1.07 (0.77–1.49) | 0.77 (0.50–1.19) | 3.29 (1.19–9.08) | 9.06 (1.78–45.96) | ||||
| MnBP | ||||||||
| Quartile 1 | 1 | 0.209 | 1 | 0.032 | 1 | 0.914 | 1 | 0.930 |
| Quartile 2 | 0.98 (0.71–1.36) | 0.86 (0.59–1.26) | 1.78 (0.58–5.48) | 1.01 (0.25–4.11) | ||||
| Quartile 3 | 0.85 (0.62–1.15) | 0.64 (0.40–1.00) | 0.75 (0.25–2.28) | 0.68 (0.16–2.88) | ||||
| Quartile 4 | 0.84 (0.60–1.16) | 0.60 (0.35–1.01) | 1.41 (0.48–4.09) | 1.06 (0.22–5.08) | ||||
| MBzP | ||||||||
| Quartile 1 | 1 | 0.210 | 1 | 0.840 | 1 | 0.043 | 1 | 0.009 |
| Quartile 2 | 1.19 (0.87–1.64) | 1.15 (0.79–1.67) | 1.97 (0.68–5.67) | 2.37 (0.58–9.55) | ||||
| Quartile 3 | 1.14 (0.84–1.56) | 1.14 (0.77–1.69) | 1.84 (0.61–5.51) | 2.14 (0.66–6.90) | ||||
| Quartile 4 | 1.23 (0.92–1.63) | 1.04 (0.70–1.56) | 3.23 (1.08–9.68) | 6.05 (1.62–22.54) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, E.-J.; Choi, B.-S.; Yang, Y.-J. Risk of Nonalcoholic Fatty Liver Disease Is Associated with Urinary Phthalate Metabolites Levels in Adults with Subclinical Hypothyroidism: Korean National Environmental Health Survey (KoNEHS) 2012–2014. Int. J. Environ. Res. Public Health 2022, 19, 3267. https://doi.org/10.3390/ijerph19063267
Yang E-J, Choi B-S, Yang Y-J. Risk of Nonalcoholic Fatty Liver Disease Is Associated with Urinary Phthalate Metabolites Levels in Adults with Subclinical Hypothyroidism: Korean National Environmental Health Survey (KoNEHS) 2012–2014. International Journal of Environmental Research and Public Health. 2022; 19(6):3267. https://doi.org/10.3390/ijerph19063267
Chicago/Turabian StyleYang, Eun-Jung, Byung-Sun Choi, and Yun-Jung Yang. 2022. "Risk of Nonalcoholic Fatty Liver Disease Is Associated with Urinary Phthalate Metabolites Levels in Adults with Subclinical Hypothyroidism: Korean National Environmental Health Survey (KoNEHS) 2012–2014" International Journal of Environmental Research and Public Health 19, no. 6: 3267. https://doi.org/10.3390/ijerph19063267
APA StyleYang, E.-J., Choi, B.-S., & Yang, Y.-J. (2022). Risk of Nonalcoholic Fatty Liver Disease Is Associated with Urinary Phthalate Metabolites Levels in Adults with Subclinical Hypothyroidism: Korean National Environmental Health Survey (KoNEHS) 2012–2014. International Journal of Environmental Research and Public Health, 19(6), 3267. https://doi.org/10.3390/ijerph19063267






